Abstract
Drugs targeting the ghrelin receptor hold therapeutic potential in anorexia, obesity and diabetes. However, developing effective drugs is challenging. To tackle this common issue across a broad drug target, this study aims to understand how anamorelin, the only approved drug targeting the ghrelin receptor, operates compared to other synthetic drugs. Our research elucidated the receptor’s structure with anamorelin and miniGq, unveiling anamorelin’s superagonistic activity. We demonstrated that ligands with distinct chemical structures uniquely bind to the receptor, resulting in diverse conformations and biasing signal transduction. Moreover, our study showcased the utility of structural information in effectively identifying natural genetic variations altering drug action and causing severe functional deficiencies, offering a basis for selecting the right medication on the basis of the individual’s genomic sequence. Thus, by building on structural analysis, this study enhances the foundational framework for selecting therapeutic agents targeting the ghrelin receptor, by effectively leveraging signaling bias and genetic variations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The cryo-EM density maps and atomic coordinates were deposited to the EM Data Bank and PDB under accession numbers EMD-36627 and 8JSR for the anamorelin-bound ghrelin receptor–miniGq complex. The initial coordinates, simulation input files and final output coordinate file from our MD simulations, conducted in four independent replicates, were deposited to the Biological Structure Model Archive under accession code BSM00078. Source data are provided with this paper.
References
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Howard, A. D. et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273, 974–977 (1996).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Masuda, Y. et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem. Biophys. Res. Commun. 276, 905–908 (2000).
Szentirmai, E., Kapas, L., Sun, Y., Smith, R. G. & Krueger, J. M. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc. Natl Acad. Sci. USA 106, 14069–14074 (2009).
Collden, G., Tschop, M. H. & Muller, T. D. Therapeutic potential of targeting the ghrelin pathway. Int. J. Mol. Sci. 18, 798 (2017).
Pantel, J. et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J. Clin. Invest. 116, 760–768 (2006).
Inoue, H. et al. Identification and functional analysis of novel human growth hormone secretagogue receptor (GHSR) gene mutations in Japanese subjects with short stature. J. Clin. Endocrinol. Metab. 96, E373–E378 (2011).
Pantel, J. et al. Recessive isolated growth hormone deficiency and mutations in the ghrelin receptor. J. Clin. Endocrinol. Metab. 94, 4334–4341 (2009).
Fredriksson, R., Lagerstrom, M. C., Lundin, L. G. & Schioth, H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 (2003).
Offermanns, S. G-proteins as transducers in transmembrane signalling. Prog. Biophys. Mol. Biol. 83, 101–130 (2003).
Downes, G. B. & Gautam, N. The G protein subunit gene families. Genomics 62, 544–552 (1999).
Wettschureck, N. & Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204 (2005).
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schioth, H. B. & Gloriam, D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 (2017).
Fazeli, P. K. et al. Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: a randomized clinical trial. J. Clin. Psychiatry 79, 17m11585 (2018).
Wiedmer, P., Nogueiras, R., Broglio, F., D’Alessio, D. & Tschop, M. H. Ghrelin, obesity and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 3, 705–712 (2007).
Temel, J. S. et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 17, 519–531 (2016).
Wang, Y. et al. Molecular recognition of an acyl-peptide hormone and activation of ghrelin receptor. Nat. Commun. 12, 5064 (2021).
Qin, J. et al. Molecular mechanism of agonism and inverse agonism in ghrelin receptor. Nat. Commun. 13, 300 (2022).
Liu, H. et al. Structural basis of human ghrelin receptor signaling by ghrelin and the synthetic agonist ibutamoren. Nat. Commun. 12, 6410 (2021).
Shiimura, Y. et al. Structure of an antagonist-bound ghrelin receptor reveals possible ghrelin recognition mode. Nat. Commun. 11, 4160 (2020).
Masuho, I. et al. Distinct profiles of functional discrimination among G proteins determine the actions of G protein-coupled receptors. Sci. Signal. 8, ra123 (2015).
Masuho, I., Skamangas, N. K. & Martemyanov, K. A. Live cell optical assay for precise characterization of receptors coupling to Gα12. Basic Clin. Pharmacol. Toxicol. 126, 88–95 (2020).
