Abstract
In this trial, long-term therapeutic effects and clinical improvements in Chinese chronic heart failure patients optimized by QuickOpt or echocardiography were compared for atrioventricular (AV) and interventricular (VV) delay optimizations after cardiac resynchronization therapy (CRT) with pacing (CRT-P) or with pacing and defibrillator (CRT-D) therapy. One hundred and ninety-six subjects (50%) had dilated cardiomyopathy, 108 (27.6%) had ischemic heart disease and 112 (28.6%) were hypertensive and were randomized into QuickOpt (198) or echocardiographic optimization (control) (194) groups at ≤2-weeks post-implantation. Programmed AV/VV delay was optimized at baseline and at 3 and 6 months. Left ventricular end-systolic volume (LVESV), New York Heart Association (NYHA) class, specific activity scale (SAS), and the six-minute walk tests (6MWT) were evaluated by blinded researchers at 12 months. Of the QuickOpt group, LVESV decreased significantly by 24.7% ± 33.9% compared with baseline, while LVESV of Controls decreased by 25.1% ± 36.1% (P = 0.924). NYHA class, SAS and 6MWT also improved similarly in both groups at 12 months. Mortality in both groups was not significantly different (11.0% vs 7.6%, P = 0.289). However, there was a significant difference in the time required for optimization by QuickOpt compared with echocardiography (3.33 ± 3.11 vs 58.79 ± 27.03 minutes, P < 0.000).
Similar content being viewed by others
Introduction
Congestive heart failure (CHF) has been estimated to affect more than 26 million people worldwide1,2 and is associated with other comorbidities (which include the symptoms of chronic obstructive pulmonary disease, renal impairment, and obstructive sleep apnea), which accounts for about 50% of all readmissions after initial hospitalization for CHF3,4. Even today, the prognosis of CHF is poor with studies from the United States (US) and Europe showing that 6-month and 5-year mortality rates remain high at 14% and 45%, respectively5,6. Due to its high incidence and mortality, CHF has been deemed to be a worldwide epidemic disease.
Currently, the use of cardiac resynchronization therapy (CRT)7,8,9,10,11, including defibrillation capability (CRT-D) or with pacing only (CRT-P), is recommended for patients with heart failure who have left ventricular dysfunction with a left ventricular ejection fraction of ≤35%, on recommended medications as per consensus guidelines for the diagnosis and treatment of CHF.
In China, nearly 3 million CHF patients require CRT-P or CRT-D devices, and proper resynchronization optimization after implantation has become increasingly challenging. Common approaches to define the best AV interval rely on repeated echocardiographic assessments with program adjustments (iterative) and other methods12,13, with the aim of interval optimization to reduce left ventricular dyssynchrony and mitral regurgitation. Optimization of both atrioventricular (AV) and ventricular-ventricular (VV) intervals improves the therapeutic effects of CRT14,15.
The key to obtaining the optimal AV interval is to synchronize ventricular systolic and diastolic phases. The optimized AV and VV intervals are calculated based on specific medical personnel perceptions and the intracardiac electrogram16. The purpose of AV interval optimization is to maximize ventricular preload as well as to allow the mitral valve to close at the proper time. The purpose of VV interval optimization is to make the peak of the left and right ventricular activation meet in the vicinity of the ventricular septum, thereby reducing ventricular dyssynchrony. The effects of this optimization can be assessed by the left ventricular end-systolic volume (LVESV) and left ventricular end-diastolic volume (LVEDV), New York Heart Association class (NYHA), 6-minute walk test (6MWT) and specific activity scale (SAS) according to the US guidelines for CHF diagnosis and therapy17.
The QuickOpt algorithm (St. Jude Medical; St. Paul, MN, USA) uses the timing cycle of the cardiac electrogram to perform rapid interval optimization, with a program for use at routine follow-up. It is a rapid (1–4 min) simple, automatic determination of optimal AV/VV delays. Compared with echocardiographic optimization, previous studies have demonstrated that the Aorta Velocity Time Index (AVTI) by QuickOpt optimization and the maximum AVTI by echocardiographic optimization are significantly correlated, with a correlation coefficient of 0.96–0.9816.
