Abstract
The heart, in addition to its primary role in blood circulation, functions as an endocrine organ by producing cardiac hormone natriuretic peptides. These hormones regulate blood pressure through the single-pass transmembrane receptor guanylyl cyclase A (GC-A), also known as natriuretic peptide receptor 1. The binding of the peptide hormones to the extracellular ___domain of the receptor activates the intracellular guanylyl cyclase ___domain of the receptor to produce the second messenger cyclic guanosine monophosphate. Despite their importance, the detailed architecture and ___domain interactions within full-length GC-A remain elusive. Here we present cryo-electron microscopy structures, functional analyses and molecular dynamics simulations of full-length human GC-A, in both the absence and the presence of atrial natriuretic peptide. The data reveal the architecture of full-length GC-A, highlighting the spatial arrangement of its various functional domains. This insight is crucial for understanding how different parts of the receptor interact and coordinate during activation. The study elucidates the molecular basis of how extracellular signals are transduced across the membrane to activate the intracellular guanylyl cyclase ___domain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The cryo-EM density maps and corresponding coordinates were deposited to the EMDB and PDB, respectively, under the following accession codes: EMD-44430 (apo full-length GC-A); EMD-44432 and PDB 9BCO (apo ICD); EMD-44433 and PDB 9BCP (apo KHD); EMD-44429 and PDB 9BCL (apo ECD (state 1)); EMD-44431 and PDB 9BCN (apo ECD (state 2)); EMD-44437 (active full-length GC-A); EMD-44436 and PDB 9BCS (active ICD); EMD-44440 and PDB 9BCV (active GCD); EMD-44434 and PDB 9BCQ (ANP-bound ECD). Source data are provided with this paper.
References
Kuhn, M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol. Rev. 96, 751–804 (2016).
Pandey, K. N. Molecular signaling mechanisms and function of natriuretic peptide receptor-a in the pathophysiology of cardiovascular homeostasis. Front Physiol. 12, 693099 (2021).
Maack, T. Receptors of atrial natriuretic factor. Annu. Rev. Physiol. 54, 11–27 (1992).
John, S. W. et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267, 679–681 (1995).
van den Akker, F. et al. Structure of the dimerized hormone-binding ___domain of a guanylyl-cyclase-coupled receptor. Nature 406, 101–104 (2000).
He, X., Chow, D., Martick, M. M. & Garcia, K. C. Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science 293, 1657–1662 (2001).
Ogawa, H., Qiu, Y., Ogata, C. M. & Misono, K. S. Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular ___domain: rotation mechanism for transmembrane signal transduction. J. Biol. Chem. 279, 28625–28631 (2004).
He, X. L., Dukkipati, A. & Garcia, K. C. Structural determinants of natriuretic peptide receptor specificity and degeneracy. J. Mol. Biol. 361, 698–714 (2006).
Misono, K. S. Atrial natriuretic factor binding to its receptor is dependent on chloride concentration: A possible feedback-control mechanism in renal salt regulation. Circ. Res. 86, 1135–1139 (2000).
Koller, K. J., Lipari, M. T. & Goeddel, D. V. Proper glycosylation and phosphorylation of the type A natriuretic peptide receptor are required for hormone-stimulated guanylyl cyclase activity. J. Biol. Chem. 268, 5997–6003 (1993).
Ramamurthy, V. et al. Interactions within the coiled-coil ___domain of RetGC-1 guanylyl cyclase are optimized for regulation rather than for high affinity. J. Biol. Chem. 276, 26218–26229 (2001).
Kelsell, R. E. et al. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum. Mol. Genet 7, 1179–1184 (1998).
Dharmaraj, S. R. et al. Mutational analysis and clinical correlation in Leber congenital amaurosis. Ophthalmic Genet 21, 135–150 (2000).
Zhao, X. et al. A novel GUCY2D mutation in a Chinese family with dominant cone dystrophy. Mol. Vis. 19, 1039–1046 (2013).
Wilson, E. M. & Chinkers, M. Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry 34, 4696–4701 (1995).
Kleinboelting, S., van den Heuvel, J. & Steegborn, C. Structural analysis of human soluble adenylyl cyclase and crystal structures of its nucleotide complexes-implications for cyclase catalysis and evolution. FEBS J. 281, 4151–4164 (2014).
