Abstract
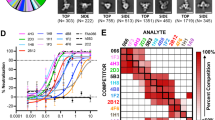
Hendra virus (HeV) and Nipah virus (NiV) are henipaviruses (HNVs) causing respiratory illness and severe encephalitis in humans, with fatality rates of 50–100%. There are no licensed therapeutics or vaccines to protect humans. HeV and NiV use a receptor-binding glycoprotein (G) and a fusion glycoprotein (F) to enter host cells. HNV F and G are the main targets of the humoral immune response, and the presence of neutralizing antibodies is a correlate of protection against NiV and HeV in experimentally infected animals. We describe here two cross-reactive F-specific antibodies, 1F5 and 12B2, that neutralize NiV and HeV through inhibition of membrane fusion. Cryo-electron microscopy structures reveal that 1F5 and 12B2 recognize distinct prefusion-specific, conserved quaternary epitopes and lock F in its prefusion conformation. We provide proof-of-concept for using antibody cocktails for neutralizing NiV and HeV and define a roadmap for developing effective countermeasures against these highly pathogenic viruses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eaton, B. T., Broder, C. C., Middleton, D. & Wang, L. F. Hendra and Nipah viruses: different and dangerous. Nat. Rev. Microbiol. 4, 23–35 (2006).
Luby, S. P. & Gurley, E. S. Epidemiology of Henipavirus disease in humans. Curr. Top. Microbiol. Immunol. 359, 25–40 (2012).
Gurley, E. S. et al. Convergence of humans, bats, trees, and culture in Nipah virus transmission, Bangladesh. Emerg. Infect. Dis. 23, 1446–1453 (2017).
Halpin, K. et al. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Tropical Med. Hyg. 85, 946–951 (2011).
Clayton, B. A. Nipah virus: transmission of a zoonotic paramyxovirus. Curr. Opin. Virol. 22, 97–104 (2017).
Pernet, O. et al. Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 5, 5342 (2014).
Navaratnarajah, C. K., Generous, A. R., Yousaf, I. & Cattaneo, R. Receptor-mediated cell entry of paramyxoviruses: mechanisms, and consequences for tropism and pathogenesis. J. Biol. Chem. 295, 2771–2786 (2020).
Bowden, T. A. et al. Structural basis of Nipah and Hendra virus attachment to their cell-surface receptor ephrin-B2. Nat. Struct. Mol. Biol. 15, 567–572 (2008).
Xu, K. et al. Host cell recognition by the henipaviruses: crystal structures of the Nipah G attachment glycoprotein and its complex with ephrin-B3. Proc. Natl Acad. Sci. USA 105, 9953–9958 (2008).
Negrete, O. A. et al. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436, 401–405 (2005).
Bonaparte, M. I. et al. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl Acad. Sci. USA 102, 10652–10657 (2005).
Negrete, O. A. et al. Two key residues in EphrinB3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathog. 2, e7 (2006).
Bishop, K. A. et al. Identification of Hendra virus G glycoprotein residues that are critical for receptor binding. J. Virol. 81, 5893–5901 (2007).
Pager, C. T. & Dutch, R. E. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J. Virol. 79, 12714–12720 (2005).
Pager, C. T., Craft, W. W., Patch, J. & Dutch, R. E. A mature and fusogenic form of the Nipah virus fusion protein requires proteolytic processing by cathepsin L. Virology 346, 251–257 (2006).
Wong, J. J. W., Paterson, R. G., Lamb, R. A. & Jardetzky, T. S. Structure and stabilization of the Hendra virus F glycoprotein in its prefusion form. Proc. Natl Acad. Sci. USA 113, 1056–1061 (2016).
Xu, K. et al. Crystal structure of the pre-fusion Nipah virus fusion glycoprotein reveals a novel hexamer-of-trimers assembly. PLoS Pathogens 11, e1005322 (2015).
Chan, Y.-P. et al. Biochemical, conformational, and immunogenic analysis of soluble trimeric forms of henipavirus fusion glycoproteins. J. Virol. 86, 11457–11471 (2012).
Liu, Q. et al. Nipah virus attachment glycoprotein stalk C-terminal region links receptor binding to fusion triggering. J. Virol. 89, 1838–1850 (2015).
Liu, Q. et al. Unraveling a three-step spatiotemporal mechanism of triggering of receptor-induced Nipah virus fusion and cell entry. PLoS Pathogens 9, e1003770 (2013).
