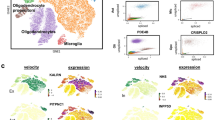
Abstract
Exercise’s protective effects in Alzheimer’s disease (AD) are well recognized, but cell-specific contributions to this phenomenon remain unclear. Here we used single-nucleus RNA sequencing (snRNA-seq) to dissect the response to exercise (free-wheel running) in the neurogenic stem-cell niche of the hippocampal dentate gyrus in male APP/PS1 transgenic AD model mice. Transcriptomic responses to exercise were distinct between wild-type and AD mice, and most prominent in immature neurons. Exercise restored the transcriptional profiles of a proportion of AD-dysregulated genes in a cell type-specific manner. We identified a neurovascular-associated astrocyte subpopulation, the abundance of which was reduced in AD, whereas its gene expression signature was induced with exercise. Exercise also enhanced the gene expression profile of disease-associated microglia. Oligodendrocyte progenitor cells were the cell type with the highest proportion of dysregulated genes recovered by exercise. Last, we validated our key findings in a human AD snRNA-seq dataset. Together, these data present a comprehensive resource for understanding the molecular mediators of neuroprotection by exercise in AD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
snRNA-seq data are available at the Sequence Read Archive with accession no. SUB13470069 and BioProject accession no. PRJNA976296 and the Broad Institute Single Cell Portal with accession no. SCP2229 and PIN 3JBO34XYYU. The human single-nucleus data from the Knight Alzheimer Disease Research Center accessed in the present study are found in the National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site with accession no. NG00108. All snRNA-seq analysis results are provided as Supplementary Data. Source data are provided with this paper.
Code availability
The relevant code can be found in Supplementary Code 1.
References
Buchman, A. S. et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78, 1323–1329 (2012).
Huuha, A. M. et al. Can exercise training teach us how to treat Alzheimer’s disease? Ageing Res. Rev. 75, 101559 (2022).
Adlard, P. A., Perreau, V. M., Pop, V. & Cotman, C. W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 25, 4217–4221 (2005).
Tapia-Rojas, C., Aranguiz, F., Varela-Nallar, L. & Inestrosa, N. C. Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s disease. Brain Pathol. 26, 62–74 (2016).
Choi, S. H. et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science https://doi.org/10.1126/science.aan8821 (2018).
De Miguel, Z. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Lee, H. et al. Genetic Alzheimer’s disease risk affects the neural mechanisms of pattern separation in hippocampal cubfields. Curr. Biol. 30, 4201–4212.e4203 (2020).
Voss, M. W., Vivar, C., Kramer, A. F. & van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 17, 525–544 (2013).
Goncalves, J. T., Schafer, S. T. & Gage, F. H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167, 897–914 (2016).
DeCarolis, N. A., Kirby, E. D., Wyss-Coray, T. & Palmer, T. D. The role of the microenvironmental niche in declining stem-cell functions associated with biological aging. Cold Spring Harbor Perspect. Med. https://doi.org/10.1101/cshperspect.a025874 (2015).
Salta, E. et al. Adult hippocampal neurogenesis in Alzheimer’s disease: a roadmap to clinical relevance. Cell Stem Cell 30, 120–136 (2023).
Hochgerner, H., Zeisel, A., Lonnerberg, P. & Linnarsson, S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 21, 290–299 (2018).
Zhou, Y. et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature 607, 527–533 (2022).
Zhong, S. et al. Decoding the development of the human hippocampus. Nature 577, 531–536 (2020).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Sadick, J. S. et al. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110, 1788–1805.e1710 (2022).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e1217 (2017).
MoTrPAC Study Group; Lead Analysts; MoTrPAC Study Group. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 629, 174–183 (2024).
Liu, L. et al. Exercise reprograms the inflammatory landscape of multiple stem cell compartments during mammalian aging. Cell Stem Cell 30, 689–705.e684 (2023).
Buckley, M. T. et al. Cell-type-specific aging clocks to quantify aging and rejuvenation in neurogenic regions of the brain. Nat. Aging 3, 121–137 (2023).
Garthe, A. & Kempermann, G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Neurosci. 7, 63 (2013).
Bak, J., Pyeon, H.-I., Seok, J.-I. & Choi, Y.-S. Effect of rotation preference on spontaneous alternation behavior on Y maze and introduction of a new analytical method, entropy of spontaneous alternation. Behav. Brain Res. 320, 219–224 (2017).
Sahay, A., Wilson, D. A. & Hen, R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588 (2011).
Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e1016 (2018).
Tucker, N. R. et al. Myocyte-specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2-mediated myocarditis. Circulation 142, 708–710 (2020).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545 (2005).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Plumpe, T. et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 7, 77 (2006).
Maliszewska-Cyna, E., Xhima, K. & Aubert, I. A comparative study evaluating the impact of physical exercise on disease progression in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 53, 243–257 (2016).
Kozareva, D. A., Cryan, J. F. & Nolan, Y. M. Born this way: hippocampal neurogenesis across the lifespan. Aging Cell 18, e13007 (2019).
Mangieri, L. R. et al. ATP6V0C knockdown in neuroblastoma cells alters autophagy–lysosome pathway function and metabolism of proteins that accumulate in neurodegenerative disease. PLoS ONE 9, e93257 (2014).
Reddy, P. H. et al. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J. Alzheimers Dis. 7, 103–117 (2005). discussion 173-180.