Sinha, S. C. & Sprang, S. R. Structures, mechanism, regulation and evolution of class III nucleotidyl cyclases. Rev. Physiol., Biochem. Pharmacol. 157, 105–140 (2006).
Steegborn, C. Structure, mechanism, and regulation of soluble adenylyl cyclases - similarities and differences to transmembrane adenylyl cyclases. Biochim. Biophys. Acta 1842, 2535–2547 (2014).
Kang, Y., Liu, R., Wu, J. X. & Chen, L. Structural insights into the mechanism of human soluble guanylate cyclase. Nature 574, 206–210 (2019).
Horst, B. G. et al. Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy. Elife 8, https://doi.org/10.7554/eLife.50634 (2019).
Tesmer, J. J., Sunahara, R. K., Gilman, A. G. & Sprang, S. R. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS [see comments]. Science 278, 1907–1916 (1997).
Zhang, G., Liu, Y., Ruoho, A. E. & Hurley, J. H. Structure of the adenylyl cyclase catalytic core. Nature 386, 247–253 (1997).
Tesmer, J. J. et al. Two-metal-Ion catalysis in adenylyl cyclase. Science 285, 756–760 (1999).
Qi, C., Sorrentino, S., Medalia, O. & Korkhov, V. M. The structure of a membrane adenylyl cyclase bound to an activated stimulatory G protein. Science 364, 389–394 (2019).
Tews, I. et al. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science 308, 1020–1023 (2005).
Seeger, F. et al. Interfacial residues promote an optimal alignment of the catalytic center in human soluble guanylate cyclase: heterodimerization is required but not sufficient for activity. Biochemistry 53, 2153–2165 (2014).
Tal, N. et al. Cyclic CMP and cyclic UMP mediate bacterial immunity against phages. Cell 184, 5728–5739 e5716 (2021).
Tesmer, J. J. et al. Molecular basis for P-site inhibition of adenylyl cyclase. Biochemistry 39, 14464–14471 (2000).
Steegborn, C., Litvin, T. N., Levin, L. R., Buck, J. & Wu, H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat. Struct. Mol. Biol. 12, 32–37 (2005).
Joubert, S., McNicoll, N. & De Lean, A. Biochemical and pharmacological characterization of P-site inhibitors on homodimeric guanylyl cyclase ___domain from natriuretic peptide receptor-A. Biochem. Pharmacol. 73, 954–963 (2007).
Chinkers, M. & Garbers, D. L. The protein kinase ___domain of the ANP receptor is required for signaling. Science 245, 1392–1394 (1989).
Taylor, S. S. et al. From structure to the dynamic regulation of a molecular switch: A journey over 3 decades. J. Biol. Chem. 296, 100746 (2021).
Kurose, H., Inagami, T. & Ui, M. Participation of adenosine 5’-triphosphate in the activation of membrane-bound guanylate cyclase by the atrial natriuretic factor. FEBS Lett. 219, 375–379 (1987).
Chinkers, M., Singh, S. & Garbers, D. L. Adenine nucleotides are required for activation of rat atrial natriuretic peptide receptor/guanylyl cyclase expressed in a baculovirus system. J. Biol. Chem. 266, 4088–4093 (1991).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Bovy, P. R. Structure activity in the atrial natriuretic peptide (ANP) family. Med Res Rev. 10, 115–142 (1990).
Miyagi, M. & Misono, K. S. Disulfide bond structure of the atrial natriuretic peptide receptor extracellular ___domain: conserved disulfide bonds among guanylate cyclase-coupled receptors. Biochim. Biophys. Acta 1478, 30–38 (2000).
Miao, Y., Feher, V. A. & McCammon, J. A. Gaussian accelerated molecular dynamics: Unconstrained enhanced sampling and free energy calculation. J. Chem. Theory Comput. 11, 3584–3595 (2015).
Wang, J. N. et al. Gaussian accelerated molecular dynamics: Principles and applications. Wires Comput Mol. Sci. 11, e1521 (2021). ARTN e1521.
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Arai, R. Design of helical linkers for fusion proteins and protein-based nanostructures. Methods Enzymol. 647, 209–230 (2021).
Li, G. et al. Construction of a linker library with widely controllable flexibility for fusion protein design. Appl. Microbiol. Biotechnol. 100, 215–225 (2016).