Connolly, S. A., Leser, G. P., Yin, H. S., Jardetzky, T. S. & Lamb, R. A. Refolding of a paramyxovirus F protein from prefusion to postfusion conformations observed by liposome binding and electron microscopy. Proc. Natl Acad. Sci. USA 103, 17903–17908 (2006).
Wong, J. J. W. et al. Monomeric ephrinB2 binding induces allosteric changes in Nipah virus G that precede its full activation. Nat. Commun. 8, 781 (2017).
Yin, H. S., Paterson, R. G., Wen, X., Lamb, R. A. & Jardetzky, T. S. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc. Natl Acad. Sci. USA 102, 9288–9293 (2005).
Bossart, K. N. et al. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 4, 146ra107 (2012).
Bossart, K. N. et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathogens 5, e1000642 (2009).
Geisbert, T. W. et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci. Trans. Med. 6, 242ra82 (2014).
Zhu, Z. et al. Exceptionally potent cross‐reactive neutralization of nipah and hendra viruses by a human monoclonal antibody. J. Infect. Dis. 197, 846–853 (2008).
Zhu, Z. et al. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J. Virol. 80, 891–899 (2006).
Xu, K. et al. Crystal structure of the Hendra virus attachment G glycoprotein bound to a potent cross-reactive neutralizing human monoclonal antibody. PLoS Pathogens 9, e1003684 (2013).
Playford, E. G. et al. Safety, tolerability, pharmacokinetics, and immunogenicity of a human monoclonal antibody targeting the G glycoprotein of henipaviruses in healthy adults: a first-in-human, randomised, controlled, phase 1 study. Lancet Infect. Dis. 20, 445–454 (2020).
Dang, H. V. et al. An antibody against the F glycoprotein inhibits Nipah and Hendra virus infections. Nat. Struct. Mol. Biol. 26, 980–987 (2019).
Mire, C. E. et al. A cross-reactive humanized monoclonal antibody targeting fusion glycoprotein function protects ferrets against lethal Nipah virus and Hendra virus infection. J. Infect. Dis. 221, S471–S479 (2020).
Drexler, J. F. et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 3, 796 (2012).
Wu, Z. et al. Novel Henipa-like virus, Mojiang paramyxovirus, in rats, China, 2012. Emerg. Infect. Dis. 20, 1064–1066 (2014).
Laing, E. D. et al. Structural and functional analyses reveal promiscuous and species specific use of ephrin receptors by Cedar virus. Proc. Natl Acad. Sci. USA 116, 20707–20715 (2019).
Kondo, N., Miyauchi, K., Meng, F., Iwamoto, A. & Matsuda, Z. Conformational changes of the HIV-1 envelope protein during membrane fusion are inhibited by the replacement of its membrane-spanning ___domain. J. Biol. Chem. 285, 14681–14688 (2010).
Tortorici, M. A. et al. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science https://doi.org/10.1126/science.abe3354 (2020).
Pinto, D. et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295 (2020).
Walls, A. C. et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell 176, 1026–1039.e15 (2019).
Borst, A. J. et al. Germline VRC01 antibody recognition of a modified clade C HIV-1 envelope trimer and a glycosylated HIV-1 gp120 core. Elife 7, e37688 (2018).
Stewart-Jones, G. B. E. et al. Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell 165, 813–826 (2016).
West, B. R. et al. Structural basis of pan-Ebolavirus neutralization by a human antibody against a conserved, yet cryptic epitope. Mbio 9, e01674–18 (2018).
Pascal, K. E. et al. Development of clinical-stage human monoclonal antibodies that treat advanced ebola virus disease in nonhuman primates. J. Infect. Dis. 218, S612–S626 (2018).
Mulangu, S. et al. A randomized, controlled trial of ebola virus disease therapeutics. N. Engl. J. Med. 381, 2293–2303 (2019).
Bornholdt, Z. A. et al. A two-antibody pan-Ebolavirus cocktail confers broad therapeutic protection in ferrets and nonhuman primates. Cell Host Microbe 25, 49–58.e5 (2019).
Wec, A. Z. et al. Development of a human antibody cocktail that deploys multiple functions to confer pan-ebolavirus protection. Cell Host Microbe 25, 39–48.e5 (2019).
Logtenberg, T. Antibody cocktails: next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 25, 390–394 (2007).
Chao, T.-Y. et al. SYN023, a novel humanized monoclonal antibody cocktail, for post-exposure prophylaxis of rabies. PLoS Negl. Trop. Dis. 11, e0006133 (2017).