Jessberger, S. et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 16, 147–154 (2009).
van Hooijdonk, L. W. et al. Lentivirus-mediated transgene delivery to the hippocampus reveals sub-field specific differences in expression. BMC Neurosci. 10, 2 (2009).
Paolicelli, R. C. et al. Microglia states and nomenclature: a field at its crossroads. Neuron 110, 3458–3483 (2022).
Sala Frigerio, C. et al. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Abeta plaques. Cell Rep. 27, 1293–1306 e1296 (2019).
Kearns, N. A. et al. Dissecting the human leptomeninges at single-cell resolution. Nat. Commun. 14, 7036 (2023).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022).
Bertram, L., McQueen, M. B., Mullin, K., Blacker, D. & Tanzi, R. E. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 39, 17–23 (2007).
Mi, W. et al. Cystatin C inhibits amyloid-beta deposition in Alzheimer’s disease mouse models. Nat. Genet. 39, 1440–1442 (2007).
Brase, L. et al. Single-nucleus RNA-sequencing of autosomal dominant Alzheimer disease and risk variant carriers. Nat. Commun. 14, 2314 (2023).
Chen, Y. & Colonna, M. Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J. Exp. Med. https://doi.org/10.1084/jem.20202717 (2021).
Deczkowska, A. et al. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173, 1073–1081 (2018).
Hou, J., Chen, Y., Grajales-Reyes, G. & Colonna, M. TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol. Neurodegen. 17, 84 (2022).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Salehipour, A., Dolatshahi, M., Haghshomar, M. & Amin, J. The role of thyroid dysfunction in Alzheimer’s disease: a systematic review and meta-analysis. J. Prev. Alzheimer’s Dis. 10, 276–286 (2023).
Song, Y. et al. Astrocytic N-methyl-d-aspartate receptors protect the hippocampal neurons against amyloid-β 142-induced synaptotoxicity by regulating nerve growth factor. J. Alzheimer’s Dis. 85, 167–178 (2022).
Habib, N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706 (2020).
Puebla, M., Tapia, P. J. & Espinoza, H. Key role of astrocytes in postnatal brain and retinal angiogenesis. Int. J. Mol. Sci. 23, 2646 (2022).
Dorrell, M. I., Aguilar, E. & Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest. Ophthalmol. Vis. Sci. 43, 3500–3510 (2002).
Oberheim, N. A. et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287 (2009).
Deming, Y. et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 11, eaau2291 (2019).
Francis, N. et al. Voluntary wheel running reduces amyloid-beta42 and rescues behavior in aged Tg2576 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 73, 359–374 (2020).
Lazarov, O. et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120, 701–713 (2005).
Brinkmalm, G. et al. A parallel reaction monitoring mass spectrometric method for analysis of potential CSF biomarkers for Alzheimer’s disease. Proteomics Clin. Appl. https://doi.org/10.1002/prca.201700131 (2018).
Li, T. et al. TFEB acetylation promotes lysosome biogenesis and ameliorates Alzheimer’s disease-relevant phenotypes in mice. J. Biol. Chem. 298, 102649 (2022).
Marksteiner, J. et al. Distribution of chromogranin B-like immunoreactivity in the human hippocampus and its changes in Alzheimer’s disease. Acta Neuropathol. 100, 205–212 (2000).
Recabarren, D. & Alarcón, M. Gene networks in neurodegenerative disorders. Life Sci. 183, 83–97 (2017).
Traxler, L. et al. Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab. 34, 1248–1263.e1246 (2022).
Ben Zablah, Y., Merovitch, N. & Jia, Z. The role of ADF/Cofilin in synaptic physiology and Alzheimer’s disease. Front. Cell Dev. Biol. 8, 594998 (2020).
Jęśko, H. et al. Age-related transcriptional deregulation of genes coding synaptic proteins in Alzheimer’s disease murine model: potential neuroprotective effect of fingolimod. Front. Mol. Neurosci. 14, 660104 (2021).
Kubiak, J. Z. & Kloc, M. Elusive role of TCTP protein and mRNA in cell cycle and cytoskeleton regulation. Results Probl. Cell Differ. 64, 217–225 (2017).
Naisbitt, S. et al. Interaction of the postsynaptic density-95/guanylate kinase ___domain-associated protein complex with a light chain of myosin-V and dynein. J. Neurosci. 20, 4524–4534 (2000).
Cheng, L. & Zhang, W. DJ-1 affects oxidative stress and pyroptosis in hippocampal neurons of Alzheimer’s disease mouse model by regulating the Nrf2 pathway. Exp. Ther. Med. 21, 557 (2021).
Blanchard, J. W. et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611, 769–779 (2022).
Eugenin von Bernhardi, J. & Dimou, L. Oligodendrogenesis is a key process for cognitive performance improvement induced by voluntary physical activity. Glia 70, 1052–1067 (2022).
O’Neill, L. M. et al. Stearoyl-CoA desaturase-2 in murine development, metabolism, and disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21228619 (2020).
Hayes, C. E. & Ntambi, J. M. Multiple sclerosis: lipids, lymphocytes, and vitamin D. Immunometabolism https://doi.org/10.20900/immunometab20200019 (2020).
Pandey, S. et al. Disease-associated oligodendrocyte responses across neurodegenerative diseases. Cell Rep. 40, 111189 (2022).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
He, W. et al. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat. Med. 10, 959–965 (2004).
Mukherjee, C. et al. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab. 32, 259–272.e210 (2020).