Su, M. et al. Structural basis of the activation of heterotrimeric gs-protein by isoproterenol-bound beta1-adrenergic receptor. Mol. Cell 80, 59–71 e54 (2020).
Alegre, K. O. et al. Structural basis and mechanism of activation of two different families of G proteins by the same GPCR. Nat. Struct. Mol. Biol. 28, 936–944 (2021).
Mastronarde, D. N. SerialEM: A program for automated tilt series acquisition on tecnai microscopes using prediction of specimen position. Microsc. Microanal. 9, 1182–1183 (2003).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, https://doi.org/10.7554/eLife.42166 (2018).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. Biol. Crystallogr 60, 2126–2132 (2004).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Goddard, T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci.: a Publ. Protein Soc. 27, 14–25 (2018).
Guo, D. et al. A Rac-cGMP signaling pathway. Cell 128, 341–355 (2007).
Guo, D., Zhang, J. J. & Huang, X. Y. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry 48, 4417–4422 (2009).
Huang, J., Sun, Y., Zhang, J. J. & Huang, X. Y. Pivotal role of extended linker 2 in the activation of Galpha by G protein-coupled receptor. J. Biol. Chem. 290, 272–283 (2015).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Guvench, O. et al. CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J. Chem. Theory Comput. 7, 3162–3180 (2011).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of <i>n</i>-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089 (1993).
Roe, D. R. & Cheatham, T. E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Acknowledgements
We thank L. Levin, J. Levitz, T. Maack, J. Meyerson and members of our research groups for helpful discussion and comments on the paper and the Antibody and Bioresource Core Facility at the Memorial Sloan Kettering Cancer Center for help with the monoclonal antibody generation. This work was supported by National Institutes of Health (NIH) grant GM138676 (X.Y.H), NIH–National Cancer Instute Cancer Center Support Grant P30 CA008748 (R.K.H) and the startup funding project 27110 at the University of North Carolina, Chapel Hill (Y.M.). The Simons EM Center and the National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center are supported by grants from the NIH National Institute of General Medical Sciences (GM103310), NYSTAR and the Simons Foundation (SF349247). Supercomputing resources were used with allocation award TG-MCB180049 through the Extreme Science and Engineering Discovery Environment, which is supported by National Science Foundation grant ACI-1548562 and project M2874 through the National Energy Research Scientific Computing Center.
Author information
Authors and Affiliations
Contributions
S.L. expressed and purified GC-A, made cryo-EM grids and performed cryo-EM screening, data collection, structure determination, model building and paper preparation. A.M.P. and N.P. performed initial cryo-EM screening and density map determination of the ECD under the supervision of R.K.H. J.W. performed and analyzed MD simulations under the supervision of Y.M. L.Z. performed cGMP assays under the supervision of W.L. E.E. helped with cryo-EM data collection. X.Y.H. supervised the project, interpreted the data and wrote the paper. All authors contributed toward the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Michaela Kuhn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification and functional characterization of the human GC-A.
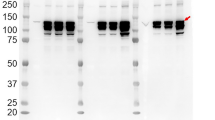
a, Size-exclusion chromatography profile of GC-A after elution from immobilized GFP-nanobody beads. b, SDS-PAGE results of peak fraction from the GC-A dimer. c, d, cGMP assays of the full-length wild-type and mutant human GC-As expressed in CHO cells. The GC-A mutants are located in the KHD and include two groups: one group is in the long extended loop region (L475G/W476G/V478G/W480G/V483G, E489G/R490G/H491G/R493G, and ΔE489-F521), and the other group includes residues involved in ATP interaction (N628A, K630A, N633A, K535A, D646A, and K666A). Data are represented as mean ± SD of three experiments.
Extended Data Fig. 2 Flow chart of Cryo-EM data processing for full-length GC-A.
The initial full-length GC-A map was determined using a small subset from the total micrographs that contributed to the final structural determination. A representative motion corrected micrograph is provided with circled particles in the “side view.” A few selected 2D classes are shown highlighting the orientation preference issue and poor particle-alignment issue even at low resolution ranges. The thumbnail maps post to refinement and classification jobs with dashed squares demonstrate the selected 3D classes and their qualities. Reference maps or particles fed into subsequent refinement or classification jobs are indicated by yellow, red and blue arrows.
