Abstract
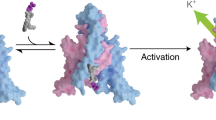
Polyunsaturated fatty acid (PUFA) lipids modulate the neuronal and microglial leak potassium channel K2P13.1 (THIK1) and other voltage-gated ion channel (VGIC) superfamily members through poorly understood mechanisms. Here we present cryo-electron microscopy structures of human THIK1 and mutants, revealing a unique two-chamber aqueous inner cavity obstructed by a hydrophilic barrier important for gating, the flow restrictor, and a P1–M4 intersubunit interface lipid at a site, the PUFA site, corresponding to the K2P small-molecule modulator pocket. This overlap, together with functional studies, indicates that PUFA site lipids are THIK1 cofactors. Comparison with a PUFA-responsive VGIC, Kv7.1, reveals a shared modulatory role for the pore ___domain intersubunit interface, providing a framework for understanding PUFA action on the VGIC superfamily. Our findings reveal the distinct THIK1 architecture, highlight the importance of the P1–M4 interface for K2P control by natural and synthetic ligands and should aid in the development of THIK subfamily modulators for neuroinflammation and autism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Coordinates and maps were deposited to the PDB and EM Data Bank under the following accession numbers: THIK1 ND, 9BSN and EMD-44870; THIK1 detergent, 9BYI and EMD-45034; THIK1-S136P ND, 9C09 and EMD-45077; THIK1-S136P detergent, 9C07 and EMD-45075; THIK1-S136P;Y273A ND, 9BWS and EMD-44978. The human THIK1 sequence was obtained from UniProt (Q9HB14). Data or materials will be provided on request from the corresponding author. Source data are provided with this paper.
Change history
15 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41594-025-01553-1
References
Enyedi, P. & Czirjak, G. Molecular background of leak K+ currents: two-pore ___domain potassium channels. Physiol. Rev. 90, 559–605 (2010).
Feliciangeli, S., Chatelain, F. C., Bichet, D. & Lesage, F. The family of K2P channels: salient structural and functional properties. J. Physiol. 593, 2587–2603 (2015).
Mathie, A., Veale, E. L., Cunningham, K. P., Holden, R. G. & Wright, P. D. Two-pore ___domain potassium channels as drug targets: anesthesia and beyond. Annu. Rev. Pharmacol. Toxicol. 61, 401–420 (2021).
Kanda, H. et al. TREK-1 and TRAAK are principal K+ channels at the nodes of Ranvier for rapid action potential conduction on mammalian myelinated afferent nerves. Neuron 104, 960–971 (2019).
Brohawn, S. G. et al. The mechanosensitive ion channel TRAAK is localized to the mammalian node of Ranvier. eLife 8, e50403 (2019).
Alloui, A. et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 25, 2368–2376 (2006).
Devilliers, M. et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat. Commun. 4, 2941 (2013).
Vivier, D. et al. Development of the first two-pore ___domain potassium channel TREK-1 (TWIK-related K+ channel 1)-selective agonist possessing in vivo anti-nociceptive activity. J. Med. Chem. 60, 1076–1088 (2017).
Yoshida, K. et al. Leak potassium channels regulate sleep duration. Proc. Natl Acad. Sci. USA 115, E9459–E9468 (2018).
Yarishkin, O. et al. TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. J. Gen. Physiol. 150, 1660–1675 (2018).
Wen, X. et al. Tandem pore ___domain acid-sensitive K channel 3 (TASK-3) regulates visual sensitivity in healthy and aging retina. Sci. Adv. 8, eabn8785 (2022).
Royal, P. et al. Migraine-associated TRESK mutations increase neuronal excitability through alternative translation initiation and inhibition of TREK. Neuron 101, 232–245 (2019).
Heurteaux, C. et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat. Neurosci. 9, 1134–1141 (2006).
Lambert, M. et al. Loss of KCNK3 is a hallmark of RV hypertrophy/dysfunction associated with pulmonary hypertension. Cardiovasc. Res. 114, 880–893 (2018).
Sörmann, J. et al. Gain-of-function mutations in KCNK3 cause a developmental disorder with sleep apnea. Nat. Genet. 54, 1534–1543 (2022).
Huang, L. S. et al. Endosomal trafficking of two-pore K+ efflux channel TWIK2 to plasmalemma mediates NLRP3 inflammasome activation and inflammatory injury. eLife 12, e83842 (2023).
