Abstract
The objective of this study was to analyze three-dimensionally the morphological characteristics of the pterygomaxillary region related to pterygoid implants. Volume, height, width and bone density were studied in relation to age, sex and dental status. This retrospective observational study analyzed the CBCT of 52 hemi-maxillas three-dimensionally (females n = 28, males n = 24; dentate = 31, edentulous = 21). Patients were exposed between September 2009 and October 2014, and data collection was performed between November 2015 and May 2016. Bone density, volume, height and width were analyzed in various locations of the maxilla and pterygoid process, and the variables age, gender and dental status patients were compared. The results show that the mean width of the pterygomaxillary joint was 7.5 mm (SD 1.00 mm), mean height was 12.51 mm (SD 1,82 mm) and mean volume was 321.7 mm3 (SD 142.02 mm3). Statistically significant differences between dentate and edentulous patients were found, showing a higher osseous density in dentate patients in the pterygoid process (758.2, SD 106.8, 95% CI 729.2 to 787.3 GSD - Gray Scale Density - compared to 689.9, SD 107.3, 95% CI 660.8 to 719.1 GSD; P = 0.022). In the maxilla, density was statistically significant lower in female subjects (571.0, SD 74.1, 95% CI 594.9 to 645.4 GSD) than in male subjects (620.2, SD 93.8, 95% CI 594.4 to 645.4 GSD, P = 0.047). In conclusion, due to the significant variation in the morphological characteristics of the pterygomaxillary region among subjects, personalized pre-surgical radiological assessment should always be performed. Gender, age and dental status are critical factors as they significantly affect bone density in this region.
Similar content being viewed by others
Introduction
The loss of superior posterior teeth and the consequent alteration of the occlusion can lead to occlusal instability, deficiencies in the masticatory function and local bone loss, among other results1. When treating long-term edentulous patients, the surgeon may face a situation in which pneumatization of the maxillary sinus, resorption of the alveolar bone and insufficient osseous density occur together. The placement of implants in the posterior maxilla can become a challenge due to, among other factors, the poor osseous quantity and quality of the maxillary tuberosity2, and it has been demonstrated that implants have a higher propensity to fail under conditions of low-density bone3. Likewise, it has been proved that, with increasing age, the number of fibroblasts in the suture decreases and the suture and surrounding bone matures4, making bone quality and repair capacity decrease. Regarding the gender of the patient, inconclusive results have been obtained when examining the relationship between this variable and the bone density of the maxilla. One study did not found statistically significant differences when comparing genders5, while other assures that female subjects had significantly denser bone compared with male subjects6.
To avoid sinus lift surgeries or bone grafts, Tulasne7 first described the pterygoid implants in 1989 as an alternative to conventional dental implants. Unlike maxillary tuberosity implants, pterygoid implants anchor in cortical bone, allowing for a better primary stabilization, which is known to be a critical factor for long-term success8.
The pterygoid implants also present some inconveniences: the learning is convoluted compared to conventional dental implants, access for surgeons and oral rehabilitators is complex and serious injuries can occur in sectioning the maxillary artery or invading the pterygomaxillary fossa2.
For the placement of these implants, it is essential to have a thorough knowledge of the anatomy involved because nearby vital structures can be injured during surgery. Special attention should be paid to the internal maxillary artery, which crosses between 10 and 23.5 mm above the pterygomaxillary suture according to authors9,10. According to Candel et al.9, any bleeding observed in the preparation of the pterygoid implant bed comes from the irrigation of the pterygoid muscles and will be controlled once the implant is inserted. When placing a pterygoid implant, it is essential to pay attention to its proximity to the greater palatine canal and nerve7,11. There are other authors who believe that none important anatomical structure can be injured during the placement of these implants12,13.
One of the most important reasons and advantages for the use of pterygoid implants is the elimination of the need to perform sinus lift surgeries or bone grafts, therefore decreasing the morbility and shortening the treatment time, as it has been proved that the osseointegration of pterygoid implants can occur in only 2 to 3 months7,14. Unlike maxillary tuberosity implants, pterygoid implants anchor in cortical bone, allowing a better primary stabilization which is known to be a critical factor for long-term success8. From a prosthetic point of view it can minimize the time to rehabilitation and bypasses the need of a distal cantilever and also makes it possible to perform, if the patient meets the indication criteria, an immediate prosthetic load15. Another anatomical region in which dental implants can be anchored in advanced resorptions of the maxilla is in the zygoma, as it has been showed to have enough thickness and trabecular density16 to support occlusal forces, but compared to pterygoid implants, the learning curve for the surgical technique is greater and the potential surgical complications could be more severe.