Extended Data Fig. 3 Cryo-EM and model qualities of apo GC-A structures.
a, Particle orientation distributions are shown in heat maps. b, Fourier correlation curves of the Cryo-EM maps calculated in Cryosparc and of the map-to-model in PHENIX are shown in black and blue curves with 0.143 and 0.5 cutoffs, respectively, that determined the final resolutions. c, Local resolutions of Cryo-EM structures shown in heat maps.
Extended Data Fig. 4 Flow chart of Cryo-EM data processing for the ECD of GC-A.
a, 2D classes are shown highlighting the highly populated orientations of the particles. The thumbnail maps post to refinement and classification jobs with dashed squares demonstrate the selected 3D classes and their qualities. Reference maps or particles fed into subsequent refinement or classification jobs are indicated by yellow and red arrows. b, Cryo-EM density and models of the previously reported and putative glycosylation sites in GC-A.
Extended Data Fig. 5 Flow chart of Cryo-EM data processing for the ICD and KHD of GC-A.
a, The thumbnail maps post to refinement and classification jobs with dashed squares demonstrate the selected 3D classes and their qualities. Reference maps or particles fed into subsequent refinement or classification jobs are indicated by yellow and blue arrows. b, Cryo-EM density and models of the ATP molecules from GC-A monomers.
Extended Data Fig. 6 Flow chart of Cryo-EM data processing for ANP-bound full-length GC-A.
The initial full-length GC-A map was determined using a small subset from the total micrographs that contributed to the final structural determination. A few selected 2D classes are shown highlighting the side-view. The thumbnail maps post to refinement and classification jobs with dashed squares demonstrate the selected 3D classes and their qualities. Reference maps or particles fed into subsequent refinement or classification jobs are indicated by arrows.
Extended Data Fig. 7 Flow chart of Cryo-EM data processing for the ANP bound ECD of full-length GC-A.
The thumbnail maps post to refinement and classification jobs with dashed squares demonstrate the selected 3D classes and their qualities. Reference maps fed into subsequent refinement or classification jobs are indicated by yellow and red arrows.
Extended Data Fig. 8 Cryo-EM and model qualities of ANP bound GC-A structures.
a, Particle orientation distributions are shown in heat maps. b, Fourier correlation curves of the Cryo-EM maps calculated in Cryosparc and of the map-to-model in PHENIX are shown in black and blue curves with 0.143 and 0.5 cutoffs, respectively, that determined the final resolutions. c, Local resolutions of Cryo-EM structures shown in heat maps.
Extended Data Fig. 9 GaMD simulations of GC-A without and with bound ANP.
a, Ensemble of the top 20 conformations of apo GC-A colored by reweighted free energy values obtained from the GaMD simulations. b, Ensemble of the top 20 conformations of ANP-bound GC-A colored by reweighted free energy values obtained from the GaMD simulations. c–g, Time courses of the RMSDs of ANP (c), GTP in Chain A (d) and Chain B (e) relative to their cryo-EM conformations calculated from the GaMD simulations of the ANP-bound GC-A. 2D free energy profiles of the ANP-bound GC-A calculated from the GaMD simulations regarding the RMSDs of ANP and GTP in Chain A (f) and in Chain B (g).
Extended Data Fig. 10 Functional studies of mutant GC-As.
a, Structure of the CC ___domain and the guanylyl cyclase catalytic ___domain of GC-A. b, The helix-turn-helix motif links the CC to the cyclase ___domain. P822 is marked in red color. c, Structural comparison of the CCs in the apo and the active states of GC-A. d, e, cGMP assays of the full-length wild-type and mutant human GC-As expressed in CHO cells. ND: not determined. Data are represented as mean ± SD of three experiments.
Supplementary information
Source data
Source Data Extended Data Fig. 1
Source data.
Source Data Extended Data Fig. 10
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Payne, A.M., Wang, J. et al. Architecture and activation of single-pass transmembrane receptor guanylyl cyclase. Nat Struct Mol Biol 32, 469–478 (2025). https://doi.org/10.1038/s41594-024-01426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-024-01426-z
This article is cited by
-
The 11th International Conference on cGMP 2024: recent trends and developments in cGMP research —meeting report
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)