Masuho, I., Xie, K. & Martemyanov, K. A. Macromolecular composition dictates receptor and G protein selectivity of regulator of G protein signaling (RGS) 7 and 9—2 protein complexes in living cells. J. Biol. Chem. 288, 25129–25142 (2013).
Offermanns, S. & Simon, M. I. Gα15 and Gα16 couple a wide variety of receptors to phospholipase C. J. Biol. Chem. 270, 15175–15180 (1995).
Masuho, I. et al. A global map of G protein signaling regulation by RGS proteins. Cell 183, 503–521 (2020).
Maeda, S., Qu, Q., Robertson, M. J., Skiniotis, G. & Kobilka, B. K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 (2019).
Holst, B. et al. Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J. Biol. Chem. 282, 15799–15811 (2007).
Roden, D. M. & George, A. L.Jr The genetic basis of variability in drug responses. Nat. Rev. Drug Discov. 1, 37–44 (2002).
Goodwin, S., McPherson, J. D. & McCombie, W. R. Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet. 17, 333–351 (2016).
Rabbani, B., Tekin, M. & Mahdieh, N. The promise of whole-exome sequencing in medical genetics. J. Hum. Genet. 59, 5–15 (2014).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Kryukov, G. V., Pennacchio, L. A. & Sunyaev, S. R. Most rare missense alleles are deleterious in humans: implications for complex disease and association studies. Am. J. Hum. Genet. 80, 727–739 (2007).
Subramanian, S. Quantifying harmful mutations in human populations. Eur. J. Hum. Genet. 20, 1320–1322 (2012).
Ashley, E. A. Towards precision medicine. Nat. Rev. Genet. 17, 507–522 (2016).
Hauser, A. S. et al. Pharmacogenomics of GPCR drug targets. Cell 172, 41–54 (2018).
Congreve, M., de Graaf, C., Swain, N. A. & Tate, C. G. Impact of GPCR structures on drug discovery. Cell 181, 81–91 (2020).
Kooistra, A. J. et al. GPCRdb in 2021: integrating GPCR sequence, structure and function. Nucleic Acids Res. 49, D335–D343 (2021).
Wang, S. et al. D4 dopamine receptor high-resolution structures enable the discovery of selective agonists. Science 358, 381–386 (2017).
Stein, R. M. et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 579, 609–614 (2020).
Lyu, J. et al. Ultra-large library docking for discovering new chemotypes. Nature 566, 224–229 (2019).
Hermans, E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol. Ther. 99, 25–44 (2003).
Inoue, A. et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell 177, 1933–1947 (2019).
Avet, C. et al. Effector membrane translocation biosensors reveal G protein and βarrestin coupling profiles of 100 therapeutically relevant GPCRs. eLife 11, e74101 (2022).
Himmelreich, S. et al. Dopamine receptor DAMB signals via Gq to mediate forgetting in Drosophila. Cell Rep. 21, 2074–2081 (2017).
de Mendoza, A., Sebe-Pedros, A. & Ruiz-Trillo, I. The evolution of the GPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity. Genome Biol. Evol. 6, 606–619 (2014).
Mende, F. et al. Translating biased signaling in the ghrelin receptor system into differential in vivo functions. Proc. Natl Acad. Sci. USA 115, E10255–E10264 (2018).
Dezaki, K., Kakei, M. & Yada, T. Ghrelin uses Gαi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes 56, 2319–2327 (2007).
Schoneberg, T. & Liebscher, I. Mutations in G protein-coupled receptors: mechanisms, pathophysiology and potential therapeutic approaches. Pharmacol. Rev. 73, 89–119 (2021).
Lango Allen, H. et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838 (2010).
Hollins, B., Kuravi, S., Digby, G. J. & Lambert, N. A. The C-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell Signal. 21, 1015–1021 (2009).
Fenech, C. et al. Ric-8A, a Gα protein guanine nucleotide exchange factor potentiates taste receptor signaling. Front. Cell. Neurosci. 3, 11 (2009).
Von Dannecker, L. E., Mercadante, A. F. & Malnic, B. Ric-8B promotes functional expression of odorant receptors. Proc. Natl Acad. Sci. USA 103, 9310–9314 (2006).