Multiple studies have shown that pterygoid implants are a predictable treatment14,16,17 but few studies have described in a precise three-dimensional way the morphological characteristics of the pterygomaxillary region.
The aim of this study was to improve the knowledge of the morphological characteristics of the pterygomaxillary region by anatomical three-dimensional analysis to improve pterygoid implants surgical planning and thus improve long-term success rates. Volume, height, width and bone density of the pterygomaxillary joint were measured to assess whether age, sex or dental status significantly affected these variables.
Methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendation guidelines18.
Study design
A retrospective cross-sectional study was conducted at Rey Juan Carlos University (Madrid, Spain). The study received approval from the Rey Juan Carlos University Review Board and have been performed in accordance with the ethical standards of 1964 Declaration of Helsinki.
Anonymization and analysis of the data
The collected data was anonymized for analysis by a data reduction technique conducted by an independent reviewer for the statistical treatment and evaluations of the variables (width, height and volume of the pterygomaxillary joint), ensuring the patients’ privacy.
Participants
Twenty-eight patients, for a total of fifty-six sides (n = 56), between 27 and 71 years old were exposed between September 2009 and October 2014, and data collection was performed between November 2015 and May 2016. Cone beam computerized tomography (CBCT) from fifty-six hemi-maxillas were studied from patients who had undergone this radiological study for surgical reasons. Informed consent statement was obtained from all individual included in the study and the patients were informed about the potential risk of CBCT.
Inclusion criteria were patients between 27 and 71 years old, treated at the Rey Juan Carlos University for surgical purposes with a CBCT scan with a FOV (field of view) that included the entire pterygomaxillary complex. To include patients in this study data on gender and age had to be registered. The exclusion criteria were traumatic, cystic or tumor lesions in the study area; the presence of systemic diseases that partially or totally contraindicate implant treatment; skeletal deformities; images that were unclear or did not include the pterygomaxillary joint in its entirety; presence of any local bone pathology; patients with non-erupted third molars in the maxilla.
Methodology
Radiographic explorations were performed with a CBCT (CS 8100 3D. Carestream). Default parameters for 3D were used: 80 kV, 2 mA, Field Of View (FOV) 8 × 9 cm, voxel size 150 µm. Patients were placed in a supine position. One cephalostat was placed stabilizing the head and palatal plane (ENA-ENP) perpendicular to the ground.
AMIRA® 6.0 software (AMIRA, Mercury Computer Systems, Berlin, Germany) was used to assess measure and create three-dimensional images of the pterygomaxillary region.
Two independent authors (J. A. M. G. and C. S. G.) performed all radiological measurements under the supervision of an expert oral and maxillofacial surgeon (J. C. P. F.).
Sample size
In previous studies, the mean width and length of the pterygomaxillary joint was 8.5 mm (SD 1.9) and 12.9 mm (SD 2.7)19. The calculation was determined with a 99% confidence level, an accuracy of 1% and a variance of 3.61 and 7.29. A sample size of 24 and 48 subjects, respectively, was necessary. When the data collection of this study began, there were no published articles using the same references or methods as we did for the variables volume and bone density. Therefore, a preliminary pilot study was conducted to obtain this data. It was determined that the sample size for the bone density variable with a margin of error of less than 10%, was 52 hemi-maxillas.
