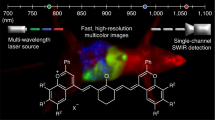
Abstract
Optical imaging provides real-time visualization of tissues and cells at high spatial and temporal resolutions through techniques such as fluorescence microscopy, optical coherence tomography and photoacoustic imaging. However, overcoming light scattering, caused by mismatches in the refractive indices of tissue components such as water and lipids, still represents a major challenge, particularly when imaging through the thicker biological tissues of living animals. Despite advances in deep-tissue imaging, many optical methods struggle to achieve diffraction-limited resolution at depth or are unsuitable for use in live animals. Here we introduce a noninvasive approach to achieving transient and reversible optical transparency in live mice using absorbing dye molecules, using tartrazine as a representative example. Rooted in the fundamental physics of light–matter interactions, this approach enables reversible optical transparency in live animals and can be further applied ex vivo in freshly dissected tissues. In this Protocol, we detail the procedures for visualizing in vivo internal organs and muscle sarcomeres in the mouse abdomen and hindlimb through their respective transparency windows, showcasing a versatile approach for a variety of optical imaging applications in live animals. The entire protocol for an in vivo application can be implemented in just over 2 weeks by users with expertise in optical imaging and animal handling.
Key points
-
The procedure includes the preparation and application of absorbing molecules to create transient optical transparency windows ex vivo and in vivo, enabling the imaging of deep tissue structures and biological processes at macroscopic and microscopic scales.
-
Alternative methods for deep tissue biological imaging, such as microendoscopes and cranial or abdominal windows, are invasive and can cause damage and disruption to the natural composition and activity of the tissue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
All relevant source data supporting this study are included in the paper and available in the accompanying source data files. Characterized and quality-controlled tartrazine solutions are available to other research laboratories upon request. Source data are provided with this paper.
References
Yun, S. H. & Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 1, 0008 (2017).
Lichtman, J. W. & Conchello, J.-A. Fluorescence microscopy. Nat. Methods 2, 910–919 (2005).
Valdastri, P., Simi, M. & Webster, R. J. 3rd Advanced technologies for gastrointestinal endoscopy. Annu. Rev. Biomed. Eng. 14, 397–429 (2012).
Boas, D. A. & Dunn, A. K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 15, 011109 (2010).
Song, G., Jelly, E. T., Chu, K. K., Kendall, W. Y. & Wax, A. A review of low-cost and portable optical coherence tomography. Prog. Biomed. Eng. 3, 032002 (2021).
Cho, S.-W. et al. Sounding out the dynamics: a concise review of high-speed photoacoustic microscopy. J. Biomed. Opt. 29, S11521 (2024).
McWade, M. A., Sanders, M. E., Broome, J. T., Solórzano, C. C. & Mahadevan-Jansen, A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159, 193–202 (2016).
Cao, R., Wallrabe, H. & Periasamy, A. Multiphoton FLIM imaging of NAD(P)H and FAD with one excitation wavelength. J. Biomed. Opt. 25, 1–16 (2020).
Tan, Y., Lin, H. & Cheng, J.-X. Profiling single cancer cell metabolism via high-content SRS imaging with chemical sparsity. Sci. Adv. 9, eadg6061 (2023).
Sun, H., Wang, S., Chen, J. & Yu, H. Label-free second harmonic generation imaging of cerebral vascular wall in local ischemia mouse model in vivo. Neuroscience 502, 10–24 (2022).
Wang, L., Jacques, S. L. & Zheng, L. MCML—Monte Carlo modeling of light transport in multi-layered tissues. Comput. Methods Prog. Biomed. 47, 131–146 (1995).
Jacques, S. L. & Pogue, B. W. Tutorial on diffuse light transport. J. Biomed. Opt. 13, 041302 (2008).
Tuchin, V. Tissue optics and photonics: light-tissue interaction II. J. Biomed. Photonics Eng. 2, 030201 (2016).
Bohren, C. F. & Huffman, D. R. Absorption and Scattering of Light by Small Particles (Wiley, 2008).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Xu, C., Nedergaard, M., Fowell, D. J., Friedl, P. & Ji, N. Multiphoton fluorescence microscopy for in vivo imaging. Cell 187, 4458–4487 (2024).
Schmidt, E. L. et al. Near-infrared II fluorescence imaging. Nat. Rev. Methods Prim. 4, 23 (2024).
Ueda, H. R. et al. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 21, 61–79 (2020).
Ou, Z. et al. Achieving optical transparency in live animals with absorbing molecules. Science 385, eadm6869 (2024).
Lucarini, V., Saarinen, J. J., Peiponen, K. -E. & Vartiainen, E. M. Kramers–Kronig Relations in Optical Materials Research (Springer, 2010).
Hecht, E. Optics (Pearson Education, 2001).
Boothe, T. et al. A tunable refractive index matching medium for live imaging cells, tissues and model organisms. eLife 6, e27240 (2017).