Small sample sizes and short evaluation periods in existing studies have mainly prevented unequivocal acceptance of the results of CRT with QuickOpt optimization in China. To compare QuickOpt and echocardiographic optimization in the short and long-term, including the clinical outcomes of the two methods across China, we designed a multicenter, prospective, randomized, double blinded, parallel controlled trial.
The main aims of our trial were to compare the therapeutic effects of optimization by QuickOpt or echocardiographic optimization on LVESV and other clinical parameters (NHYA class, SAS, and the 6MWT) in a 31-hospital collaborative cohort of Chinese patients, including adverse events (AE) over one year after CRT-P/D implantation.
Methods
Patient selection and study design
All patients were enrolled according to the following criteria for CRT-P/D indications: 1) left ventricular ejection fraction (LVEF) ≤35%; 2) New York Heart Association (NYHA) class III-IV heart failure; 3) QRS complex duration ≥120 ms.
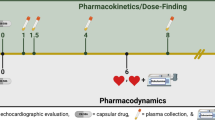
Based on the inclusion criteria, 400 patients with CHF in 31 different hospitals from May 2010 to February 2015 were implanted with CRT-P (260 patients) or CRT-D (140 patients) and then they were automatically assigned into the QuickOpt and Echo groups for optimization in a 1:1 ratio by software which linked in all the sites. One hundred and ninety-eight patients were randomized to the QuickOpt group, 194 patients to the echocardiography group after two weeks implantation, and 8 declined randomization because they could not follow up to the end of the research period. We performed CRT-P/D interval optimization during the first two weeks, at 3 months (±2 weeks) and 6 months (±2 weeks) after implantation, and programmed the optimal AV/VV interval in the CRT-P/D (Fig. 1).
Patients who gave informed consent for the study were implanted with CRT-P (Frontier II 5596) or CRT-D (Epic + HF V350, Atlas + HF V341, Atlas II HF V365, Atlas II + HF V366, Atlas II + HF V367, Promote 3107–30, Promote 3107–36) and lead systems (St. Jude Medical, USA) and received optimal medical therapy.
Patients were excluded if they were suffering from severe sinus bradycardia (sinus rate ≤40 bpm) or had persistent chronic atrial tachyarrhythmia, including atrial fibrillation, atrial flutter and atrial tachycardia, or second- or third-degree AV block, or a QRS complex duration less than 120 ms. Other exclusion criteria were:
-
patients <18-years;
-
life expectancy <1 year;
-
received intravenous-positive inotropic drug treatment;
-
participating in other device or drug trials;
-
being prepared for cardiac transplantation;
-
hypertrophic cardiomyopathy;
-
severe aortic or mitral stenosis or regurgitation without valve replacement; -pregnant or lactating women;
-
coronary artery bypass surgery, percutaneous coronary intervention or cardiomyoplasty in previous 6 weeks;
-
acute coronary syndrome;
-
stroke;
-
pre-excitation.
Echocardiography group
The apical four-chamber view was used to measure the mitral inflow velocity profile at the mitral valve to optimize AV delay. The AV delay was initially set between 60 ms and 200 ms, and was varied in 20 ms steps. The EA duration was measured at each AV interval, and the optimal AV delay was defined as the maximal EA duration without truncating the A wave11.
To optimize VV delay, an apical five-chamber view was used to determine the velocity-time integral (VTI) of the left ventricular outflow tract. The VV delay was set between −40 ms and +40 ms, with programmed adjustments in 20 ms increments; optimal VV delay resulted in a maximal VTI11.
QuickOpt group
AV/VV delay optimization was performed at the intervals stated above with devices being programmed according to data obtained during QuickOpt optimization, namely optimized sensed AV, paced AV and VV intervals.