Analysis variables
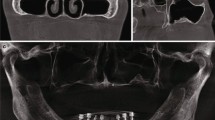
The anatomical and radiological variables studied in this paper are described below. (1) Sex: male or female. (2) Age. (3) Side: right or left. (4) Joint width: this element was described as the joint width of the maxillary tuberosity and the pterygoid process in millimeters (mm), and the pyramidal process if present, measured on the first axial plane where a full joint contact was observed (Fig. 1). (5) Joint height: this element was described as the measurement in millimeters (mm) between the most caudal and most cranial points of the pterygomaxillary joint, which was considered the pterygomaxillary column (Fig. 2). (6) Bone volume: this element was described as the total bone volume in cubic millimeters (mm3). The area of study was delimited by the following limits: the posterior wall, the maxillary sinus, the pterygoid and scaphoid process, the inferior limit of the pterygomaxillary fossa and the separation of the tuberosity and pterygoid process. (7) Bone density: nine points were measured in total, three points at the inferior part of the pterygomaxillary column, three points at the superior part and three in a medium zone equidistant from the two previously described. Each of these areas was measured at the anterior limit (posterior sinus wall), the joint between the maxillae and the pterygoid/pyramidal process and the posterior limit (scaphoid or pterygoid fossae). As surgical radiological assessment was proceeded by CBCT, bone density was measured in gray values (GSD). According to a previous study20, gray values from CBCT could be used to assess bone quality and had been used previously in various studies21,22,23. (8) Dental status: dentate/edentulous: if the second or third upper molars were present, the patient was considered dentate. If none of these molars were present, the patient was considered edentulous.
Statistical analysis
Two observers (J. A. M. G. and C. S. G.) performed the measurements. Student’s paired t test, non-parametric Wilcoxon contrast and the intraclass correlation coefficient (ICC) were used to analyze agreement between observers in all the variables.
A descriptive statistical analysis was performed using IBM SPSS® version 19.0 statistical package (IBM Corp., Armonk, NY, USA), in which the variables of joint width, joint height, bone volume and bone density were crossed with genre, age, side and dentate/edentulous status.
First, normality was tested for all the variables with Kolmogorov-Smirnov and Shapiro-Wilk tests. The Student’s T-Test was applied if normality was met, and a non-parametric U Mann-Whitney test was used if it was not met. To evaluate concordance between measurement and age, Pearson’s correlation coefficient, or Spearman’s non-parametric correlation when applicable, was used. Results were considered statistically significant when P < 0.05.
The required sample size was calculated to obtain precise volume and linear measurements so that the confidence interval did not vary by more than 10% of the corresponding mean value and presented a confidence level of 95%.
Results
Of 28 CBCTs available (56 sides), 26 CBCTs (52 sides) met the inclusion criteria (Fig. 3). The two CBCTs that were not included were due to the presence of bone pathology in the study area. Females represented 53.8% (n = 28) of the study sample, and 46.2% (n = 24) were males. The subjects’ mean age was 49.7 (SD 10.3). Right and left sides were equally distributed representing 50% (n = 26) of the sample each.
The intraclass correlation coefficient (ICC) varied from 0.82 (95% CI = 0.60 to 0.92) and 0.99 (95% CI = 0.81 to 0.94), which is considered excellent24.
Normality tests were applied, and all the variables but “volume” met this condition. Non-parametric tests were used when necessary. No missing data was identified in any of the variables.
The mean width of the joint was 7.51 mm (SD 1.0 mm, 95% CI 7.14 to 7.78 mm); mean width for females was 7.51 mm (SD 0.82, 95% CI 7.29 to 7.73 mm) and for males 8.07 mm (SD 1.23, 95% CI 7.74 to 8.40 mm). Mean height was 12.5 mm (SD 1.8 mm, 95% CI 12.01 to 12.99 mm); mean height for females was 12.55 mm (SD 1.60, 95% CI 12.12 to 12.98 mm) and for males 12.72 mm (SD 2.06, 95% CI 12.16 to 13.28 mm). Mean volume was 321.76 mm3 (SD 142.02 mm3, 95% CI 283.2 to 360.4 mm3); mean volume for females was 395.39 mm3 (SD 177.13, 95% CI 347.25 to 443.53 mm3) and for males 274.18 mm3 (SD 50.11, 95% CI 260.56 to 287.80 mm3).
Age, sex or dental status had no impact on the length or width of the pterygomaxillary joint. According to our study, these variables would not have an effect on the volume of the pterygomaxillary region either, however, for this variable a larger sample is needed to contrast the data.
Bone density values can be observed in Table 1. Considering male subjects and female subjects together, for the maxillary tuberosity density in the most caudal measurement, Pearson’s correlation index was −0.34, which was statistically significant (P = 0.015) and can be interpreted as older patients having less density in the tuberosity at its lowest point.