Miller, J. A., Woltjer, R. L., Goodenbour, J. M., Horvath, S. & Geschwind, D. H. Genes and pathways underlying regional and cell type changes in Alzheimer’s disease. Genome Med. 5, 48 (2013).
Wiegreffe, C. et al. Bcl11a (Ctip1) controls migration of cortical projection neurons through regulation of Sema3c. Neuron 87, 311–325 (2015).
Zhang, L. et al. Roles and mechanisms of axon-guidance molecules in Alzheimer’s disease. Mol. Neurobiol. 58, 3290–3307 (2021).
Fishell, G. & Kepecs, A. Interneuron types as attractors and controllers. Annu. Rev. Neurosci. 43, 1–30 (2020).
Cassano, T. et al. PDIA3 expression is altered in the lmbic brain regions of triple-transgenic mouse model of Alzheimer’s disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24033005 (2023).
Satoh, J. et al. TMEM106B expression is reduced in Alzheimer’s disease brains. Alzheimers Res. Ther. 6, 17 (2014).
Zarouchlioti, C., Parfitt, D. A., Li, W., Gittings, L. M. & Cheetham, M. E. DNAJ proteins in neurodegeneration: essential and protective factors. Philos. Trans. R. Soc. B Biol. Sci. 373, 20160534 (2018).
Vanlandewijck et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018).
Jeong, H.-W. et al. Single-cell transcriptomics reveals functionally specialized vascular endothelium in brain. eLife 11, e57520 (2022).
van Praag, H., Shubert, T., Zhao, C. & Gage, F. H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 (2005).
Pereira, A. C. et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA 104, 5638–5643 (2007).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603, 885–892 (2022).
McAvoy, K. M. et al. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron 91, 1356–1373 (2016).
Sahay, A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011).
Taupin, P. Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev. 2, 213–219 (2006).
Novak, C. M., Burghardt, P. R. & Levine, J. A. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci. Biobehav. Rev. 36, 1001–1014 (2012).
Cooper, C., Moon, H. Y. & van Praag, H. On the run for hippocampal plasticity. Cold Spring Harbor Perspect. Med. https://doi.org/10.1101/cshperspect.a029736 (2017).
Islam, M. R. et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 3, 1058–1070 (2021).
Nuber, S. et al. Abrogating native α-synuclein tetramers in mice causes a L-DOPA-responsive motor syndrome closely resembling Parkinson’s disease. Neuron 100, 75–90.e75 (2018).
Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 (1984).
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006).
Shi, Q. et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J. Neurosci. 35, 13029–13042 (2015).
Wolf, A., Bauer, B., Abner, E. L., Ashkenazy-Frolinger, T. & Hartz, A. M. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS ONE 11, e0147733 (2016).
Zhao, X. & van Praag, H. Steps towards standardized quantification of adult neurogenesis. Nat. Commun. 11, 4275 (2020).
Franklin, K. B. J. The Mouse Brain in Stereotaxic Voordinates, 3rd edn (Elsevier/Academic Press, 2008).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Ohno, Y., Murphy, R., Choi, M., Ou, W. & Sumbria, R. K. Full- versus sub-regional quantification of amyloid-beta load on mouse brain sections. J. Vis. Exp. https://doi.org/10.3791/63669 (2022).
Ocañas, S. R. et al. Minimizing the ex vivo confounds of cell-isolation techniques on transcriptomic and translatomic profiles of purified microglia. eNeuro https://doi.org/10.1523/ENEURO.0348-21.2022 (2022).
Bordt, E. A. et al. Isolation of microglia from mouse or human tissue. STAR Protoc. 1, 100035 (2020).
Tang, Y., Garson, K., Li, L. & Vanderhyden, B. C. Optimization of lentiviral vector production using polyethylenimine-mediated transfection. Oncol. Lett. 9, 55–62 (2015).
Tropepe, V. et al. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev. Biol. 208, 166–188 (1999).
Reynolds, B. A. & Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 (1992).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Guo, W., Patzlaff, N. E., Jobe, E. M. & Zhao, X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat. Protoc. 7, 2005–2012 (2012).
Tucker, N. R. et al. Transcriptional and cellular diversity of the human heart. Circulation 142, 466–482 (2020).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Li, B. et al. Cumulus provides cloud-based data analysis for large-scale single-cell and single-nucleus RNA-seq. Nat. Methods 17, 793–798 (2020).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal https://doi.org/10.14806/ej.17.1.200 (2011).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Sierksma, A. et al. Novel Alzheimer risk genes determine the microglia response to amyloid-beta but not to TAU pathology. EMBO Mol. Med. 12, e10606 (2020).
Reimand, J. et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 14, 482–517 (2019).
Xie, Z. et al. Gene set knowledge discovery with Enrichr. Curr. Protoc. 1, e90 (2021).
Ietswaart, R., Gyori, B. M., Bachman, J. A., Sorger, P. K. & Churchman, L. S. GeneWalk identifies relevant gene functions for a biological context using network representation learning. Genome Biol. 22, 55 (2021).
Zalocusky, K. A. et al. Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer’s disease. Nat. Neurosci. 24, 786–798 (2021).
Bilsland, J. G. et al. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology 33, 685–700 (2008).
Kim, W. B. & Cho, J.-H. Encoding of contextual fear memory in hippocampal–amygdala circuit. Nat. Commun. 11, 1382 (2020).