Di, A. et al. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity 49, 56–65 (2018).
Drinkall, S. et al. The two pore potassium channel THIK-1 regulates NLRP3 inflammasome activation. Glia 70, 1301–1316 (2022).
Madry, C. et al. Microglial ramification, surveillance, and interleukin-1β Release are regulated by the two-pore ___domain K+ channel THIK-1. Neuron 97, 299–312 (2018).
Kyrargyri, V. et al. P2Y13 receptors regulate microglial morphology, surveillance, and resting levels of interleukin 1β release. Glia 68, 328–344 (2020).
Bagriantsev, S. N., Peyronnet, R., Clark, K. A., Honore, E. & Minor, D. L. Jr. Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 30, 3594–3606 (2011).
Piechotta, P. L. et al. The pore structure and gating mechanism of K2P channels. EMBO J. 30, 3607–3619 (2011).
Cohen, A., Ben-Abu, Y., Hen, S. & Zilberberg, N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J. Biol. Chem. 283, 19448–19455 (2008).
Lolicato, M. et al. K2P2.1 (TREK-1)–activator complexes reveal a cryptic selectivity filter binding site. Nature 547, 364–368 (2017).
Lolicato, M. et al. K2P channel C-type gating involves asymmetric selectivity filter order–disorder transitions. Sci. Adv. 6, eabc9174 (2020).
Schewe, M. et al. A non-canonical voltage-sensing mechanism controls gating in K2P K+ channels. Cell 164, 937–949 (2016).
Natale, A. M., Deal, P. E. & Minor, D. L. Jr. Structural insights into the mechanisms and pharmacology of K2P potassium channels. J. Mol. Biol. 433, 166995 (2021).
Brohawn, S. G., Campbell, E. B. & MacKinnon, R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc. Natl Acad. Sci. USA 110, 2129–2134 (2013).
Dong, Y. Y. et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science 347, 1256–1259 (2015).
Miller, A. N. & Long, S. B. Crystal structure of the human two-pore ___domain potassium channel K2P1. Science 335, 432–436 (2012).
Turney, T. S., Li, V. & Brohawn, S. G. Structural basis for pH-gating of the K+ channel TWIK1 at the selectivity filter. Nat. Commun. 13, 3232 (2022).
Li, B. B., Rietmeijer, R. A. & Brohawn, S. G. Structural basis for pH gating of the two-pore ___domain K+ channel TASK2. Nature 586, 457–462 (2020).
Rödström, K. E. J. et al. A lower X-gate in TASK channels traps inhibitors within the vestibule. Nature 582, 443–447 (2020).
Lazarenko, R. M. et al. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K+ current. J. Neurosci. 30, 9324–9334 (2010).
Sakamaki, K. et al. Dysregulation of a potassium channel, THIK-1, targeted by caspase-8 accelerates cell shrinkage. Biochim. Biophys. Acta 1863, 2766–2783 (2016).
Campanucci, V. A. et al. O2 sensing by recombinant TWIK-related halothane-inhibitable K+ channel-1 background K+ channels heterologously expressed in human embryonic kidney cells. Neuroscience 135, 1087–1094 (2005).
Chatelain, F. C. et al. Silencing of the tandem pore ___domain halothane-inhibited K+ channel 2 (THIK2) relies on combined intracellular retention and low intrinsic activity at the plasma membrane. J. Biol. Chem. 288, 35081–35092 (2013).
Kang, D., Hogan, J. O. & Kim, D. THIK-1 (K2P13.1) is a small-conductance background K+ channel in rat trigeminal ganglion neurons. Pflugers Arch. 466, 1289–1300 (2014).
Rajan, S. et al. THIK-1 and THIK-2, a novel subfamily of tandem pore ___domain K+ channels. J. Biol. Chem. 276, 7302–7311 (2001).
Zou, X. et al. The phosphodiesterase inhibitor IBMX blocks the potassium channel THIK-1 from the extracellular side. Mol. Pharmacol. 98, 143–155 (2020).
Izquierdo, P., Shiina, H., Hirunpattarasilp, C., Gillis, G. & Attwell, D. Synapse development is regulated by microglial THIK-1 K+ channels. Proc. Natl Acad. Sci. USA 118, e2106294118 (2021).