Dentate subjects represented 59.6% of the study sample (n = 31), and 40.4% (n = 21) were edentulous. When dentate and edentulous patients were studied separately, in dentate patients, the bone density of the middle section of the pterygomaxillary fissure increased with age (Pearson’s correlation = 0.46, P = 0.01). Also, in dentate patients, a statistically significant difference was observed between males’ and females’ middle sections of the pterygoid process (males: 734.7 GSD, SD 91.9 GSD, 95% CI = 709.7 to 759.7; females: 649.7 GSD, SD 105.1 GSD, 95% CI = 621.1 to 678.2 GSD; P = 0.026). In edentulous patients, density in the maxillary tuberosity’s middle section was significantly lower in female subjects (559.7 GSD, SD 85.4 GSD, 95% CI = 536.5 to 582.9 GSD) than in male subjects (631.1 GSD, SD 68.9 GSD, 95% CI = 612.4 to 649.8; P = 0.047). Also, the cancellous bone density of the maxillary tuberosity in male subjects was significantly higher (448.1 GSD, SD 74.6 GSD, 95% CI = 427.8 to 468.4) than in female subjects (366.1 GSD, 85.9 GSD, 95% CI = 342.8 to 389.4; P = 0.031).
Discussion
This research shows three critical factors affect osseous density locally: gender (female subjects had statistically less density), age (density decreased as age progressed) and dental status (edentulous patients presented an inferior density).
In a study performed by Lee et al.25, the height of the pterygopalatine suture has been reported to be 13.1 mm, which is in concordance with the measurement in our study, 12.5 mm (SD 1.8 mm). Studies have suggested that implants measuring 13.0 to 18.0 mm could be appropriated to ensure an engagement in the cortical bone of the pterygoid process17,26.
The width of the pterygomaxillary joint has been previously studied in articles related to surgical techniques such as Le Fort I osteotomies27,28. However, we have not been able to find research focused on implantology that evaluates this characteristic. Our findings are slightly shorter than those measured by Dadwal et al.27 and Chin et al.28. This may be due to the differences in the measurement method, since in our study the measurement was always made of the most caudal region and in the previously mentioned articles they do not specify at what point of the pterygopalatine fissure the measurements were made or how they considered the exact point of measurement.
We have not found other studies in the literature that measure the volume of the maxillary tuberosity, therefore, we cannot compare the results.
Regarding bone densities, Rodríguez et al.29 reported an average of 307.4 GSD (SD 155.9 GSD) in the maxillary tuberosity and 632.0 GSD (SD 209.7 GSD) in the pterygoid area. Our measurements were slightly higher for both maxillary and pterygoid region (370.3 GSD, SD 71.4 GSD; and 692.4 GSD, SD 85.5 GSD; respectively), which could be due to mean age, which is not specified in Rodríguez’s study. In this study, bone density in the pterygoid area was 139.2% higher than in the tuberosity area, proving that it is an area of better choice for the anchorage of the implant head29. It has been proven that implants in type I and II bone had a higher survival rate and could support sand with a higher occlusal load for which type IV bone was unsuitable30, which supports our recommendation of using pterygoid implants for the rehabilitation of the posterior maxillae.
In our study, it was observed a statistically significant difference at the upper area on the pterygoid process, where dentate subjects presented a density of 758.2 GSD (SD 106.8 GSD. 95% CI 729.2 to 787.3 GSD) and edentulous patients presented 686.9 GSD (SD 107.3, 95% CI 657.8 to 716.1 GSD) (p < 0.05). This is believed to be explained because dentate patients have proven to have a greater muscular strength which develops into a mayor osseous density. A minor electromyographic activity of the masticatory muscles is related to the deterioration of the dental status31,32. Also, the middle section of the pterigomaxilar column had a greater density along with age; the older the subject was, the greater the density of the bone (Pearson’s correlation = 0.46, p = 0.01). This is also believed to be a result of muscular strength33. The osseous density of this middle section of the pterygoid process is important because is one of the main sites of anchorage of the pterygoid implants. These implants are anchored in the pterygoid plate of the sphenoid bone, moving backwards and upwards through the maxillary tuberosity and palatine bone13,15,34. It was observed that an implant placed in the pterigomaxilar area with an angulation on 45 degrees would increase a mean of 8–9 mm the bone to implant contact13.