Acknowledgements
This work was supported by the NIH (grant nos. NS117694, AG062904, AG064580 and AG072054 to C.D.W., HL140187 to N.R.T., AG066171 to K.V.K., AG057777 and AG072464 to O.H. and NS118146 and NS127211 to B.A.B.); the Cure Alzheimer’s Fund, an Alzheimer Association Research Grant, a SPARC Award from the McCance Center for Brain Health, the Hassenfeld Clinical Scholar Award and the Claflin Distinguished Scholar Award to C.D.W.; the BIDMC 2023 Translational Research Hub Spark Grant Award to B.A.B.; and the MGH Fund for Medical Discovery (grant no. 2024A022508) to J.F.d.R. C.D.W. is an ADDF-Harrington Scholar. O.H. is an Archer Foundation Research Scientist. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank all members of C.D.W. and N.R.T.’s labs for helpful discussions. We thank the Neurobiology Imaging Facility at Harvard Medical School, Boston, MA, for their support with the RNAscope, and the Center of Excellence for Molecular Imaging at Mass General Brigham for access to the Nikon Ti2 Microscope with Yokogawa W1 and SoRa Module. We thank T. Kafri, Director of the UNC Lenti-shRNA Core Facility, for helpful advice.
Author information
Authors and Affiliations
Contributions
R.L. and P.S. contributed equally. M.L.L., R.L., P.S., R.S.G., P.K., J.F.d.R. and N.R.T. performed the bioinformatic analyses. J.F.d.R., L.M., M.A.I., S.V., M.R.I. and C.D.W. performed and analyzed the in vivo experiments. J.F.d.R., M.A.I, S.A., K.V.K. and C.D.W. performed and analyzed the in vitro experiments. L.M., J.F.d.R., M.A.I., P.S., R.L., G.M.G., N.B.H., K.V.M.-F., E.B.H., K.H., S.M., S.S. and P.K. performed tissue analysis. S.V. and R.D.P. performed the nuclei isolation. R.D.P. prepared the libraries for sequencing. L.B., J.F.d.R., P.K., P.S., O.H. and B.A.B. performed the human data analysis. J.F.d.R., L.M., M.A.I., S.V. and R.L. contributed to the experimental design. N.R.T. supervised the bioinformatic analysis. C.D.W. directed the research. J.F.d.R., R.L., N.R.T. and C.D.W. co-wrote the paper with assistance from all other authors.
Corresponding author
Ethics declarations
Competing interests
C.D.W. is an academic co-founder and consultant for Aevum Therapeutics and has a financial interest in Aevum Therapeutics, a company developing drugs that harness the protective molecular mechanisms of exercise to treat neurodegenerative and neuromuscular disorders. Her interests were reviewed and are managed by the MGH and Mass General Brigham in accordance with their conflict-of-interest policies. The other authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Naomi Habib and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Neurogenic niche response to exercise and AD at the single-nuclei level.
a, MWM latency to reach the escape platform in acquisition (Three-way ANOVA, Exercise n.s. P = 0.0964, Genotype ****P < 0.0001, Exercise x genotype n.s. P = 0.2664, Exercise x genotype x time *P = 0.0263). b, Acquisition 24 h probe trial (Two-way ANOVA, Exercise **P = 0.0074, Genotype n.s. P = 0.2919, Exercise x genotype n.s. P = 0.8698), and c, 24 h probe trial in reversal (Two-way ANOVA, Exercise **P = 0.0067, Genotype n.s. P = 0.3812, Exercise x genotype n.s. P = 0.904). d, Daily running activity (Two-way repeated measures ANOVA, Time ****P < 0.0001, Genotype n.s. P = 0.2706, Time x genotype n.s. P = 0.3457). e, Open field (OPF) (Two-way ANOVA, Exercise n.s. P = 0.8558, Genotype n.s. P = 0.2011, Exercise x genotype n.s. P = 0.6738), f, Spontaneous alternation behavior (SAB) (Two-way ANOVA, Exercise n.s. P = 0.1004, Genotype n.s. P = 0.1187, Exercise x genotype n.s. P = 0.2082), and g, Contextual fear conditioning (CFC) test in all mice (Two-way repeated measures ANOVA, Context ****P < 0.0001, Group n.s. P = 0.438, Context x group n.s. P = 0.4465, followed by Sidak’s multiple comparisons WT-Sed AvsB ***P = 0.0002, WT-Run AvsB ***P = 0.0004, APP/PS1-Sed AvsB n.s. P = 0.0519, APP/PS1-Run AvsB n.s. P = 0.1497). h, Number of cells per cell cluster within each group. i, PCA analysis of pseudobulk data from all samples. j, UMAP representation of marker genes expression in different clusters. Color represents expression level according to the scale bar on the right. k, Percentage of cells per cell cluster within each group. l, The scatter plot shows regulator genes based on GeneWalk analysis observed in WSvsAS. Each dot represents a regulator gene, and the color represents the cell cluster. For all behavior experiments WT-Sed n = 12, WT-Run n = 12, APP/PS1-Sed n = 9, APP/PS1-Run n = 9 (a-g), for snRNAseq WT-Sed, WT-Run, APP/PS1-Run n = 5, APP/PS1-Sed n = 4 (h and k). Data represent the mean ± s.e.m. of biologically independent samples.
Extended Data Fig. 2 Representative enriched pathways across different cell types.
GSEA pre-ranked on gene ontology results for all cell types between the ASvsAR and WSvsAS in neuronal cells (a) and glia cells (b). The representative enriched pathways were selected based on FDR < 0.25.