Raveh, A., Cooper, A., Guy-David, L. & Reuveny, E. Nonenzymatic rapid control of GIRK channel function by a G protein-coupled receptor kinase. Cell 143, 750–760 (2010).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Ferreira, F. P., Goulart, M. D., de Almeida-Pedrin, R. R., Conti, A. C. & Cardoso, M. A. Treatment of class III malocclusion: atypical extraction protocol. Case Rep. Dent. 2017, 4652685 (2017).
Bepler, T., Kelley, K., Noble, A. J. & Berger, B. Topaz-Denoise: general deep denoising models for cryoEM and cryoET. Nat. Commun. 11, 5208 (2020).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Masuho, I., Martemyanov, K. A. & Lambert, N. A. Monitoring G protein activation in cells with BRET. Methods Mol. Biol. 1335, 107–113 (2015).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Acknowledgements
We thank B. Kobilka and K. Suzuki for helpful discussion, H. Hisano and H. Ikeda for expression and purification of the ghrelin receptor–miniGq complex and N. Nomura for the supply of cells expressing Nb35 or scFv16 and for advice on purification. This work was supported by startup funding from Sanford Research (to I.M.), JSPS KAKENHI grants 18K16217 (to Y.S.), 20K06531 (to M.K. and Y.S.), 20KK0359 (to Y.S.), 22KK0099 (to H.A., Y.S. and D.I.), 24K01965 (to Y.S.) and 24K01984 (to S.Y.), the Takeda Science Foundation (to Y.S. and M.K.), the Kobayashi Foundation (Y.S.), the Naito Foundation (M.K.), and the Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research) from AMED under grant numbers JP23ama121007 (to S.I), JP23ama121001 (to D.I.) and JP24ama121013 (to T.M.).
Author information
Authors and Affiliations
Contributions
Y.S. designed the expression construct, optimized the purification procedure and conducted the cell surface expression assay. Y.S. and H.A. purified the ghrelin receptor–miniGq protein complex. D.I., H.A. and J.K. carried out the cryo-EM analysis under the guidance of T.K. D.I. and H.A. determined the structure with supervision from S.I. Y.S. and K.M. performed a Ca2+ mobilization assay with guidance from M.K. R.T. was responsible for most BRET experiments and data analysis. R.K. investigated the intrinsic biochemical properties of ghrelin-bound ghrelin receptor. E.K. analyzed the binding mechanisms of ligands to the ghrelin receptor, as well as the structural changes associated with ligand binding. S.Y. performed the MD simulation. H.W.-M. undertook the analyses of natural genetic variations and binding mechanisms of ligands to the ghrelin receptor and maintained the cells for BRET experiments. Y.S., D.I. and I.M. wrote the paper with input from all authors. The project was overseen by T.M., M.K., S.I. and I.M. M.K. and S.I. were responsible for structural analyses. I.M. was responsible for project design, data interpretation and writing of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Osamu Nureki, Jin-Peng Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Single-particle cryo-EM analysis procedure.
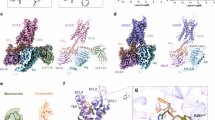
(a) Representative elution profile of the purified anamorelin-bound ghrelin receptor-miniGq complex from a size exclusion column, along with its Coomassie Brilliant Blue (CBB) staining. Peak I represent the void peak. Purification and CBB staining were performed more than five times, consistently yielding similar results. (b) Representative cryo-EM micrographs and two-dimensional classification averages of the anamorelin-bound ghrelin receptor-miniGq complex. A total of 11,068 micrographs were acquired using a Titan Krios microscope. (c) Cryo-EM data processing workflows of the anamorelin-bound ghrelin recepro-miniGq complex. The final cryo-EM map is colored according to local resolution (Å). (d) Euler angle distributions of the particles used for final reconstruction (left) and the global GSFSC curve for the final 3D density map (right).
Extended Data Fig. 2 Motif comparisons.