As for the angulation of the pterygoid implants with respect to the Frankfort plane, there is no consensus. Different authors defend their placement with an approximate angle of 45°13,15,35 while in more recent studies their placement is defended at an angle of 70°27,28,29,30,31,32,33,34,35. In a study developed by Rodríguez et al.36, where 454 implants were placed in 392 patients, the mean angulation was 70.4° ± 7.2°. Anatomical studies support this technique: the average inclination of the bone pillar composed of the pterygoid process and the tuberosity of the maxilla is between 67.3° and 75.1° in relation to the Frankfort plane37. Therefore, previous studies that suggested placing the pterygoid implants with an approximate inclination of 45° with respect to the Frankfurt plane would be relegated13,15,34. This traditional angulation of the pterygoid implants has different disadvantages, the main one being that the transmission of the occlusal forces does not occur in an axial direction, and may compromise the long-term result of the prosthetic rehabilitation36. An inclination of 45° decreases its load capacity by up to 50% with respect to the same implant placed at 90°34. Therefore, a 20 mm pterygoid implant with an angle of 45° would have the same occlusal loading capacity as a 10 mm implant placed at 90° 36.
Pterygoid implants were first described by Tulasne2 as a reliable method for the rehabilitation of the atrophied posterior maxilla. It was described as an alternative to rehabilitate the atrophic maxilla avoiding the need for previous surgeries such as maxillary sinus augmentation or alternative bone graft9,15. In 1992, Tulasne described a success rate of 80%7, nonetheless during the past years several studies have reported a much higher success rate, reporting from 90.7% to 99% of success9,14,16,17,38,39.
These implants are proven to compensate for the posterior maxilla’s poor osseous quality by anchoring themselves to the pterygoid process’s cortical bone2,7,21. Dental implants placed in the tuberosity have also been widely described34,40,41, as it is possible to place an implant completely and solely in the maxillary tuberosity if the bone’s quantity and quality are adequate, but is also well-known that in most of the cases, this anatomical region is conformed by Type III or Type IV cancellous bone21. Among others, loss of the dental implants is related to low-primary stability, which is at the same time related to bone density40. This research confirms that the tuberosity has a very low bone density compared to the pterygoid region, agreeing with previous studies9. It has also been shown that increasing dental implant length plays a fundamental role in increasing dental implant primary stability42.
In patients with atrophic maxillae who are not subsidiaries of receiving bone grafts or short implants, the placement of zygomatic implants should also be assessed43. The main indication of these implants is the rehabilitation of completely edentulous patients, with severe pneumatization of the maxillary sinuses and severe resorption of the posterior alveolar ridge43. The volume and extension of the maxillary sinus along with the angulation of the anterior wall will determine the position of the most distal implant and its angulation. This, unless pterygoid implants are also placed, will define the distal extension of the prosthesis43,44. The survival rate of this type of implants is high (97.86% in a 36-month follow-up period), according to the systematic review by Goiato et al.45. The placement of zygomatic implants is complex due to the intricate anatomy of the maxillofacial region and the greater length of these implants (from 30 to 52.5 mm)44. Although some authors argue that the surgical and postoperative complications of these implants are infrequent44, if any of them appear they can be serious. Among the main complications are: rhinosinuisitis46, lack of osseointegration47, soft tissue infection48,49, paraesthesia48,49 and oroantral fistula48,50.
For the placement of these implants, it is imperative to have a thorough knowledge of the anatomy involved because nearby vital structures can be injured during surgery. Special attention should be paid to the internal maxillary artery7,9,10,11.
Conclusions
Pterygomaxillary region morphology has a wide range of variation between individuals, and every time a pterygoid implant is planned to be placed in this area, a personalized pre-surgical radiological assessment should be performed. Various factors such as dental status (edentulous patients presented an inferior density), age (density decreased as age progressed) and gender (female subjects had statistically less density) have an impact on bone density in the pterygomaxillary region. Nevertheless, it has been shown that the bone density of the pterygoid process is always greater than the bone density of the maxillary tuberosity and, therefore, it should be the choice for implant anchoring in atrophic maxillae.