Nagarajan, T. et al. in Human Antibody Therapeutics for Viral Disease (ed. Dessain, S. K.) 67–101 (Springer, 2008); https://doi.org/10.1007/978-3-540-72146-8_3
Amaya, M. & Broder, C. C. Vaccines to emerging viruses: Nipah and Hendra. Annu. Rev. Virol. 7, 447–473 (2020).
Avanzato, V. A. et al. A structural basis for antibody-mediated neutralization of Nipah virus reveals a site of vulnerability at the fusion glycoprotein apex. Proc. Natl Acad. Sci. USA 116, 25057–25067 (2019).
Earl, P. L., Broder, C. C., Doms, R. W. & Moss, B. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71, 2674–2684 (1997).
Huynh-Do, U. et al. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J. Cell Sci. 115, 3073–3081 (2002).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Zhang, K. GCTF: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Scheres, S. H. W. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Goddard, T. D., Huang, C. C. & Ferrin, T. E. Visualizing density maps with UCSF Chimera. J. Struct. Biol. 157, 281–287 (2007).
Brown, A. et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D Biol. Crystallogr. 71, 136–153 (2015).
Dimaio, F. et al. Atomic-accuracy models from 4.5-Å cryo-electron microscopy data with density-guided iterative local refinement. Nat. Methods 12, 361–365 (2015).
DiMaio, F., Leaver-Fay, A., Bradley, P., Baker, D. & André, I. Modeling symmetric macromolecular structures in Rosetta3. PLoS ONE 6, e20450 (2011).
Wang, R. Y. R. et al. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. Elife 5, e17219 (2016).
Frenz, B. et al. Automatically fixing errors in glycoprotein structures with Rosetta. Structure 27, 134–139.e3 (2019).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Barad, B. A. et al. EMRinger: side chain–directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Agirre, J. et al. Privateer: software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 22, 833–834 (2015).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Laing, E. D. et al. Rescue and characterization of recombinant cedar virus, a non-pathogenic Henipavirus species. Virol. J. 15, 56 (2018).
Mishra, A. K. et al. Structure and characterization of Crimean-Congo hemorrhagic fever virus GP38. J. Virol. https://doi.org/10.1128/jvi.02005-19 (2020).
Ferrara, F. & Temperton, N. Pseudotype neutralization assays: from laboratory bench to data analysis. Methods Protoc. 1, 8 (2018).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Voss, N. R., Yoshioka, C. K., Radermacher, M., Potter, C. S. & Carragher, B. DoG Picker and TiltPicker: software tools to facilitate particle selection in single particle electron microscopy. J. Struct. Biol. 166, 205–213 (2009).
Acknowledgements
This study was supported by the National Institute of Allergy and Infectious Diseases (grant nos. DP1AI158186 and HHSN272201700059C to D.V. and grant nos. AI054715, AI077995 and AI142764 to C.C.B.), the National Institute of General Medical Sciences (grant no. GM120553 to D.V.), an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund (D.V.), a Pew Biomedical Scholars Award (D.V.) and the University of Washington Arnold and Mabel Beckman cryo-EM center. Operations support of the Galveston National Laboratory was supported by NIAID/NIH grant no. UC7AI094660.
Author information
Authors and Affiliations
Contributions
H.V.D., R.W.C., T.W.G., C.C.B. and D.V. designed the experiments. A.S.D., L.Y. and Y.-P.C. designed and cloned the HeV F and NiV F constructs and produced and isolated 1F5, 5B3 and 12B2 mouse hybridoma and mAbs. H.V.D. expressed and purified the soluble HNV F proteins used in this study. B.R.W., L.Z. and Z.A.B. performed humanization of murine mAbs and produced the humanized mAbs. H.V.D. performed IgG fragmentation and binding assays. H.V.D. conducted the cryo-EM sample preparation, data collection and data processing. H.V.D. and D.V. built and refined the atomic models. R.W.C. carried out the neutralization assays. V.B., C.M. and T.W.G. performed escape mutant analyses. C.K.N. carried out the membrane fusion inhibition assay. M.A. prepared a stable cell line. H.V.D., R.W.C., S.C.D.S., C.C.B. and D.V. analyzed the data. H.V.D. and D.V. prepared the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
Z.A.B., B.R.W. and L.Z. are employees and shareholders in Mapp Biopharmaceutical Inc., and L.Z. is a co-owner of Mapp Biopharmaceutical, Inc. D.V. is a consultant for Vir Biotechnology Inc. The Veesler laboratory has received an unrelated sponsored research agreement from Vir Biotechnology Inc. The other authors declare no competing interests.