A comparison of conserved motifs in structures bound to anamorelin (PDB ID: 8JSR), ghrelin (PDB ID: 7F9Y), and CPD21 (PDB ID: 6KO5). The locations of the motifs are indicated by colored boxes: magenta, salt bridge; blue, aromatic cluster; green, PI(V)F; purple, NPxxY; and red, D(E)RY. The movements of side chains that undergo significant changes upon activation of the ghrelin receptor are indicated with red arrows.
Extended Data Fig. 3 Cryo-EM density map.
(a) The transmembrane helices 1-7, and the α5 helix of the miniGq of the anamolerin-bound ghrelin receptor-miniGq complex. (b) The agonist-binding site of the anamolerin-bound ghrelin receptor-miniGq complex. (c) The density map of amino acid residues showing the orientation specific to the anamorelin-bound structure.
Extended Data Fig. 4 Dose response analysis and expression levels of functional receptors on the plasma membrane.
(a) Ca2+ mobilization assay for ghrelin receptor mutants. Wild-type and alanine mutants are shown in gray and black, respectively. The change in maximum [Ca2+]i upon the addition of each concentration of anamorelin was determined in quadruplicate (n = 4 wells). Symbols and error bars represent mean and standard error. A representative result from three independent experiments with similar results was presented. (b) Cell surface expression levels of wild-type and mutant ghrelin receptors. The wild-type and mutant receptors were fluorescently labeled through an N-terminal Flag tag on each receptor. The expression levels of the mutants were quantified by calculating the ratio of green fluorescence intensity to that of the wild type. The expression levels of each mutant were 50–145% of the wild type, except for L2105.36A, S2175.43A, and F3127.42A. Statistics: results are expressed as mean ± SD (One-way ANOVA followed by Dunnett multiple comparison test, P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; n = 3 wells). Adjusted P values in comparison with the wild-type receptor are as follows: <0.0001 for E124E, R283A, S123A, S217A, F309A, F312A, and L210A; 0.0001 for D99A; 0.0003 for N305A; 0.0009 for F286A; 0.0012 for Q120A; 0.0041 for L103A; 0.0400 for R102A; 0.0608 for L181A; 0.2281 for M213A; 0.9076 for I178A; >0.9999 for V214A.
Extended Data Fig. 5 Investigating the ghrelin-induced G protein-coupling profile of the ghrelin receptor.
(a-c) Real-time monitoring of G protein activation by the ghrelin receptor. The saturated concentration of ghrelin (10 μM) was applied at time point 0, and the change in BRET signal was monitored (a). The maximum amplitude of the response (b) and activation rates (c) are presented as bar graphs. The traces represent the mean values of three independent experiments (a). Mean ± SEM of three independent experiments is shown (b and c).
Extended Data Fig. 6 Quantitative analysis of ibutamoren and AwFw-LL properties on the ghrelin receptor.
(a-f) Quantitative characterization of the response induced by ibutamoren (a-c) and AwFw-LL (d-f) compared to ghrelin using the in cellulo GEF assay. (a) Normalized BRET responses to the maximum amplitude induced by ghrelin are shown. (b and c) The maximum amplitude (b) and activation rates (c) induced by ibutamoren, presented as fold change in comparison to the ghrelin-induce response, are shown as bar graphs. (d) Normalized BRET responses to the maximum amplitude induced by ghrelin are shown. (e and f) The maximum amplitude (e) and activation rates (f) induced by AwFw-LL, presented as fold change in comparison to the ghrelin-induced response, are shown as bar graphs. The traces represent the mean values of five independent experiments (a and d). Mean ± SEM of five independent experiments are shown (b-c and e-f). ‘N.D.’ indicates not detected (e). Statistical significance was determined by One-Way ANOVA followed by Dunnett multiple comparison test (b and c) and Two-Way ANOVA with Šídák multiple comparison test (e and f), with significance indicated by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Adjusted P values are as follows: 0.0003 for Gq vs. GoA, 0.0290 for Gq vs. G15, and 0.0307 for Gq vs. G13 in panel b; 0.0463 for Gq vs. GoA, 0.0002 for Gq vs. G15, and 0.9907 for Gq vs. G13 in panel c; <0.0001 for GoA, G15, and G13, and 0.0055 for Gq in panel e; 0.0002 for GoA, 0.0034 for Gq, <0.0001 for G15, and 0.0009 for G13 in panel f. "N.D." indicates that the response was not determined (f) due to small responses that could not be accurately quantified for activation rates.