Extended Data Fig. 3 Remodeling of adult hippocampal neurogenesis in exercise and AD.
a, Quantification of BrdU+NeuN+ adult-born neurons in the dorsal and ventral DG and representative higher magnification confocal images with anti-BrdU (green) and NeuN (red) from WT and APP/PS1, sedentary or running, mice. Scale bar, 50 µm. n = 6 per group. Two-way ANOVA, Dorsal DG: Exercise ****P < 0.0001, Genotype *P = 0.0269, Exercise x genotype n.s. P = 0.058, Ventral DG: Exercise *P < 0.0101, Genotype n.s. P = 0.1629, Exercise x genotype n.s. P = 0.305. b, Quantification of DCX+ cells in the dorsal and ventral DG from WT and APP/PS1, sedentary or running, mice. n = 6 per group. Two-way ANOVA, Dorsal DG: Exercise n.s. P = 0.0914, Genotype **P = 0.0013, Exercise x genotype n.s. P = 0.1552, Ventral DG: Exercise **P = 0.0069, Genotype ****P < 0.0001, Exercise x genotype n.s. P = 0.1426. c, Heatmap shows the normalized mean expression (z-score) of neurogenesis-related genes reported by Hochgerner et al. in our dataset. d, UMAP representations of early neuronal marker genes expression. Color represents expression level according to the scale bar on the right. e, f, Scatter plots showing the correlation between AD and exercise effects in Neuroblast I (e) and II (f). Each dot represents a statistically significant DEG in AD (WSvsAS). Dots with black borders represent statistically significant DEGs with exercise in AD mice (ASvsAR). The color gradient illustrates the recovery score (|logFC ASvsAR | ). The dot size represents the fraction of non-zero count nuclei in the AR group. g, Dot plots showing Immature Neurons’ recDEGs for all neurogenic cell types. In each, the hue and size of the dot represent the mean expression and fraction of non-zero count nuclei, respectively. Data represent the mean ± s.e.m. (a and b) of biologically independent samples.
Extended Data Fig. 4 Immature Neurons recDEG Atpif1 knock-down disrupts neuronal proliferation and differentiation in vitro.
a-d, Primary cortical neurons were transduced with LV-shRNA for five days. PrestoBlue HS normalized cell viability (a, one-way ANOVA followed by Dunnett’s against shCtrl: shSlc25a4 n.s. P = 0.9967, shAtp6v0c n.s. P = 0.0573, shAtpif1 *P = 0.013), confirmation of the gene knock-down (KD) by qPCR (b, two-way ANOVA, KD ****P < 0.0001, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl ****P < 0.0001), and gene expression of neuronal markers in response to Atp6v0c (c, two-way ANOVA, KD ****P < 0.0001, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl: Neurod1 ****P < 0.0001, Dcx **P = 0.0017, Tubb3 n.s. P = 0.8694, Map2 ***P = 0.0003, Dlg4 **P = 0.0071, Syn1 **P = 0.0037, Bax *P = 0.028) and Atpif1 knock-down (d, two-way ANOVA, KD ****P < 0.0001, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl: Neurod1, Dcx, Map2, Dlg4, and Syn1 ****P < 0.0001, Tubb3 **P = 0.0046, Bax **P = 0.0022). shAtp6v0c and shSlc25a4 n = 6, shAtpif1 n = 4 (a), shAtp6v0c and shAtpif1 n = 6, shSlc25a4 n = 5 (b-d). e, Representative confocal images of EdU (red) and Nestin (green) staining of embryonic neural stem and progenitor cells transduced with LV-shRNA and maintained in proliferating media for 5 days. Scale bar, 50 µm. f-h, Neurospheres were transduced with LV-shRNA and maintained in differentiation media for five days. Confirmation of the gene knock-down by qPCR (f, two-way ANOVA, KD ****P < 0.0001, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl ****P < 0.0001), and gene expression of neuronal markers in response to Atp6v0c (g, two-way ANOVA, KD n.s. P = 0.0877, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl: Neurod1 and Tubb3 ****P < 0.0001, Dcx n.s. P = 0.3704, Map2 n.s. P = 0.0826, Dlg4 n.s. P = 0.1893, Syn1 n.s. P = 0.1901) and Atpif1 knock-down (h, two-way ANOVA, KD ****P < 0.0001, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl: Neurod1, Dcx, Tubb3, and Map2 ****P < 0.0001, Dlg4 **P = 0.0015, Syn1***P = 0.0004). n = 11 per group. i-l, Primary cortical neurons were transduced with LV-shRNA for five days and treated with 20 µM recombinant amyloid-beta 42 for the last 16 h (i and j), or Abeta-enriched Tg2576 conditioned-media for the last 3 h (k and l). Normalized calcein fluorescent signal indicative of live cells after 16 h amyloid-beta 42 treatment (i, Welch’s ANOVA followed by Dunnett’s T3 against shCtrl ****P < 0.0001), normalized EthD1 fluorescent signal indicative of dead cells after 16 h amyloid-beta 42 (j, Welch’s ANOVA followed by Dunnett’s T3 against shCtrl, shAtp6v0c **P = 0.0055, shAtpif1 ***P = 0.0002), normalized calcein fluorescent signal after 3 h Tg2576 conditioned-media (k, Welch’s ANOVA followed by Dunnett’s T3 against shCtrl ****P < 0.0001), and normalized EthD1 fluorescent signal after 3 h Tg2576 conditioned-media (l, one-way ANOVA followed by Dunnett’s against shCtrl, shAtp6v0c n.s. P = 0.0726, shAtpif1 n.s. P = 0.0754). n = 6 per group. m-o, Adult hippocampus derived neurospheres were transduced with LV-shRNA and maintained in differentiation media for three days. Confirmation of the gene knock-down by qPCR (m, two-tailed unpaired t-test ****P < 0.0001), PrestoBlue HS normalized cell viability (n, two-tailed unpaired t-test **P = 0.0065), and gene expression of neuronal markers in response to Atpif1 knock-down (o, two-way ANOVA, KD ****P < 0.0001, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl: Neurod1, Dcx, and Map2 ****P < 0.0001, Tubb3 n.s. P = 0.681, Dlg4 *P = 0.0152, Syn1 n.s. P = 0.7519, Bax n.s. P = 0.9609). n = 4 (n) and 12 per group (m, o). Data represent the mean ± s.e.m. of biologically independent samples.