Follow-up
Within 2 weeks after CRT-P/D implantation, we used Merlin 3650 PCS (St. Jude Medical, USA) to implement the QuickOpt algorithm, and separately kept 12-lead ECG data of dual chamber pacing for subsequent analysis (Fig. 1). The follow-up data included echocardiography values (only within 12 months), the 6MWT and SAS data (only at 12 months), the NYHA class at each follow-up, 12-lead ECG (pacing), pacemaker follow-up data, and optimization results after QuickOpt optimization (at 3 and 6 months).
This trial complied with the Declaration of Helsinki. Our protocol was approved by the Research Ethics Committee of all 31 hospitals participating (Anhui Provincial Hospita; Fuwai Hospital, Chinese Academy of medical Sciences; The First Affiliated Hospital of Xi’an Jiaotong University; Tianjin Chest Hospital; The First Affiliated Hospital, Zhejiang University; The General Hospital of Lanzhou Military; The First Hospital of Lanzhou University; The General Hospital of Shenyang Military; The First Affiliated Hospital of Xinjiang Medical University; Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School; The Second Affiliated Hospital of Zhejiang University School of Medicine; Beijing Anzhen Hospital, Capital Medical University; Sun Yat-sen Memorial Hospital of Sun Yat-sen University; The First Affiliated Hospital of Kunming Medical University; General Hospital of Ningxia Medical University; The First Affiliated Hospital of Sun Yat-sen University; Renmin Hospital of Wuhan University; Sir Run Run Shaw Hospital of Medicine, Zhejiang University; The First Affiliated Hospital of Guangxi Medical University; Fujian Provincial Hospital; The 251st Hospital of PLA; Hangzhou First People’s Hospital; The second people’s Hospital of Yunnan Province; Hebei General Hospital; People’s Hospital of Yuxi City; Chinese PLA General Hospital; Xiangya Hospital Central South University; Zhejiang Greentown Hospital; The Second Hospital of Tianjin Medical University; Daqing Oilfield General Hospital; Shaoxing People’s Hospital) in this trial. The trial register number was NCT01172067 (First received: July 28, 2010; Last updated: July 28, 2016; Last verified: July 2016).
Evaluation of therapeutic effects
Primary efficacy
Comparison of changes in LVESV between the 2 groups, 12 months after implantation. All echocardiography images were collected and measured in one core laboratory to reduce any measurement bias.
Secondary efficacy
A blinded researcher evaluated changes in clinical parameters of patients at 12 months, including NHYA class, SAS and the 6MWT. SAS is a simple method to assess body functions.
Other efficacy
Safety indicators; AE (any adverse medical events) since the patients signed consent until the end of the study, regardless of the cause. Severe AEs that occurred during the course of the clinical study, such as the need for hospitalization, prolonged hospitalization, disability affecting patient’s work, life threatening events or death.
AE related to CRT-P/D included, but were not limited to:
-
worsening heart failure;
-
pacing system infection;
-
elevated pacing threshold;
-
lead dislocation;
-
lead fracture;
-
lead insulation damage;
-
death.
Each center had a physician specifically responsible for collecting data and then filling in the Electronic Data Capture (EDC). The data were reliable and every record of death and hospitalization was documented.
The report of death incidence consisted of two procedures. Once informed of a death, physicians evaluated the cause and reported it to the ethics department in the hospital within 24 hours. Then the ethics department reported to the China Food and Drug Administration (CFDA). In the meanwhile, physicians filled the death table in the electronic CRF within 3 days, and kept the original medical record as the initial document.
Statistical analysis
We hypothesized that 12 months after implantation, the improvement in LVESV in the QuickOpt group would equal that of the echocardiography group. As the presumed lower limit value was −10%, the assumed significant difference was 0.05 (1-sided), the detection efficacy was 80%, the overall standard deviation was 0.35, and the difference between the two group averages was 0.1, we needed 306 cases in total and 153 cases for each group. Taking into account 20% loss of cases due to death, heart transplantation, loss to follow-up and other causes, we finally enrolled 200 cases in each group, with a total cohort size of 400 cases. For primary efficacy, the difference between the two types of optimization was evaluated by Student’s t-test.