Extended Data Fig. 7 Impact of natural genetic variations in the orthosteric ligand-binding pocket on agonist actions.
(a) Schematic representation of the BRET-based kinetic assay used for real-time monitoring of the ghrelin receptor’s activity on Gq following agonist stimulation. (b-e) The response of 14 ghrelin receptor mutants to various agonists (10 μM ghrelin, 10 μM GHRP-6, 10 μM ibutamoren, and 10 μM anamorelin) was examined, focusing on the activation of Gq as a functional readout of the mutant receptors. Bar graphs represent the fold change compared to the wild-type receptor-induced response, illustrating the maximum amplitude and the speed of G protein activation induced by each agonist. The basal activity of the ghrelin receptor was also examined (f). Mean ± SEM of four independent experiments (e), three independent experiments (b-d), or thirteen independent experiments (f) are shown. Statistical significance was determined by One-Way ANOVA followed by Dunnett multiple comparison test (b-f), with significance indicated by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Adjusted P values in comparison with the wild-type receptor are as follows: <0.0001 for S123G, A204E, V214L, S217P, F279L, and N305K, 0.0005 for P200S, 0.0028 for M213I, 0.0066 for I178T, 0.0121 for E124D, 0.0769 for L103F, 0.2792 for R102P, 0.7626 for D99E, and >0.9999 for V214A in panel b (amplitude); <0.0001 for D99E, R102P, S123G, E124D, I178T, A204E, S217P, F279L, and N305K, 0.0002 for V214A, 0.0014 for V214L, 0.0605 for M213I, 0.4336 for L103F, and 0.6960 for P200S in panel b (activation rate); <0.0001 for R102P, S123G, E124D, P200S, A204E, M213I, V214L, S217P, and F279L, 0.0004 for I178T, 0.0037 for L103F, 0.9437 for V214A, and 0.9999 for D99E in panel c (amplitude); <0.0001 for D99E, R102P, E124D, A204E, and F279, 0.0026 for S217P, 0.0219 for I178T, 0.0405 for P200S, 0.6703 for V214L, 0.9963 for M213I, 0.9995 for L103F, and >0.9999 for S123G and V214A in panel c (activation rate); <0.0001 for R102P, S123G, A204E, S217P, and F279L, 0.0003 for E124D and V214L, 0.0071 for P200S, 0.0085 for M213I, 0.0231 for I178T, 0.0667 for L103F, 0.9766 for V214A, 0.9945 for D99E, and >0.9999 for N305K in panel d (amplitude); <0.0001 for R102P and N305K, 0.0002 for D99E, 0.0008 for A204E, 0.0066 for E124D, 0.0197 for L103F, 0.0243 for F279L, 0.0343 for V214L, 0.0537 for S217P, 0.1246 for V214A, 0.2056 for P200S, 0.3043 for S123G, 0.9998 for I178T, and >0.9999 for M213I in panel d (activation rate); <0.0001 for R102P, S123G, E124D, I178T, P200S, A204E, M213I, V214L, S217P, F279L, and N305K, 0.0055 for L103F, 0.9053 for V214A, and 0.9773 for D99E in panel e (amplitude); <0.0001 for D99E, R102P, E124D, A204E, M213I, S217P, and F279L, 0.0046 for N305K, 0.0312 for P200S, 0.0427 for S123G, 0.0948 for I178T, 0.3042 for L103F, 0.6292 for V214L, and 0.9963 for V214A in panel e (activation rate); and <0.0001 for D99E, R102P, S123G, E124D, I178T, P200S, A204E, M213I, V214L, S217P, F279L, and N305K, 0.0007 for V214A, and 0.10172 for L103F in panel f. ‘N.D.’ indicates not detected.
Extended Data Fig. 8 Impact of natural genetic variations in the orthosteric ligand-binding pocket on antagonist/inverse agonist actions.