Izquierdo, P., Attwell, D. & Madry, C. Ion channels and receptors as determinants of microglial function. Trends Neurosci. 42, 278–292 (2019).
Ossola, B. et al. Characterisation of C101248: a novel selective THIK-1 channel inhibitor for the modulation of microglial NLRP3-inflammasome. Neuropharmacology 224, 109330 (2023).
Deal, P. E. et al. Development of covalent chemogenetic K2P channel activators. Cell Chem. Biol. 31, 1305–1323 (2024).
Elinder, F. & Liin, S. I. Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Front. Physiol. 8, 43 (2017).
Jowais, J. J. et al. Mechanistic understanding of KCNQ1 activating polyunsaturated fatty acid analogs. J. Gen. Physiol. 155, e202313339 (2023).
Yazdi, S. et al. Identification of PUFA interaction sites on the cardiac potassium channel KCNQ1. J. Gen. Physiol. 153, e202012850 (2021).
Golluscio, A. et al. Binding of PUFA stabilizes a conductive state of the selectivity filter in IKs channels. eLife 13, RP95852 (2024).
Liin, S. I., Yazdi, S., Ramentol, R., Barro-Soria, R. & Larsson, H. P. Mechanisms underlying the dual effect of polyunsaturated fatty acid analogs on Kv7.1. Cell Rep. 24, 2908–2918 (2018).
Pope, L., Lolicato, M. & Minor, D. L. Jr. Polynuclear ruthenium amines inhibit K2P channels via a ‘finger in the dam’ mechanism. Cell Chem. Biol. 27, 511–524 (2020).
Schmidpeter, P. A. M. et al. Membrane phospholipids control gating of the mechanosensitive potassium leak channel TREK1. Nat. Commun. 14, 1077 (2023).
Lolicato, M., Riegelhaupt, P. M., Arrigoni, C., Clark, K. A. & Minor, D. L. Jr. Transmembrane helix straightening and buckling underlies activation of mechanosensitive and thermosensitive K2P channels. Neuron 84, 1198–1212 (2014).
Brohawn, S. G., Campbell, E. B. & MacKinnon, R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 516, 126–130 (2014).
Brohawn, S. G., del Marmol, J. & MacKinnon, R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441 (2012).
Brohawn, S. G., Su, Z. & MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl Acad. Sci. USA 111, 3614–3619 (2014).
Rietmeijer, R. A., Sorum, B., Li, B. & Brohawn, S. G. Physical basis for distinct basal and mechanically gated activity of the human K+ channel TRAAK. Neuron 109, 2902–2913 (2021).
Catterall, W. A., Wisedchaisri, G. & Zheng, N. The chemical basis for electrical signaling. Nat. Chem. Biol. 13, 455–463 (2017).
Fink, M. et al. A neuronal two P ___domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 17, 3297–3308 (1998).
Patel, A. J. et al. A mammalian two pore ___domain mechano-gated S-like K+ channel. EMBO J. 17, 4283–4290 (1998).
Renigunta, V., Zou, X., Kling, S., Schlichthorl, G. & Daut, J. Breaking the silence: functional expression of the two-pore-___domain potassium channel THIK-2. Pflugers Arch. 466, 1735–1745 (2014).
Neves, M. A., Yeager, M. & Abagyan, R. Unusual arginine formations in protein function and assembly: rings, strings, and stacks. J. Phys. Chem. B. 116, 7006–7013 (2012).
Magalhaes, A., Maigret, B., Hoflack, J., Gomes, J. N. & Scheraga, H. A. Contribution of unusual arginine–arginine short-range interactions to stabilization and recognition in proteins. J. Protein Chem. 13, 195–215 (1994).
Bagriantsev, S. N., Clark, K. A. & Minor, D. L. Jr. Metabolic and thermal stimuli control K2P2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 31, 3297–3308 (2012).
Richardson, J. S. & Richardson, D. C. Amino acid preferences for specific locations at the ends of α helices. Science 240, 1648–1652 (1988).
Sandoz, G., Douguet, D., Chatelain, F., Lazdunski, M. & Lesage, F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc. Natl Acad. Sci. USA 106, 14628–14633 (2009).
Yohannan, S., Faham, S., Yang, D., Whitelegge, J. P. & Bowie, J. U. The evolution of transmembrane helix kinks and the structural diversity of G protein-coupled receptors. Proc. Natl Acad. Sci. USA 101, 959–963 (2004).