References
Araújo, M. & Lindhe, J. Socket grafting with the use of autologous bone: an experimental study in the dog. Clin. Oral Implants Res. 22, 9–13 (2010).
Tulasne, J. F. Implant treatment of missing posterior dentition in The Branemark Osseointegrated Implant (eds Albrektsson, T. & Zarb, G. A.) 103 (Chicago, IL: Quintessence, 1989).
Fischer, K., Bäckström, M. & Sennerby, L. Immediate and early loading of oxidized tapered implants in the partially edentulous maxilla: a 1-year prospective clinical, radiographic, and resonance frequency analysis study. Clin. Implant. Dent. Relat. Res. 11, 69–80 (2009).
Yin, J. et al. Changes in the Zygomaticomaxillary Suture During Aging. J. Craniofac. Surg. 26, 2201–2206 (2015).
Bertl, K. et al. MicroCT-based evaluation of the trabecular bone quality of different implant anchorage sites for masticatory rehabilitation of the maxilla. J. Craniomaxillofac. Surg. 43, 961–968 (2015).
Ohiomoba, H., Sonis, A., Yansane, A. & Friedland, B. Quantitative evaluation of maxillary alveolar cortical bone thickness and density using computed tomography imaging. Am. J. Orthod. Dentofacial. Orthop. 151, 82–91 (2017).
Tulasne, J. F. Osseointegrated fixtures in the pterygoid region in Advanced Osseointegration Surgery: Applications in the Maxillofacial Region. (eds Worthington, P. & Branemark, P.I.) 182–188. Chicago, IL: Quintessence (1992).
Fuh, L. J. et al. Variations in bone density at dental implant sites in different regions of the jawbone. J. Oral Rehabil. 37, 346–351 (2010).
Candel, E., Peñarrocha, D. & Peñarrocha, M. Rehabilitation of the atrophic posterior maxilla with pterygoid implants: a review. J. Oral Implantol. 38, 461–466 (2012).
Apinhasmit, W. et al. Anatomical study of the maxillary artery at the pterygomaxillary fissure in a Thai population: Its relationship to maxillary osteotomy. J Med Assoc Thail. 87, 1212–7 (2004).
Suzuki, M. et al. Regional Anatomical Observation of Morphology of Greater Palatine Canal and Surrounding Structures. Bull Tokyo Dent Coll. 57, 223–231 (2016).
Balshi, T. J., Wolfinger, G. J., Slauch, R. W. & Balshi, S. F. Brånemark system implant lengths in the pterygomaxillary region: a retrospective comparison. Implant Dent 22(6), 610–2 (2013).
Graves, S. L. The pterygoid plate implant: a solution for restoring the posterior maxilla. Int. J. Periodontics. Restor. Dent. 14, 512–523 (1994).
Balshi, T. J., Wolfinger, G. J. & Balshi, S. F. Analysis of 356 pterygomaxillary implants in edentulous arches for fixed prosthesis anchorage. Int. J. Oral Maxillofac. Implants 14, 398–406 (1999).
Bidra, A. S. & Huynh-Ba, G. Implants in the pterygoid region: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 40, 773–781 (2011).
Kato, Y., Kizu, Y., Tonogi, M. & Yamane, G. Y. Internal structure of zygomatic bone related to zygomatic fixture. J. Oral Maxillofac. Surg. 63, 1325–1329 (2005).
Balshi, S. F., Wolfinger, G. J. & Balshi, T. J. Analysis of 164 titanium oxide-surface implants in completely edentulous arches for fixed prosthesis anchorage using the pterygomaxillary region. Int. J. Oral Maxillofac. Implants 20, 946–952 (2005).
Von Elm, E. et al. & STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 12, 1495–1499 (2014).
Lee, S. H. et al. Evaluation of pterygomaxillary anatomy using computed tomography: Are there any structural variations in cleft patients? J Oral Maxillofac Surg. 10, 2644–2649 (2011).
Parsa, A., Ibrahim, N., Hassan, B., van der Stelt, P. & Wismeijer, D. Bone quality evaluation at dental implant site using multislice CT, micro-CT, and cone beam CT. Clin. Oral Implants Res. 26, e1–7 (2013).
Wang, F. et al. Accuracy of cone beam computed tomography grayscale density in determining bone architecture in the posterior mandible: an in vivo study with microcomputed tomography validation. Int. J. Oral Maxillofac. Implant 32, 1074–1079 (2017).