Extended Data Fig. 5 Exercise regulates DAM-like microglia in AD mouse models.
a, b, Quantification of IBA-1+ microglia per mm2 in the dorsal DG (a, two-way ANOVA, Exercise **P = 0.0021, Genotype ****P < 0.0001, Exercise x genotype **P = 0.0031, followed by Fisher’s LSD, WT-Sed vs Run ns P = 0.8979, APP/PS1 Sed vs Run ***P = 0.0001) and ventral DG (b, two-way ANOVA, Exercise *P = 0.0495, Genotype ****P < 0.0001, Exercise x genotype n.s. P = 0.1564) (WT-Sed n = 6, WT-Run n = 6, APP/PS1-Sed n = 5, APP/PS1-Run n = 6). c, UMAP representation of marker genes for perivascular macrophages (Mrc1, Cd163, Cd74), monocytes (S100a4), B cells (Cd79b, Rag1), T cells (Trbc2, Cd3g), and natural killer cells (Nkg7). Color represents expression level according to the scale bar on the right. d, Cell composition in percentage for each subcluster shown in Fig. 4g–i. Two-way ANOVA, Exercise n.s. P = 0.9956, Genotype ****P < 0.0001, Exercise x genotype n.s. P = 0.8881. e, Heatmap shows the normalized mean expression (z-score) group of IFN, MHC, and Cyc-M microglia genes reported by Chen et al. in our dataset. f, Dot plots showing microglia subcluster 1 markers. In each, the hue and size of the dot represent the mean expression and fraction of non-zero count nuclei, respectively. g, Violin plots of gene signatures for DAM (Irf8, Trem2, Igf1, Axl, and Csf1) and Homeostatic (P2ry12, Cd33, Tmem119, Csf1r, Cx3cr1) microglia using snRNAseq data of microglia subcluster 1 from AD mice DG. Gene signature = sum of normalized gene expression for all genes of the gene signature per cell (n = 150 and 229 cells for ‘Sed’ and ‘Run’, respectively). Two-tailed Mann Whitney, DAM P = 0.0195 and Homeostatic P = 0.9858. h, Bar plots of gene signatures for DAM (Irf8, Trem2, Igf1, Axl, and Csf1) and Homeostatic microglia (P2ry12, Cd33, Tmem119, Csf1r, Cx3cr1) in isolated CD11b+ cells (microglia) from the cortex and hippocampus of the 5xFAD mouse model using QPCR. Gene signature = sum of normalized gene expression for all genes of the gene signature per animal (n = 8 and 7 for ‘Sed’ and ‘Run’, respectively). Two-tailed unpaired t-test, DAM P = 0.0616 and Homeostatic P = 0.9462. Data represented by the mean ± s.e.m. (a, b, d, h) or by the median (middle bold line) and upper and lower quartiles (lighter dotted lines) (g) of biologically independent samples.
Extended Data Fig. 6 Exercise shifts the transcriptional state of astrocytes.
a, b, Quantification of GFAP+ astrocytes per mm2 in the dorsal DG (a, two-way ANOVA, Exercise n.s. P = 0.9007, Genotype n.s. P = 0.5594, Exercise x genotype n.s. P = 0.3558) and ventral DG (b, two-way ANOVA, Exercise n.s. P = 0.6633, Genotype n.s. P = 0.9575, Exercise x genotype n.s. P = 0.9111) (WT-Sed n = 6, WT-Run n = 6, APP/PS1-Sed n = 5, APP/PS1-Run n = 6). c, Heatmap shows the normalized mean expression (z-score) of the marker genes for each astrocyte subcluster (subcluster 0 in blue and subcluster 1 in orange). d, UMAP representation of the expression of the high-confidence markers for Radial Glia-like cells in astrocytes. Yellow dots are all nuclei in the astrocyte cluster; grey and green dots represent potentially Radial Glia-like cells based on the expression of listed markers. e, Heatmap shows the normalized mean expression (z-score) per group of previously identified disease-associated astrocytes (DAA) markers in our dataset (subcluster 0 in blue and subcluster 1 in orange). f, Heatmap shows the normalized mean expression (z-score) per group of previously identified reactive astrocytes markers in our dataset (subcluster 0 in blue and subcluster 1 in orange). g, Bar chart of the relevant enriched terms for the astrocyte subcluster 1 marker genes from Enrichr. Enriched terms displayed presented an adjusted p-value < 0.05 determined by Fisher exact test with the Benjamini-Hochberg correction for multiple hypotheses. h, CDH4 counts in astrocytes subclusters from human parietal cortex snRNA-seq in Brase et al.42. The subclusters presented are the originally described ones from42. Linear mixed effect model (covariates, sex and cluster; random effect, sample). i, Violin plots of gene signatures in the astrocyte subcluster 1 (Mfge8, Plxna2, Grin2b, Bmper, Dab1, Pde1c, and Cdh4) using snRNAseq data of astrocytes subcluster 1 from AD mice DG. Gene signature = sum of normalized gene expression for all genes of the gene signature per cell (n = 36 and 60 cells for ‘Sed’ and ‘Run’, respectively). Two-tailed Mann Whitney P = 0.0028. j, Bar plots of gene signatures for astrocyte subcluster 1 (Mfge8, Plxna2, Grin2b, Bmper, Dab1, Pde1c, and Cdh4) in isolated ACSA2+ cells (astrocytes) from the cortex and hippocampus of the 5xFAD mouse model using qPCR. Gene signature = sum of normalized gene expression for all genes of the gene signature per animal (n = 7 for ‘Sed’ and ‘Run’). Two-tailed unpaired t-test P = 0.0240. Data represented by the mean ± s.e.m. (a, b, j) or by the median (middle bold line) and upper and lower quartiles (lighter dotted lines) (i) of biologically independent samples.