Ben Soussia, I. et al. Mutation of a single residue promotes gating of vertebrate and invertebrate two-pore ___domain potassium channels. Nat. Commun. 10, 787 (2019).
Pope, L. et al. Protein and chemical determinants of BL-1249 action and selectivity for K2P channels. ACS Chem. Neurosci. 9, 3153–3165 (2018).
Wague, A. et al. Mechanistic insights into volatile anesthetic modulation of K2P channels. eLife 9, e59839 (2020).
Huffer, K., Tan, X. F., Fernandez-Marino, A. I., Dhingra, S. & Swartz, K. J. Dilation of ion selectivity filters in cation channels. Trends Biochem. Sci. 49, 417–430 (2024).
Blin, S. et al. Tandem pore ___domain halothane-inhibited K+ channel subunits THIK1 and THIK2 assemble and form active channels. J. Biol. Chem. 289, 28202–28212 (2014).
Khoubza, L., Chatelain, F. C., Feliciangeli, S., Lesage, F. & Bichet, D. Physiological roles of heteromerization: focus on the two-pore ___domain potassium channels. J. Physiol. 599, 1041–1055 (2021).
Levitz, J. et al. Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc. Natl Acad. Sci. USA 113, 4194–4199 (2016).
Czirjak, G. & Enyedi, P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore ___domain potassium channel subunits. J. Biol. Chem. 277, 5426–5432 (2002).
Blin, S. et al. Mixing and matching TREK/TRAAK subunits generate heterodimeric K2P channels with unique properties. Proc. Natl Acad. Sci. USA 113, 4200–4205 (2016).
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360, 376 (1996).
Sun, J. & MacKinnon, R. Structural basis of human KCNQ1 modulation and gating. Cell 180, 340–347 (2020).
Liao, M., Cao, E., Julius, D. & Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 (2013).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Morales-Perez, C. L., Noviello, C. M. & Hibbs, R. E. Manipulation of subunit stoichiometry in heteromeric membrane proteins. Structure 24, 797–805 (2016).
Ritchie, T. K. et al. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 (2009).
Lee, H., Lolicato, M., Arrigoni, C. & Minor, D. L. Jr. Production of K2P2.1 (TREK-1) for structural studies. Methods Enzymol. 653, 151–188 (2021).
Shaya, D. et al. Voltage-gated sodium channel (NaV) protein dissection creates a set of functional pore-only proteins. Proc. Natl Acad. Sci. USA 108, 12313–12318 (2011).
Edelhoch, H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6, 1948–1954 (1967).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in PHENIX. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng. 8, 127–134 (1995).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413 (2016).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Parrinello, M. & Rahman, A. Polymorphic transitions in single-crystals—a new molecular-dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald—an N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Gowers, R. J. et al. MDAnalysis: a Python package for the rapid analysis of molecular dynamics simulations. In Proceedings of the 15th Python in Science Conference (eds Benthall, S. & Rostrup, S.) (SciPy, 2016).
Michaud-Agrawal, N., Denning, E. J., Woolf, T. B. & Beckstein, O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011).
Grossfield, A. WHAM: the weighted histogram analysis method, version 2.0.10. http://membrane.urmc.rochester.edu/wordpress/?page_id=126 (2024).
Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001).
Acknowledgements
We thank J. Geo for molecular biology support, H. Khant, Y. Liu and A. Cassago at the SLAC Cryo-EM Center for help with microscope handling and data acquisition, A. Mondal for critical advice on cryo-EM data analysis and model building, J.M. Rosenberg for advice on the free energy simulations and K. Brejc for comments on the manuscript. This work was supported by National Institutes of Health grant R01-MH093603 to D.L.M. Some of this work was performed at the Stanford-SLAC Cryo-EM Center (S2C2), which is supported by the National Institute of General Medical Sciences (1R24GM154186). Instruments at the UCSF Cryo-EM facility are partially supported by grants from the NIH (S10OD020054, S10OD021741 and S10OD026881). UCSF cryo-EM facility is managed by D. Bulkley and G. Gilbert.