For secondary efficacy, the improvements in the NYHA class and SAS evaluation between the two types of optimization were compared with a full analysis set (FAS) and a Cochran-Mantel-Haenszel test. If the results of a chi-square test yielded a homogeneous distribution, the Fisher exact test was used. The improvement in the 6MWT between the two optimization methods was also compared with FAS and any significant differences evaluated by means of a t-test. If the result was an obvious non-normal distribution and heterogeneity of variance according to the distribution of final data, we adopted one of the following non-parametric statistics: Wilcoxon rank sum test or the Kolmogorov-Smirbov test. The FAS was an ideal group of participants as close to the Intention To Treat (ITT) principle as possible, which included nearly all subjects after randomization. The data were analyzed according to the intention-to-treat principle. The per protocol set (PPS) was a subset of the full analysis set, and each subject in this data set showed good compliance without violating the program. In our study, there were 392 cases consistent with the FAS set and 226 consistent with the PPS set.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Clinical data
The research cohort in this trial was comprised of 392 patients with CHF, in which 289 patients were male and 103 female. The mean age of the QuickOpt group was 61.38 ± 11.77 years and the echocardiography group 59.21 ± 11.36 years. 196 patients were diagnosed with dilated cardiomyopathy and 112 patients with hypertension, 47 patients with diabetes and <10 cases of hypertriglyceridemia, valvular heart disease, stroke or paroxysmal atrial fibrillation; however, there were 15 cases of stroke in both groups. In comparison of demographics, baseline data in the two groups showed no significant differences (Table 1). Among them, 27.6% (108) had ischemic heart disease and 72.4% (283) non-ischemic heart disease (Table 2). However, there were more patients with first-degree AV block in the QuickOpt than in the echocardiography group, and the mean LVEF was higher in the QuickOpt than in the echocardiography control patients (Table 2). There were no significant differences in the duration of heart failure, heart rate, QRS duration, different types of bundle branch block, or mean LVEDDs between the two groups (Table 2).
Comparison of CRT implantation methods and optimization
In the QuickOpt optimization group, 99 patients received CRT-Ps and 99 patients CRT-Ds. In the echocardiography control group, 95 patients received CRT-Ps and 99 patients CRT-Ds. 99.5% of the CRTs were implanted on the left side and the leads were placed through the left subclavian vein. The positions of the electrodes in the posterior lateral veins in the QuickOpt and echocardiography control group were 50.0% and 47.9%, respectively and in the lateral veins 38.1% and 38.4%, respectively. The depth of the electrodes in the veins middle segment accounted for 65.2% and 72.2%, respectively, and the depth of electrodes in the veins distal segment 31.3% and 23.7%, with the depth in the proximal segment being 3.5% and 4.1%. Therefore, no significant differences were observed between the QuickOpt optimization group and the echocardiography control group, with regard to the device implantation and leads positions (Supplementary Table 1). Supplementary Table 2 lists the baseline conditions of the patients. There were no significant differences between the two groups in the positions of the right atrial, right ventricular and left ventricular leads, as well as in the 3 month, 6 month and 12 month program controls (data not shown).
A comparison of the optimization process, optimization results and programmed control data in the two groups (shown in Table 3), revealed that the optimization time of the QuickOpt group at baseline, 3 months and 6 months required a median time of 2 minutes, whereas the echocardiography group had a mean time of 54.25–58.79 minutes, and the median time was 43.50–55.00 minutes.
Optimization results showed that the PV (paced atrial to paced ventricular) interval in the QuickOpt group was significant longer than in the echocardiography group at baseline, 3 months and 6 months (169.85 ± 15.13; 162.20 ± 21.16; 168.53 ± 16.84 vs 159.18 ± 23.49; 169.61 ± 14.68 vs 159.81 ± 24.84 ms). The VV interval in the QuickOpt group was only significantly longer at baseline (32.53 ± 16.99 vs. 27.32 ± 15.72 ms), but there were no significant differences between the QuickOpt and the echocardiography groups in the AV (atrial sense to ventricular pace) interval at any stage.