Extended Data Fig. 7 Astrocytes recDEGs knock-down alters astrocytes states in vitro.
Primary cortical mixed glia cultures were transduced with LV-shRNA for five days in standard growth media (a-d) or treated in the last 24 h with a reactive astrocyte activating cocktail of cytokines (e-h) or amyloid-β 42 peptide (i-l). Confirmation of the gene knock-down by QPCR (a, e, i), and gene expression of reactive astrocyte markers and markers of our subcluster 0 and 1 in response to Nme7 (b, f, j), St7 (c, g, k), and Thra (d, h, l) knock-down. n = 3 for shCtrl in e-l, n = 4 for all other groups. Two-way ANOVA followed by Fisher’s LSD. *p < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s., not significant. Data represent the mean ± s.e.m. of biologically independent samples. a: two-way ANOVA, KD and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl ****P < 0.0001; b: two-way ANOVA, KD and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl Gfap, Cdh20, Rorb, and Cdh4 ****P < .0001, C3 n.s. P = 0.3171, Serpina3n ***P = 0.0009, Hspb1 **P = 0.004, Csmd1 **P = 0.0068; c: two-way ANOVA, KD n.s. P = 0.2011, KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl Gfap, C3, Serpina3n, Cdh20, and Cdh4 ****P < 0.0001, Hspb1 *P = 0.0277, Rorb n.s. P = 0.1921, Csmd1 n.s. P = 0.9147; d: two-way ANOVA, KD and KD x gene ****P < .0001, followed by Fisher’s LSD compared to shCtrl Gfap, Serpina3n, Rorb, Cdh4, and Csmd1****P < 0.0001, C3 ***P = 0.0001, Hspb1 n.s. P = 0.283, Cdh20 *P = 0.0174; e: two-way ANOVA, KD and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl ****P < .0001; f: two-way ANOVA, KD and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl Gfap, Serpina3n, Cdh20, and Rorb ****P < 0.0001, C3 n.s. P = 0.7271, Hspb1 n.s. P = 0.107, Cdh4 n.s. P = 0.0856, Csmd1 n.s. P = 0.1875; g: two-way ANOVA, KD and KD x gene ****P < .0001, followed by Fisher’s LSD compared to shCtrl Gfap **P = 0.0092, C3, Cdh20, Cdh4, and Csmd1 ****P < .000, Serpina3n **P = 0.0012, Hspb1 ***P = 0.001, Rorb n.s. P = 0.159; h: two-way ANOVA, KD and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl Gfap, Serpina3n, Cdh20, Rorb, and Cdh4****P < 0.0001, C3 n.s. P = 0.8352, Hspb1 n.s. P = 0.9755, Csmd1 n.s. P = 0.1847; i: two-way ANOVA, KD and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl ****P < 0.0001; j: two-way ANOVA, KD and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl Gfap ***P = 0.0002, C3 n.s. P = 0.2851, Serpina3n **P = 0.003, Hspb1 n.s. P = 0.5116, Cdh20, Rorb, and Cdh4 ****P < 0.0001, Csmd1 **P = 0.0093; k: two-way ANOVA, KD n.s. P = 0.7698 and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl Gfap, ***P = 0.0001, C3, Cdh20, and Cdh4 ****P < 0.0001, Serpina3n n.s. P = 0.137, Hspb1 n.s. P = 0.0896, Rorb n.s. P = 0.6144, Csmd1 n.s. P = 0.6805. l: two-way ANOVA, KD n.s. P = 0.4382 and KD x gene ****P < 0.0001, followed by Fisher’s LSD compared to shCtrl Gfap, Serpina3n, Hspb1, Cdh20, Rorb, and Cdh4 ****P < 0.0001, C3 n.s. P = 0.9321, Csmd1 * P = 0.0236.