Alkhader, M., Hudieb, M. & Khader, Y. (2017) Predictability of bone density at posterior mandibular implant sites using cone-beam computed tomography intensity values. Eur. J. Dent. 11, 311–316 (2017).
Guerra, E. N. S. et al. Capability of CBCT to identify patients with low bone mineral density: a systematic review. Dentomaxillofac. Radiol. 46, 20160475 (2017).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Lee, S. P., Paik, K. S. & Kim, M. K. Anatomical study of the pyramidal process of the palatine bone in relation to implant placement in the posterior maxilla. J. Oral Rehabil. 28, 125–132 (2001).
Rodríguez, X. et al. Anatomical study of the pterygomaxillary area for implant placement: cone beam computed tomographic scanning in 100 patients. Int. J. Oral Maxillofac. Implants 29, 1049–1052 (2014).
Dadwal, H., Shanmugasundaram, S., Krishnakumar Raja, V. B., Preoperative & Postoperative, C. T. Scan Assessment of Pterygomaxillary Junction in Patients Undergoing Le Fort I Osteotomy: Comparison of Pterygomaxillary Dysjunction Technique and Trimble Technique-A Pilot Study. J Maxillofac Oral Surg. 14, 713–719 (2015).
Chin, Y. et al. The pterygomaxillary junction: An imaging study for surgical information of LeFort I osteotomy. Sci Rep. 7, 9953 (2017).
Rodríguez, X. et al. Anatomical and radiological approach to pterygoid implants: a cross-sectional study of 202 cone beam computed tomography examinations. Int. J. Oral Maxillofac. Surg. 45, 636–640 (2016).
Linetskiy, I., Demenko, V., Linetska, L. & Yefremov, O. Impact of annual bone loss and different bone quality on dental implant success – a finite element study. Comput. Biol. Med. 91, 318–325 (2017).
Gaszynska, E., Kopacz, K., Fronczek-Wojciechowska, M., Padula, G. & Szatko, F. Electromyographic activity of masticatory muscles in elderly women – a pilot study. Clin. Interv. Aging. 12, 111–116 (2017).
Takagi, D. et al. Factors affecting masticatory function of community-dwelling older people: Investigation of the differences in the relevant factors for subjective and objective assessment. Gerodontology 34, 357–364 (2017).
Sato, H., Kawamura, A., Yamaguchi, M. & Kasai, K. Relationship between masticatory function and internal structure of the mandible based on computed tomography findings. Am. J. Orthod. Dentofac. Orthop. 128, 766–773 (2015).
Venturelli, A. A modified surgical protocol for placing implants in the maxillary tuberosity: clinical results at 36 months after loading with fixed partial dentures. Int. J. Oral Maxillofac. Implant. 11, 743–749 (1996).
Curi, M. M. & Cardoso, C. L. Retrospective Study of Pterygoid Implants in the Atrophic Posterior Maxilla: Implant and Prosthesis Survival Rates Up to 3 Years. Int. J. Oral Maxillofac. Implant 30, 3–8 (2015).
Rodriguez, X., Mendez, V., Vela, X. & Segala, M. Modified surgical protocol for placing implants in the pterygomaxillary region: clinical and radiologic study of 454 implants. Int J Oral Maxillofac Implant. 27, 1547–53 (2012).
Yamaura, T., Abe, S., Tamatsu, Y., Rhee, S. & Hashimoto, M. Anatomical study of the maxillary tuberosity in Japanese men. Bull Tokyo Dent Coll 39(4), 287–92 (1998).
Peñarrocha, M., Carrillo, C., Boronat, A. & Peñarrocha, M. Retrospective study of 68 implants placed in the pterygomaxillary region using drills and osteotomes. Int. J. Oral Maxillofac. Implant. 24, 720–726 (2009).
Valerón, J. F. & Valeron, P. F. Long-term results in placement of screw-type implants in the pterygomaxillary-pyramidal region. Int. J Oral Maxillofac. Implant. 22, 195–200 (2007).
Goiato, M. C., Santos, D. M., Santiago, J. F. Jr., Moreno, A. & Pellizzer, E. P. Longevity of dental implants in type IV bone: a systematic review. Int. J. Oral Maxillofac. Surg. 43, 1108–1116 (2014).