Comparison of the therapeutic effects on the two groups
Twelve months after optimization, the LVESV of the QuickOpt group was significantly decreased by 24.7 ± 33.9% compared with baseline, while the LVESV of the control group was decreased by 25.1 ± 36.1%, P = 0.924. The LVEDV of the 2 groups over 12 months showed significant improvement but there was no significant difference between the two optimization groups. The LVEF was significantly increased 12 months after optimization in the QuickOpt and control groups, being 38.4 ± 44.3% and 38.4 ± 44.8%, respectively, which demonstrates that the primary efficacy of the QuickOpt optimization was not inferior to the efficacy of the echocardiography (Table 4).
In addition, we found that the NYHA class for QuickOpt optimization patients was significantly improved 3, 6 and 12 months after CRT-P/D implantation. The number of NYHA class III and IV patients was significantly reduced, but there was no significant difference in improvement between the QuickOpt and echocardiography control groups (Table 5).
The SAS evaluation scores in both the QuickOpt and echocardiography groups were not significantly different throughout the study.
Finally, twelve months after CRT-P/D implantation, the 6MWT improved in both groups. However, there was no significant difference, which was consistent with the secondary efficacy of QuickOpt optimization being not inferior to echocardiography optimization (Table 6).
Comparison of the incidence of adverse events and mortality rates between the two groups
There was no significant difference in the incidence of device-related AE between the QuickOpt and echocardiography groups (Table 7), although there were 27 SAEs in the QuickOpt group and 19 in the echocardiography group. Three AEs were associated with the device in the QuickOpt group, 1 with increased LV pacing threshold, 1 electrode dislocation, and 1 junctional rhythm and low blood pressure. In the echocardiography group, there was 1 patient with an increased LV pacing threshold, and 1 patient with exacerbation of heart failure. There was no significant difference in mortality rates between the QuickOpt and echocardiography group (11.0% vs 7.6%, P = 0.289) (Table 7).
Discussion
In the present study, we confirmed in a large-scale clinical trial using QuickOpt optimization against the standard echocardiographic method in Chinese congestive heart failure patients treated by cardiac resynchronization, that the hemodynamic efficacy of QuickOpt optimization was not inferior to the echocardiographic method. These results were achieved by using QuickOpt in a time as rapidly as 3 min compared with 56 min by echocardiography (P < 0.05).
The AV/VV interval optimizations, assessed blindly, were associated with improvement in heart function and a reduction in mitral regurgitation. Ritte et al.18 reported that the optimal AV delay was 100–120 ms. We found using QuickOpt, that the optimized AV delay was 122.98 ± 16.21 ms at baseline, 121.29 ± 16.16 ms at 3 months and 120.59 ± 13.60 ms at 6 months, which were not significantly different from echocardiographically determined intervals. By extending Left Ventricular Filling Time (LVFT) and thus raising LVEF, mitral valve regurgitation was reduced. The two methods together with programmed control of AV/PV and VV have improved heart function (NYHA scores) and left ventricular end-systolic volume (LVESV) (Table 4). The 6MWT and SAS scores were also significantly improved at 12-months compared with baseline.
The PV delay time exhibited a significant difference between QuickOpt and echocardiography optimizations at baseline, 3 months and 6 months, due to the optimal AV delay defined as the maximal EA duration without truncating the A wave, combined with the optimal VV delay producing a maximal LV outflow velocity-time integral. This difference in PV was also seen in the small cohort study of Wang et al.10 without adverse effects on the parameters of LV function.