Extended Data Fig. 8 Exercise remodels AD-dysregulated pathways in mGCs.
a, Average size of amyloid-beta plaques (3D6 staining) in ventral DG sections (APP/PS1-Sed n = 5, APP/PS1-Run n = 6, three section per animal; Two-tailed unpaired t-test P = 0.6679). Data is represented by the median (middle bold line) and upper and lower quartiles (lighter dotted lines). b, Schematic representation of amyloid precursor protein (APP) processing and amyloid beta degradation pathways. Adapted from the KEGG pathway database. c, Expression level of chimeric mouse/human APPswe and the human PS1-dE9 transgene in the APP/PS1 mice, and the fraction of cells expressing the gene. d, Expression levels of alpha-secretase (Adam10), beta-secretase (Bace1), and gamma-secretase (Psenen, Ncstn, Aph1a) coding- genes in all neuronal clusters and the fraction of cells expressing the gene. e, Expression levels of the Aβ-degrading enzyme coding-gene Ide and Mme in all cell clusters and the fraction of cells expressing the gene. f, Immediate early gene expression in the different neuronal cell types by group. In each dotplot, the hue and size of the dot represent the mean expression and fraction of non-zero count nuclei, respectively. Data represented by biologically independent samples. Panel b created using BioRender.com.
Extended Data Fig. 9 Exercise and AD responses in interneurons and vascular cells.
a, Scd2 average expression of all groups in oligodendrocyte progenitor cells (OPCs) and oligodendrocytes. b, Scd2 expression in oligodendrocytes in different groups. c, Cell compositional analysis for the OPCs subclusters. Two-way ANOVA, Exercise n.s. P = 0.1966, Genotype n.s. P = 0.3081, Exercise x genotype n.s. P = 0.2483. d, Comparison of our exercise effects in DG oligodendrocytes and OPCs with reported exercise effects in the subventricular zone in Buckley et al.20. Genes common to both projects, and those we found differentially expressed in exercise vs. sedentary conditions with an FDR-adjusted p value < 0.05 are displayed. Genes with > 0.5 Log2FC change in both projects are labeled. e-g, Interneuron subcluster UMAP representation (e), mean proportion (f), and marker genes for each subcluster (g). h, Scatter plot showing the correlation between AD and exercise effects in Interneurons. Each dot represents a statistically significant DEG in AD (WSvsAS). Dots with black borders represent statistically significant DEGs with exercise in AD mice (ASvsAR). The color gradient illustrates the recovery score (|log(FC ASvsAR) | ). The dot size represents the fraction of non-zero count nuclei in the AR group. i, Dot plots showing recDEGs in Interneurons. j, Cell compositional analysis for the Vascular cell subclusters. Two-way ANOVA, subcluster 0: Exercise *P = 0.0266, Genotype n.s. P = 0.9986, Exercise x genotype n.s. P = 0.2262, subcluster 1: Exercise *P = 0.0113, Genotype n.s. P = 0.378, Exercise x genotype n.s. P = 0.5948, subcluster 2: Exercise n.s. P = 0.9024, Genotype n.s. P = 0.492, Exercise x genotype n.s. P = 0.4417. k, Sema3c was a significant recDEGs shared by different cell types. l, Body weights at the start and end of the experiment. Two-way repeated measures ANOVA, Group n.s. P = 0.0603, Time *P = 0.0232, Group x time **P = 0.0018. WT-Sed n = 5, WT-Run n = 5, APP/PS1-Sed n = 4, APP/PS1-Run n = 5 (c and j). WT-Sed n = 12, WT-Run n = 12, APP/PS1-Sed n = 9, APP/PS1-Run n = 9 (l). In each dotplot, the hue and size of the dot represent the mean expression and fraction of non-zero count nuclei, respectively. Data represent the mean ± s.e.m. of biologically independent samples (c, j, l).
Supplementary information
Supplementary Code 1
Jupyter Notebook with relevant code.
Supplementary Data 1
snRNA-seq quality control metrics.
Supplementary Data 2
Marker genes for the global cell clusters.
Supplementary Data 3
DEGs for each cell type.
Supplementary Data 4
GeneWalk analysis.
Supplementary Data 5
GSEA analysis of AD and exercise effects.
Supplementary Data 6
CellChat analysis.
Supplementary Data 7
Marker genes for the neurogenic cell types.
Supplementary Data 8
Genes dysregulated in AD and recovered with exercise (recDEGs).
Supplementary Data 9
Comparison with human AD snRNA-seq from ref. 42.
Supplementary Data 10
Marker genes for cellular subclusters.
Supplementary Data 11
Nuclei counts for subclusters.
Supplementary Data 12
Functional enrichment analysis for astrocyte subcluster 1.
Supplementary Table 1
Antibodies, primers and demographics of human samples used in the present study.
Source data
Source Data Fig. 1
Numerical source data and statistical details.
Source Data Fig. 2
Numerical source data and statistical details.
Source Data Fig. 3
Numerical source data and statistical details.
Source Data Fig. 4
Numerical source data and statistical details.
Source Data Fig. 5
Numerical source data and statistical details.
Source Data Fig. 6
Numerical source data and statistical details.
Source Data Extended Data Fig. 1
Numerical source data and statistical details.
Source Data Extended Data Fig. 3
Numerical source data and statistical details.
Source Data Extended Data Fig. 4
Numerical source data and statistical details.
Source Data Extended Data Fig. 5
Numerical source data and statistical details.
Source Data Extended Data Fig. 6
Numerical source data and statistical details.
Source Data Extended Data Fig. 7
Numerical source data and statistical details.
Source Data Extended Data Fig. 8
Numerical source data and statistical details.
Source Data Extended Data Fig. 9
Numerical source data and statistical details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Rocha, J.F., Lance, M.L., Luo, R. et al. Protective exercise responses in the dentate gyrus of Alzheimer’s disease mouse model revealed with single-nucleus RNA-sequencing. Nat Neurosci (2025). https://doi.org/10.1038/s41593-025-01971-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41593-025-01971-w