Oliveira, L. M. C. & Tuchin, V. V. The Optical Clearing Method: A New Tool for Clinical Practice and Biomedical Engineering (Springer, 2019).
Tuchin, V. V. Optical Clearing of Tissues and Blood (SPIE, 2005).
Talei Franzesi, G. et al. In vivo optical clearing of mammalian brain. Preprint at bioRxiv https://doi.org/10.1101/2024.09.05.611421 (2024).
König, J. in Colour Additives for Foods and Beverages (ed. Scotter, M. J.) 35–60 (Woodhead Publishing, 2015).
FAO Nutrition Meetings Report Series (Food and Agriculture Organization of the United Nations, World Health Organization, 1966).
Narawane, A. et al. Optical clearing with tartrazine enables deep transscleral imaging with optical coherence tomography. J. Biomed. Opt. 29, 120501 (2024).
Miller, D. et al. Enhanced penetration depth in optical coherence tomography and photoacoustic microscopy in vivo enabled by absorbing dye molecules. Optica 12, 24–30 (2025).
Cai, R. et al. Whole-mouse clearing and imaging at the cellular level with vDISCO. Nat. Protoc. 18, 1197–1242 (2023).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Susaki, E. A. et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014).
He, R. et al. Effects of optical clearing agents on noninvasive blood glucose monitoring with optical coherence tomography: a pilot study. J. Biomed. Opt. 17, 101513 (2012).
Murakami, T. C. et al. A three-dimensional single-cell-resolution whole-brain atlas using CUBIC-X expansion microscopy and tissue clearing. Nat. Neurosci. 21, 625–637 (2018).
Feng, W., Liu, C.-J., Wang, L. & Zhang, C. An optical clearing imaging window: realization of mouse brain imaging and manipulation through scalp and skull. J. Cereb. Blood Flow. Metab. 43, 2105–2119 (2023).
Inagaki, S. et al. Isotonic and minimally invasive optical clearing media for live cell imaging ex vivo and in vivo. Preprint at bioRxiv https://doi.org/10.1101/2024.09.13.612584 (2024).
Antaris, A. L. et al. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun. 8, 15269 (2017).
Theer, P., Hasan, M. T. & Denk, W. Two-photon imaging to a depth of 1000 micron in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 28, 1022–1024 (2003).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photon. 7, 205–209 (2013).
Costantini, I., Cicchi, R., Silvestri, L., Vanzi, F. & Pavone, F. S. In-vivo and ex-vivo optical clearing methods for biological tissues: review. Biomed. Opt. Express 10, 5251–5267 (2019).
Iijima, K., Oshima, T., Kawakami, R. & Nemoto, T. Optical clearing of living brains with MAGICAL to extend in vivo imaging. iScience 24, 101888 (2021).
Tuchin, V. V. Tissue optics and photonics: light-tissue interaction. J. Biomed. Photonics Eng. 1, 98–134 (2015).
Zhu, D., Larin, K. V., Luo, Q. & Tuchin, V. V. Recent progress in tissue optical clearing. Laser Photon. Rev. 7, 732–757 (2013).
Dragicevic, N. & Maibach, H. I. (eds.) Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement (Springer, 2015).
Roberts, M. S. et al. Topical drug delivery: history, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 177, 113929 (2021).
Dragicevic, N. & Maibach, H. I. (eds.) Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement (Springer, 2017).
Hale, C. S. et al. Development and applications of a concentrating membrane osmometer for colloid solutions. Rev. Sci. Instrum. 90, 034102 (2019).
Sweeney, T. E. & Beuchat, C. A. Limitations of methods of osmometry: measuring the osmolality of biological fluids. Am. J. Physiol. 264, R469–R480 (1993).
Nair™ How to Use Hair Removal Creams https://www.naircare.com/en/education/how-to-use-hair-removal-creams (2025, accessed 15 Apr 2025).
Thompson, S. & Slaga, T. J. Mouse epidermal aryl hydrocarbon hydroxylase. J. Invest. Dermatol. 66, 108–111 (1976).
Benaouda, F. et al. Needleless administration of advanced therapies into the skin via the appendages using a hypobaric patch. Proc. Natl Acad. Sci. USA 119, e2120340119 (2022).
Wang, C. et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science 377, 517–523 (2022).
Diao, S. et al. Biological imaging without autofluorescence in the second near-infrared region. Nano Res 8, 3027–3034 (2015).