The significant difference in the optimization time by QuickOpt compared with echocardiography (3 min vs 56 min, P < 0.05) is a valuable clinical benefit with this methodology, which is non-inferior to echocardiography and needing circa 50 min less time than echocardiographic analysis. In a busy medical environment, this time saving allows physicians and technologists to reallocate echocardiography services for other patients.
Recent clinical studies using QuickOpt have unequivocally demonstrated that the Aorta Velocity Time Index (AVTI) determined by QuickOpt and the maximum AVTI by echocardiography optimization are significantly correlated (correlation coefficient r = 0.96–0.98)10. During echocardiographic optimization, optimal AV/VV delays are determined by the mitral inflow velocity and left ventricular outflow tract velocity profiles, which are measured from Doppler signals. The ideal AV delays show separation of the E and A waves on transmitral inflow Doppler signals19 while QuickOpt optimization is an algorithm which rapidly determines optimal AV and VV intervals based on heart electrical activity as measured by intracardiac electrography20. This approach should ensure that both intrinsic wave front and pacing stimuli arrive at the ventricular septum simultaneously to optimize the AV/VV delays. Thus, the use of QuickOpt optimization is easier to implement and far less time consuming10. Wang’s group also suggested that those patients who do not respond to CRT should receive echocardiographic optimization, which may result in a better hemodynamic outcome.
The other major aspect of our study is that we were able to demonstrate that the efficacy of QuickOpt optimization on parameters of cardiac function was comparable with that of the echocardiographic method. Further, LVEF was significantly increased at 12 months in both optimization groups, being 38.4 ± 44.3% and 38.4 ± 44.8%, respectively. Wang and colleagues10 reported similar findings in their smaller number of patients when assessed at 12 months after optimization. In our study, we clearly demonstrated that there were no significant differences in LVESV, LVEDV and LVEF between the two groups at 12 months (Table 4). Another important point from this trial is that patients from 31 hospitals were followed up at 3, 6 and 12 months, presenting a detailed and robust longitudinal clinical study with clearly effective clinical utility. The follow-up data included echocardiography (within 12 months), the 6MWT assessment (at 12 months), the NYHA class at each follow-up, 12 lead ECG (pacing), pacemaker follow-up data, and optimization results after QuickOpt optimization (at 3 and 6 months).
Finally, it is important to note that there were no significant differences in the incidence of device-related AEs and mortality rates between the QuickOpt and echocardiography groups (Table 7).
Conclusions
This multi-center and large cohort trial has shown that QuickOpt is a rapid and simple method to carry out optimization of AV and VV delays, which is non-inferior compared to the echocardiographic method in terms of resulting cardiac function and adverse events. For busy cardiology departments this will significantly free up precious clinical time.
References
Braunwald, E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 337, 1360–1369 (1997).
Hai, J. J. et al. Clinical Characteristics, Management, and Outcomes of Hospitalized Heart Failure in a Chinese Population-The Hong Kong Heart Failure Registry. J Card Fail (2016).
Redfield, M. M. et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289, 194–202 (2003).
Francis, G. S. et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 82, 1724–1729 (1990).
Mosterd, A. & Hoes, A. W. Clinical epidemiology of heart failure. Heart 93, 1137–1146 (2007).
Desai, A. S. & Stevenson, L. W. Rehospitalization for heart failure: predict or prevent? Circulation 126, 501–506 (2012).
Pu, L. J. et al. Cardiac resynchronization therapy (CRT) with right ventricular sense triggered left ventricular pacing benefits for the hemodynamics compared with standard CRT for chronic congestive heart failure: A cross-over study. Cardiol J 22, 80–86 (2015).
Starling, R. C. et al. Impact of a Novel Adaptive Optimization Algorithm on 30-Day Readmissions: Evidence From the Adaptive CRT Trial. JACC Heart Fail 3, 565–572 (2015).
Brugada, J. et al. Automatic optimization of cardiac resynchronization therapy using SonR-rationale and design of the clinical trial of the SonRtip lead and automatic AV-VV optimization algorithm in the paradym RF SonR CRT-D (RESPOND CRT) trial. Am Heart J 167, 429–436 (2014).