Author information
Authors and Affiliations
Contributions
S.R.-C., S.J., F.A.-A., F.N., M.G. and D.L.M. conceptualized the study and designed the experiments. S.R.-C. expressed and purified the proteins and prepared the cryo-EM samples. S.R.-C. and F.A.-A. collected and analyzed the cryo-EM data. S.R.-C.and F.A.-A. built and refined the models. S.J. collected and analyzed the TEVC data. F.N. and M.G. designed and analyzed the computational studies. S.R.-C., S.J., F.N. M.G. and D.L.M. analyzed the data. M.G. and D.L.M. provided guidance and support. S.R.-C., S.J., F.A.-A., F.N., M.G. and D.L.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Youxing Jiang, Marcos Matamoros and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Functional characterization of THIK1EM and cryo-EM analysis in MSP1E1 nanodiscs.
a, Exemplar Two Electrode Voltage Clamp (TEVC) recordings of K2P13.1(THIK-1) (grey) and THIK1EM (orange) in Xenopus oocytes. 0 mV trace is indicated. Inset shows protocol. b, Representative TEVC current-voltage responses for K2P13.1(THIK-1) (black) and THIK1EM (orange). Inset shows protocol. c, Average currents I/I<FL> at 0 mV where I<FL> = average currents for full length K2P13.1 (THIK-1). n.s. p > 0.05. Statistical analysis was performed using Kruskal-Wallis test (nonparametric ANOVA) followed by Dunn’s multiple comparisons test. Error bars are S.E.M. d, Exemplar SEC (Superose 6 Increase 10/300 GL) for THIK1EM:MSP1E1 nanodiscs. e, peak fraction SDS-PAGE from ‘d’. More than three purifications were carried out for consistency. f, electromicrograph (~105,000x magnification) from 14,254 movies, and g, 2D class averages. h, Workflow for cryo-EM data processing for the THIK1EM:MSP1E1 nanodisc complex in cryoSPARC-3.286. Red arrow indicates the class of particles extracted without Fourier cropping after the initial cleanup. These were further subjected to heterogeneous, non-uniform, and local refinement jobs that resulted in the final map at 2.65 Å (red box). i, Particle distribution plot and j, gold-standard Fourier Shell Correlation (FSC) curves for the THIK1EM:MSP1E1 nanodisc complex.
Extended Data Fig. 2 THIK1EM:MSP1E1 nanodisc (ND) cryo-EM map and model quality.
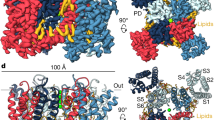
a, Cryo-EM map of the THIK1EM:ND complex. THIK1EM and associated lipids are colored magenta and turquoise. Locations of select channel elements are indicated. Nanodisc density is transparent. b, Cryo-EM maps for indicated THIK1EM elements. Select residues are indicated. Channel elements are deep salmon. Linoleic acid (EIC) is yelloworange. Maps are rendered at 4-5σ. c, THIK1EM:ND local resolution. Select channel elements and lipid belt are labeled. d, THIK1EM:ND local B-factor.
Extended Data Fig. 3 THIK1EM:ND cryo-EM maps of key features.
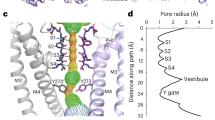
a, Selectivity filter and flow restrictor densities (6σ) for THIK1EM:ND (deep teal and salmon). Select residues are labeled. Potassium ions are shown as purple spheres. b, Cyro-EM map of the THIK1EM:ND complex showing the channel cartoon (magenta and turquoise) and ___location of PUFA density (red and yelloworange). Turquoise subunit transmembrane helices are indicated. Nanodisc density is transparent. c, Slice through the THIK1EM:ND complex cyro-EM map (7σ) showing ___location of PUFA density (red and yelloworange). Inset shows local density. PUFA site shows linoleic acid (EIC). Grey bars denote the membrane.
Extended Data Fig. 4 K2P13.1 (THIK-1) structure comparisons.
Superpositions of K2P13.1(THIK-1) (salmon) (PDB:9BSN) with: a, K2P2.1(TREK-1):ML335 (light blue) (PDB:6CQ6)24 (RMSDCa = 1.1613 Å) b, K2P10.1(TREK-2):norfluoxetine (Nfx) (hot pink) (PDB:4XDK)29 (RMSDCa = 1.580 Å). c, K2P5.1(TASK-2) pH 6.5 (orange) (PDB:6WLV)32 (RMSDCa = 1.816) and pH 8.5 (teal) (PDB:6WM0) (RMSDCa = 1.639 Å)32. d, K2P1.1(TWIK-1) (deep olive) (PDB:3UKM)30 (RMSDCa = 2.277 Å). e, K2P3.1(TASK-1) (grey) (PDB:6RV2)33 (RMSDCa = 1.323 Å). For simplicity, only one K2P13.1(THIK-1) Arg sandwich position and PUFA site are labeled. Site of the K2P3.1 (TASK-1) X-gate is labeled in ‘e’. Each panel shows side (left) and cytoplasmic (right) views. Grey bars denote the membrane.