Acknowledgements
Ellipsometry characterizations were performed at the Stanford Nano Shared Facilities, supported by the National Science Foundation (grant no. ECCS-1542152). Any opinions, findings, conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. In vivo multiphoton imaging experiments were performed at Stanford Wu Tsai Neuroscience Microscopy Service, with help from G. Wang. G.H. acknowledges three awards from the NIH (grant nos. 5R00AG056636-04, 1R34NS127103-01 and R01NS126076), an NSF CAREER award (grant no. 2045120), an NSF EAGER award (grant no. 2217582), a Rita Allen Foundation Scholars Award, a Beckman Technology Development Grant, a grant from the Focused Ultrasound Foundation, a gift from the Spinal Muscular Atrophy Foundation, gifts from the Pinetops Foundation, two seed grants from the Wu Tsai Neurosciences Institute, two seed grants from the Bio-X Initiative of Stanford University and a teacher-scholar award from the Camille and Henry Dreyfus Foundation. M.L.B. acknowledges a grant from the Air Force Office of Scientific Research (grant no. FA9550-21-1-0312). C.H.C.K. acknowledges the National Science Foundation Graduate Research Fellowships program (grant no. 1656518) and the Wu Tsai Neuroscience NeuroTech Training program. E.L.S. acknowledges a TIME fellowship. L.Z. acknowledges a Knight-Hennessy Fellowship. H.C. acknowledges a Stanford Interdisciplinary Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
G.H. and M.L.B. contributed ideas and designed research. C.H.C.K., E.L.S. and G.H. made tartrazine solutions and characterized their optical properties. C.H.C.K., E.L.S. and G.H. prepared mouse skin samples and performed ex vivo transparency experiments. C.H.C.K., S.Z., Z.L., L.Z. and G.H. prepared live mice and performed in vivo transparency experiments. M.C., X.C. and C.W. prepared bioadhesive hydrogels. C.H.C.K., E.L.S., M.L.B. and G.H. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
G.H., M.L.B. and S.Z. are inventors on patent application (patent no. WO2023122534A1) submitted by Stanford University that covers the principles of achieving optical transparency by applying the Kramers–Kronig relations.
Peer review
Peer review information
Nature Protocols thanks Arthur Petusseau, Myunghwan Choi, Conor Evans and Wonsang Hwang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Ou, Z. et al. Science 385, eadm6869 (2024): https://doi.org/10.1126/science.adm6869
Miller, D. A. et al. Optica 12, 24–30 (2025): https://doi.org/10.1364/OPTICA.546779
Extended data
Extended Data Fig. 1 Instability of tartrazine solutions.
a, Pure tartrazine solution after 96 h at room temperature, featuring complete precipitation. b, Pure tartrazine solution after complete precipitation, followed by 10 min in an 80 °C oven to redissolve the precipitates. c, Tartrazine/PVA solutions after 96 h at room temperature, featuring complete precipitation. d, Tartrazine/PVA solution after complete precipitation, followed by 10 min in an 80 °C oven to redissolve the precipitates.
Extended Data Fig. 2 The transparency window in the mouse abdomen appears orange to red after the skin has fully absorbed the tartrazine molecules.
a, An orange-to-red transparency window in the abdomen of a 23-day-old female C57Bl/6J mouse weighing 9.8 g. Excess tartrazine was removed before taking the photo. Note the orange-red color in this video comes from the tartrazine dye, not blood. b, A close-up view of the transparency window is shown by pressing a coverslip onto the abdomen. c, A white dashed line is overlaid on the photo to mark the red/yellow boundary in the skin. Scale bars: 1 cm.
Extended Data Fig. 3 Dissected skin after sufficient absorption of tartrazine becomes red and transparent.
In this example, a 22-day-old female C57Bl/6J mouse weighing 10.5 g was used. Tartrazine was applied for 10 min, and excess tartrazine was removed before the skin was dissected.
Supplementary information
Supplementary Video 1
Achieving and reversing optical transparency in a live mouse. This video shows the entire process of depilation, exfoliation, tartrazine application, imaging and transparency reversal.
Supplementary Video 2
A clear boundary can be observed between the transparent (red) and opaque (yellow) regions of the skin on the mouse abdomen. After the skin has fully absorbed tartrazine molecules, it appears red and translucent, while depilated areas that have been in contact with the dye but have not absorbed sufficient amounts still appear yellow once the excess dye is removed. Note that when the skin on the left side of the mouse abdomen is moved by dragging with fingers, the underlying gut structures become masked or revealed, depending on the transparency of the skin above.
Supplementary Video 3
Reversing optical transparency in a live mouse. This video begins with a live mouse featuring a transparency window in the abdomen, enabling imaging through the skin. It then demonstrates the process of extracting tartrazine from the skin to reverse the transparency effect, applying a hydrogel to maintain skin hydration after reversal and securing the hydrogel with medical tape to protect it before the mouse is recovered from anesthesia.
Source data
Source Data Fig. 3
Baseline-subtracted UV-visible absorption spectrum of the 0.62 mM tartrazine solution with an optical path length of 1 mm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keck, C.H.C., Schmidt, E.L., Zhao, S. et al. Achieving transient and reversible optical transparency in live mice with tartrazine. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01187-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01187-z