(a) Schematic representation of the BRET-based kinetic assay used for real-time monitoring of the ghrelin receptor’s activity on Gq following antagonist/inverse agonist stimulation. (b-e) The response of 14 ghrelin receptor mutants to various ligands (100 μM YIL 781, 32 μM CPD21, 100 μM JMV 2959, and 10 μM PF-05190457) was examined, focusing on the activation of Gq as a functional readout of the mutant receptors. Bar graphs illustrate the maximum amplitude and the speed of G protein activation induced by each ligand. Gq deactivation induced by an inverse agonist was quantified (e). Mean ± SEM of four independent experiments (b) or three independent experiments (c-e) are shown. Statistical significance was determined by One-Way ANOVA with Dunnett multiple comparison test (b, c, d, and e), with significance indicated by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Adjusted P values in comparison with the wild-type receptor are as follows: <0.0001 for I178T, P200S, and S217P, 0.0005 for D99E, 0.0023 for S123G, 0.0024 for V214A, 0.0245 for M213I, and 0.9996 for A204E in panel b (amplitude); <0.0001 for D99E, I178T, P200S, and V214A, 0.0011 for S217P, 0.0020 for A204E, 0.7644 for S123G, and >0.9999 for M213I in panel b (activation rate); <0.0001 for I178T and S217P, 0.0002 for M213I, 0.0173 for D99E, 0.0330 for E124D, 0.5048 for V214A, 0.7065 for P200S, and 0.9998 for S123G in panel c (amplitude); <0.0001 for D99E, S123G, E124D, I178T, P200S, and S217P, 0.0038 for V214A, and 0.0080 for M213I in panel c (activation rate); 0.0002 for E124D, 0.0003 for R102P and I178T, and 0.0007 for V214A in panel d (amplitude); <0.0001 for R102P, E124D, I178T, and V214A in panel d (activation rate); <0.0001 for no GHSR, S213G, E124D, I178T, A204E, M213I, V214L, S217P, F279L, and N305K, 0.0002 for P200S, 0.6001 for L103F, 0.9942 for D99E, and 0.9994 for V214A in panel e. ‘N.D.’ indicates not detected.
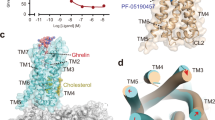
Extended Data Fig. 9 Impact of N3057.35K mutations on drug actions.
(a) Assay design to examine the antagonist/inverse agonist action of the N3057.35K mutant compared to the wild-type ghrelin receptor. (b-i) Effects of JMV 2959, PF-05190457, YIL 781, or CDP21 on wild-type ghrelin receptor (b, d, f, and h) and N3057.35K mutant (c, e, g, and i). Transfected cells were pre-incubated with 100 μM JMV 2959, 10 μM PF-05190457, 10 μM YIL 781, or 32 μM CPD21 for 1 min and then stimulated with 10 μM anamorelin. The traces represent the mean values of three independent experiments. Mean ± SEM are presented as bar graphs. Statistical significance was determined by unpaired two-tailed t test, with significance indicated by asterisks (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Adjusted P values are as follows: 0.0004 (b), 0.0005 (c), <0.0001 (d), 0.0009 (e), <0.0001 (f), 0.1809 (g), and <0.0001 (h). (j) Comparison of the chemical structures of YIL 781 and CPD21. (k) Binding modes of N3057.35 and CPD21 or PF-05190457.
Supplementary information
Supplementary Information
Table of contents, Supplementary Figs. 1–5 and Supplementary Table 1.
Supplementary Video 1
Dynamics of anamorelin–ghrelin receptor interactions.
Supplementary Tables
Supplementary Table 2. Interaction between ligands and GHSR1a. Supplementary Table 3. MVs identified in the GHSR gene.
Supplementary Data 1
Source data for Supplementary Fig. 3.
Supplementary Data 2
Source data for Supplementary Fig. 4.
Supplementary Data 3
Source data for Supplementary Fig. 5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shiimura, Y., Im, D., Tany, R. et al. The structure and function of the ghrelin receptor coding for drug actions. Nat Struct Mol Biol 32, 531–542 (2025). https://doi.org/10.1038/s41594-024-01481-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-024-01481-6
This article is cited by
-
Personalized medicine for cancer cachexia via the ghrelin receptor
Nature Structural & Molecular Biology (2025)