Extended Data Fig. 5 K2P13.1(THIK-1) structural and sequence features.
a, Cytoplasmic view of the K2P13.1(THIK-1) Asn ring (Asn134 and Asn227) and flow restrictor barrier (Ile139 and Tyr273). Ser136 is also shown. Linoleic acid (EIC) is shown in space filling. b, Lateral view of the K2P13.1(THIK-1) central cavity. Asn ring (Asn134 and Asn227) and flow restrictor barrier (Ile139 and Tyr273) are labeled. EIC and S4 potassium ion (violet) are shown in space filling. Pond and inner vestibule regions are shown in blue. c, M4 sequence comparison. Labels indicate: K2P13.1(THIK-1) EIC contact residues (orange), flow restrictor (red), Asn ring (green), and K2P2.1(TREK-1) K2P modulator pocket residues that contact the ML335 activator (light blue)24. Black asterisk denotes Arg sandwich residue. d, M2 sequence comparison. Labels indicate K2P13.1(THIK-1) EIC contact residues (orange) and Asn ring (green). Red ‡ indicates S136P mutation site. e, PUFA site showing hydrogen bond and salt bridge interactions (dashed lines) and van der Waals contacts ≤ 5 Å. Colors indicate residues from the M1 (teal), M2 (magenta), P1 (salmon), and M4 (violet) helices. EIC is yelloworange and black. f, P1 sequence comparison. Labels indicate K2P13.1(THIK-1) EIC contact residues (orange) and K2P2.1(TREK-1) K2P modulator pocket residues that contact the ML335 activator (light blue)24. Black asterisk denotes Arg sandwich residue. g, M1 sequence comparison. Labels indicate K2P13.1(THIK-1) EIC contact residues (orange). h, Superposition of the SF1-M2 regions of K2P13.1(THIK-1) (salmon), K2P13.1(THIK-1) S136P (cyan), and K2P13.1(THIK-1) S136P/Y273A (magenta) with K2P2.1(TREK-1):ML335 (light blue) (PDB:6CQ6)24, K2P10.1(TREK-2):norfluoxetine (Nfx) (hot pink) (PDB:4XDK)29, K2P5.1(TASK-2) pH 6.5 (orange) (PDB:6WLV)32 and pH 8.5 (teal) (PDB:6WM0)32, K2P1.1(TWIK-1) (deep olive) (PDB:3UKM)30, pH 7.4 (7SK0)31, and pH 5.5 (7SK1)31, and K2P3.1(TASK-1) (grey) (PDB:6RV2)33. Cyan lines show the range of M2 movements indicated by the 15° double headed arc. Potassium ions are shown as purple spheres. GenBank Sequences in c, d, f, and g are for human: K2P13.1(THIK-1) 16306555; K2P13.2(THIK-2) 11545761; K2P2.1(TREK-1) 14589851; K2P10.1(TREK-2) 20143944; K2P4.1(TRAAK) 15718767; K2P3.1(TASK-1) 4504849; K2P9.1 (TASK-3) 542133161; K2P15.1(TASK-5) 333440483; K2P5.1(TASK-2) 333440483; K2P16.1(TALK-1) 14149764; K2P17.1(TALK-2) 17025230; K2P1.1(TWIK-1) 4504847; K2P6.1(TWIK-2) 4758624; and K2P18.1(TRESK) 32469495.
Extended Data Fig. 6 THIK1EM electrostatic surface potentials.
a, Electrostatic surface potentials calculated using APBS105. Side, cytoplasmic, and extracellular views are shown. b, Slice through the center of K2P13.1EM showing the inner cavity. Selectivity filter ions are shown as purple spheres. Location of Tyr273 is indicated. One subunit is shown as transparent. Select channel elements are labeled. Grey bars denote the membrane.
Extended Data Fig. 7 Structural analysis of THIK1EM in nanodiscs and detergent micelles.