Ridell, A., Gröndahl, K. & Sennerby, L. Placement of Brånemark implants in the maxillary tuber region: anatomical considerations, surgical technique and long-term results. Clin. Oral Implants Res. 20, 94–98 (2009).
Bataineh, A. B. & Al-Dakes, A. M. The influence of length of implant on primary stability: An in vitro study using resonance frequency analysis. J. Clin. Exp. Dent. 9, e1–e6 (2017).
Aparicio, C. et al. Zygomatic implants: Indications, techniques and outcomes, and the Zygomatic Success Code. Periodontol 2000. 66, 41–58 (2014).
Chana, H., Smith, G., Bansal, H. & Zahra, D. A Retrospective Cohort Study of the Survival Rate of 88 Zygomatic Implants Placed Over an 18-year Period. Int J Oral Maxillofac Implants 34, 461–70 (2019).
Goiato, M. C. et al. Implants in the zygomatic bone for maxillary prosthetic rehabilitation: A systematic review. Int J Oral Maxillofac Surg 43, 748–57 (2014).
Bothur, S., Kullendorff, B. & Olsson-Sandin, G. Asymptomatic Chronic Rhinosinusitis and Osteitis in Patients Treated with Multiple Zygomatic Implants: A Long-Term Radiographic Follow-up. Int J Oral Maxillofac Implants. 30, 161–8 (2016).
Molinero-Mourelle, P. et al. Surgical complications in zygomatic implants: A systematic review. Med Oral Patol Oral Cir Bucal 21, e751–7 (2016).
Chrcanovic, B. R., Albrektsson, T. & Wennerberg, A. Survival and Complications of Zygomatic Implants: An Updated Systematic Review. J Oral Maxillofac Surg 74, 1949–64 (2016).
Kahnberg, K. E. et al. Clinical Evaluation of the Zygoma Implant: 3-Year Follow-Up at 16 Clinics. J Oral Maxillofac Surg. 65, 2033–8 (2007).
Stiévenart, M. & Malevez, C. Rehabilitation of totally atrophied maxilla by means of four zygomatic implants and fixed prosthesis: a 6–40-month follow-up. Int J Oral Maxillofac Surg. 39, 358–63 (2010).
Acknowledgements
We would like to acknowledge to Dr. Ortega Araneguí (Diagnóstico Bucofacial Dr. Ortega Inc) for his help in the radiographic data harvesting. We also thank the Papercheck Proofreading & Editing Services for the English editing of this manuscript. This study was funded by Grant A-274 (Intradent Iberia SA- Rey Juan Carlos University). Principal Research: Juan Carlos Prados Frutos.
Author information
Authors and Affiliations
Contributions
C.S.-G, R.R. and J.C.P.-F. conceived and designed the study. C.S.-G., J.A.M.-G. and J.C.P.-F. were responsible for the data collection. C.S.-G., R.R. and J.A.M.-G. were responsible for the data analysis. C.S.-G., R.R. and J.C.P.-F. wrote the paper. All authors finally approved the paper before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salinas-Goodier, C., Rojo, R., Murillo-González, J. et al. Three-dimensional descriptive study of the pterygomaxillary region related to pterygoid implants: A retrospective study. Sci Rep 9, 16179 (2019). https://doi.org/10.1038/s41598-019-52672-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52672-x
This article is cited by
-
Morphometric Analysis of Pterygomaxillary Fissure in Dry Skulls and its Clinical Significance During Head and Neck Injury
Indian Journal of Otolaryngology and Head & Neck Surgery (2025)
-
Three-dimensional digital anatomical measurements of pterygoid plates and posterior maxillary region
BMC Oral Health (2024)
-
Anatomical study of pterygoid implants: artery and nerve passage through bone dehiscence of the greater palatine canal
International Journal of Implant Dentistry (2024)
-
Tomographic Analysis of the Pterygomaxillary Region in Atrophic Maxilla for the Placement of Pterygoid Implants
Journal of Maxillofacial and Oral Surgery (2024)
-
Virtual pterygoid implant planning in maxillary atrophic patients: prosthetic-driven planning and evaluation
International Journal of Implant Dentistry (2023)