Wang, D. et al. Long-term clinical effects of programmer-guided atrioventricular and interventricular delay optimization: Intracardiac electrography versus echocardiography for cardiac resynchronization therapy in patients with heart failure. J Int Med Res 41, 115–122 (2013).
Kamdar, R. et al. A prospective comparison of echocardiography and device algorithms for atrioventricular and interventricular interval optimization in cardiac resynchronization therapy. Europace 12, 84–91 (2010).
Ishikawa, T. et al. Prediction of optimal atrioventricular delay in patients with implanted DDD pacemakers. Pacing Clin Electrophysiol 22, 1365–1371 (1999).
Cazeau, S. et al. Echocardiographic modeling of cardiac dyssynchrony before and during multisite stimulation: a prospective study. Pacing Clin Electrophysiol 26, 137–143 (2003).
Abraham, W. T. et al. Rationale and design of a randomized clinical trial to assess the safety and efficacy of frequent optimization of cardiac resynchronization therapy: the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am Heart J 159, 944–948 e941 (2010).
Tracy, C. M. et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 60, 1297–1313 (2012).
Hua, W. et al. A prospective study to evaluate the efficacy of an intracardiac electrogram-based atrioventricular and interventricular intervals optimization method in cardiac resynchronization therapy. Chin Med J (Engl) 125, 428–433 (2012).
European Society of, C. et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 15, 1070–1118 (2013).
Ritter, P., Padeletti, L., Gillio-Meina, L. & Gaggini, G. Determination of the optimal atrioventricular delay in DDD pacing. Comparison between echo and peak endocardial acceleration measurements. Europace 1, 126–130 (1999).
Whinnett, Z. I. et al. Maximizing efficiency of alternation algorithms for hemodynamic optimization of the AV delay of cardiac resynchronization therapy. Pacing Clin Electrophysiol 34, 217–225 (2011).
Strauss, M. et al. Impact of moderate exercise workload on predicted optimal AV and VV delays determined by an intracardiac electrogram-based method for optimizing cardiac resynchronization therapy. Clin Res Cardiol 99, 735–741 (2010).
Acknowledgements
This study was supported by funding from St. Jude Medical. We sincerely thank Jihong Qu and Yanhong Li from St. Jude Medical for text revision.
Author information
Authors and Affiliations
Contributions
Shu Zhang and Dejia Huang were responsible for the conception and design of the study. Ji Yan, Xiaolin Xue, Shu Zhang, Jing Xu, Qianmin Tao, Weize Zhang, Zheng Zhang, Wei Hua, Yanchun Liang, Baopeng Tang, Wei Xu, Geng Xu, Xuejun Ren, Jingfeng Wang, Tao Guo, Shaobin Jia, Yugang Dong, Hong Jiang, Guosheng Fu, Liguang Zhu, Lin Chen, Fuli Tian, Feng Ling, Jianmei Li, Xiaoyong Qi, Yinglu Hao, Yutang Wang, Liangrong Zheng, Xiaoqun Pu, Farong Shen, Guangping Li, Hui Li and Fang Peng were responsible for acquisition of data. Shu Zhang and Dejia Huang performed the data analysis. Ji Yan, Shu Zhang and Dejia Huang drafted the manuscript. All authors participated in interpretation of the findings and all authors read and approved the final version of the manuscript. All authors confirm that the content has not been published elsewhere and does not overlap with or duplicate their published work.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, J., Zhang, S., Huang, D. et al. Evaluation of the therapeutic effects of QuickOpt optimization in Chinese patients with chronic heart failure treated by cardiac resynchronization. Sci Rep 8, 4259 (2018). https://doi.org/10.1038/s41598-018-22525-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22525-0
This article is cited by
-
Acute recoordination rather than functional hemodynamic improvement determines reverse remodelling by cardiac resynchronisation therapy
The International Journal of Cardiovascular Imaging (2021)