Superposition of THIK1EM:DDM:CHS:GDN, THIK1EM:Det (orange) and THIK1EM:Nanodisc, THIK1EM:ND (deep teal) structures showing a, side and b, cytoplasmic views. c, Comparison of THIK1EM:Det (orange) and THIK1EM:ND (deep teal) flow restrictor regions. Linoleic acid (EIC) is shown in space filling. d, THIK1EM:DDM:CHS:GDN (deep teal and salmon) selectivity filter and flow restrictor densities (9σ). Select residues are labeled. Potassium ions are purple spheres. e, Comparison of modeled lipid positions for THIK1EM:Det (orange) THIK1EM:ND (deep teal). Common lipid positions are shown in space filling. Lipid belt, inner site lipid, and PUFA are indicated. Select channel elements are labelled. f, Extracellular view of ‘e’. Circle indicates lipid belt boundary. Potassium ions shown as purple spheres.
Extended Data Fig. 8 Structural comparison of THIK1EM S136P in nanodiscs and detergent micelles.
Superposition of THIK1EM S136P:DDM:CHS:GDN, THIK1EM S136P:Det (deep blue) and THIK1EM S136P:Nanodisc, THIK1EM S136P:ND (cyan) structures showing a, side and b, cytoplasmic views. c, Comparison of THIK1EM S136P:ND (cyan) and THIK1EM S136P:Det (deep blue) flow restrictor regions. Distances between Tyr273 hydroxyl oxygens are indicated. Linoleic acid (EIC) is shown in space filling. d, and e, Selectivity filter and flow restrictor densities (7-7.5σ) for d, THIK1EM S136P:ND (cyan) and e, THIK1EM S136P:Det. Select residues are labeled.
Extended Data Fig. 9 THIK1EM S136P;Y273A:ND structural comparisons.
a, and b, Superposition of THIK1EM S136P;Y273A:ND (magenta), THIK1EM S136P:ND (cyan) and THIK1EM:ND (salmon) a, side and b, cytoplasmic views. c, Comparison of THIK1EM S136P;Y273A:ND (magenta), THIK1EM S136P:ND (cyan). Distance between Ile139 closest approach is indicated. d, Comparison of THIK1EM S136P:ND (cyan) and THIK1EM S136P;Y273A:ND (magenta) flow restrictor regions. Distances between Ala273 methyl groups and Tyr273 hydroxyls are indicated. EIC is shown in space filling. e, Selectivity filter and flow restrictor densities (8σ) for THIK1EM S136P;Y273A:ND (magenta). Select residues are labeled. Potassium ions are shown as purple spheres.
Extended Data Fig. 10 K2P13.1(THIK-1) PUFA site simulations show binding plasticity.
a, Cα RMSD of residues lining the lipid binding pocket for each PUFA tested. b, PUFA RMSD with respect to the initial build (Cα residues 97-110 and 254-270). c-f, representative PUFA site structures from the distributions in ‘a’ for: c, EIC (yellow), d, α-LNL (firebrick), e, DGLA (orange), and f, AA (purple). In c-f Distances for AA carboxylate and Arg258 are indicated by the arrows. Starting structures are shown as transparent. Lipid carboxylate displacements are indicated by the red arrows. g-i, Observed distance distributions for: g, PUFAC1-R92CZ, h, PUFAC1-R92CZ, and i, R92CZ-R258CZ atoms.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Table 1, Legends for Videos 1–4, Data quantification and Statistical analysis.
Supplementary Video 1
THIK1 structural changes.
Supplementary Video 2
Cytoplasmic view of THIK1 structural changes.
Supplementary Video 3
Dynamics of THIK1 Y273 during umbrella sampling with potassium at the flow restrictor.
Supplementary Video 4
Dynamics of THIK1-Y273F during umbrella sampling with potassium at the flow restrictor.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Excel spreadsheets.
Source Data Extended Data Fig. 1
Uncropped gel.
Source Data Extended Data Fig. 10
Excel spreadsheets.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roy-Chowdhury, S., Jang, S., Abderemane-Ali, F. et al. Structure of the human K2P13.1 channel reveals a hydrophilic pore restriction and lipid cofactor site. Nat Struct Mol Biol (2025). https://doi.org/10.1038/s41594-024-01476-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01476-3
This article is cited by
-
Gating mechanism of the two-pore-___domain potassium channel THIK1
Nature Structural & Molecular Biology (2025)