Additional information
Peer review information Nature Structural & Molecular Biology thanks Dimiter Dimitrov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Beth Moorefield was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM characterization of NiV F in complex with the 12B2 Fab fragment.
a, Representative micrograph. Scale bar, 100 nm. b, Reference-free 2D class averages. Scale bar, 100 Å. c, Gold-standard (black) and map-model (red) Fourier shell correlation curves. Dotted line indicates the 0.143 and 0.5 thresholds. d, Two orthogonal views of the cryo-EM map colored by local resolution estimated using cryoSPARC. e, Cryo-EM data processing flow chart. Selected groups of particles at different steps are boxed. NUR: Non-Uniform refinement.
Extended Data Fig. 2 Binding of the 12B2 Fab fragment to immobilized NiV F S69A ectodomain (N67 glycan mutant) analyzed by biolayer interferometry.
Raw data are colored according to the key and fitted curves are displayed as black dashed lines. The vertical dotted lines correspond to the transition between the association and dissociation phases.
Extended Data Fig. 3 CryoEM characterization of HeV F in complex with the 1F5 Fab fragment.
a, Representative micrograph. Scale bar, 100 nm. b, Reference-free 2D class averages. Scale bar, 100 Å. c, Gold-standard (black) and map-model (red) Fourier shell correlation curves. Dotted line indicates the 0.143 and 0.5 thresholds. d, Two orthogonal views of the cryo-EM map colored by local resolution estimated using cryoSPARC. e, Cryo-EM data processing flow chart. Selected groups of particles at different steps are boxed. NUR: Non-Uniform refinement.
Extended Data Fig. 4 EM characterization of the negatively stained ternary complex of NiV F/12B2/5B3 and NiV F/12B2/1F5.
a,c, A representative micrograph and 2D class averages of NiV F/12B2/5B3 complex (a) and NiV F/12B2/1F5 complex (c) from the corresponding negative staining dataset of NiV F incubated with excess of 12B2/1F5 Fabs or 12B2/5B3 Fabs (See Materials & Methods). Micrograph scale bar: 100 nm; 2D class average scale bar: 100Å. b,d, Three-dimensional reconstructions of the ternary complex of NiV F/12B2/5B3 (b) and NiV F/12B2/1F5 (d), representing a complex with the highest stoichiometry of Fabs:NiV F from the corresponding negative staining dataset.
Extended Data Fig. 5 Comparison of the footprints of the 12B2 and the 1F5 antibody on prefusion F and postfusion F and among HNV F proteins.
a, b, Molecular surface representation of the NiV F prefusion trimer (a) and the homology model of NiV F postfusion (b) showing the 12B2 footprint in orange. c-d, Molecular surface representation of the HeV F prefusion trimer (c) and the homology model of NiV F postfusion (d) showing the 1F5 footprint in purple. The homology model of NiV F postfusion in (b) and (d) was obtained by threading the NiV F sequence onto the human parainfluenza postfusion F structure23 (PDB: 1ZTM). e, Sequence alignment of HNV F glycoproteins (NiV, HeV, GhV: Ghana bat virus; CedV: Cedar virus; MojVF: Mojiang virus). Residues on HNV F constituting the 12B2 or 1F5 epitope are denoted with an orange or purple asterisk, respectively.
Supplementary information
Rights and permissions
About this article
Cite this article
Dang, H.V., Cross, R.W., Borisevich, V. et al. Broadly neutralizing antibody cocktails targeting Nipah virus and Hendra virus fusion glycoproteins. Nat Struct Mol Biol 28, 426–434 (2021). https://doi.org/10.1038/s41594-021-00584-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-021-00584-8
This article is cited by
-
Machine learning assisted in Silico discovery and optimization of small molecule inhibitors targeting the Nipah virus glycoprotein
Scientific Reports (2025)
-
From antibiotic to antiviral: computational screening reveals a multi-targeting antibiotic from Streptomyces spp. against Nipah virus fusion proteins
Molecular Diversity (2025)
-
A potent Henipavirus cross-neutralizing antibody reveals a dynamic fusion-triggering pattern of the G-tetramer
Nature Communications (2024)
-
The cryo-EM structure of homotetrameric attachment glycoprotein from langya henipavirus
Nature Communications (2024)
-
Recent Advances in Immunological Landscape and Immunotherapeutic Agent of Nipah Virus Infection
Cell Biochemistry and Biophysics (2024)