Abstract
Kikuchi-Fujimoto disease (KFD) is usually self-limiting, but prolonged systemic symptoms often result in frequent hospital visits, long admission durations, or missed workdays. We investigated the role of fluorine-18 fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in assessing KFD severity. We reviewed the records of 31 adult patients with pathologically confirmed KFD who underwent 18F-FDG PET/CT between November 2007 and April 2018 at a tertiary-care referral hospital. Disease severity was assessed using criteria based on clinical manifestations of advanced KFD. Systemic activated lymph nodes and severity of splenic activation were determined using semi-quantitative and volumetric PET/CT parameters. The median of the mean splenic standardized uptake value (SUVmean) was higher in patients with severe KFD than those with mild KFD (2.38 ± 1.18 vs. 1.79 ± 0.99, p = 0.058). Patients with severe KFD had more systemically activated volume and glycolytic activity than those with mild KFD (total lesion glycolysis: 473.5 ± 504.4 vs. 201.6 ± 363.5, p = 0.024). Multivariate logistic regression showed that myalgia (odds ratio [OR] 0.035; 95% confidence interval [CI] 0.001–0.792; p = 0.035), total lymph node SUVmax (cutoff 9.27; OR 24.734; 95% CI 1.323–462.407; p = 0.032), and spleen SUVmean (cutoff 1.79; OR 37.770; 95% CI 1.769–806.583; p = 0.020) were significantly associated with severe KFD. 18F-FDG PET/CT could be useful in assessing KFD severity.
Similar content being viewed by others
Introduction
Kikuchi-Fujimoto disease (KFD), also known as histiocytic necrotizing lymphadenitis, is a disease endemic to Asia and of unknown etiology1,2,3. It usually develops in young adult women and is most commonly characterized by cervical lymphadenopathy and fever4,5. KFD presents with various clinical features, ranging from absence of systemic symptoms to significant symptoms like night sweats, myalgia, weight loss, arthralgia, and hemophagocytic lymphohistiocytosis (HLH)6,7,8,9.
Although KFD is usually benign and self-limiting, patients with prolonged systemic symptoms are plagued with frequent hospital visits, long durations of admission, or missed workdays10. Immunomodulating drugs, such as high-dose corticosteroids or intravenous immunoglobulins, which aid in shortening the clinical course of the disease, have been administered for treating patients with severe KFD11,12. However, as there are no established markers for KFD severity, the determination of treatment options for KFD is dependent only on the clinician’s discretion, which may result in delayed treatment or a prolonged symptom duration.
Fluorine-18 fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) can be used to evaluate glucose utilization in multiple organs. Although 18F-FDG uptake has been predominately used to evaluate cancer metabolism, 18F-FDG PET/CT is used in clinical settings to assess localized inflammatory foci and infectious diseases, such as tuberculosis, Q fever, infective endocarditis, vascular graft infection, chronic active Epstein–Barr virus infection, invasive fungal infection, and surgical site infection13,14,15,16,17. Another advantage of 18F-FDG PET/CT is that PET metrics, such as standardized uptake value (SUV), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), allow for the evaluation of the severity and quantification of glycolysis in multiple organs, which serve as prognostic prediction parameters for survival in patients with solid tumors18.
The spleen is an important immune organ in both innate and adaptive immune responses and in regulating immune homeostasis19. Studies have reported diffuse increased splenic 18F-FDG uptake in patients with lymphoma, infections, tuberculosis, and autoimmune diseases20,21,22,23. Moreover, recent findings suggest that a diffuse increased FDG uptake was observed not only in the lymph nodes but also in the spleen in patients with KFD24,25. However, the relationship between abnormal 18F-FDG uptake in patients with KFD and disease severity has not been established to date.
We hypothesized that glucose metabolism in the spleen and pathologic lymph nodes varies according to KFD severity because of the presence of systemic inflammation. Hence, we aimed to investigate the 18F-FDG uptake in the spleen and lymph nodes in patients with KFD and evaluate its performance as a disease severity parameter.
Methods
Patient selection
Inclusion criteria
We retrospectively reviewed the electronic medical records between November 2007 and April 2018 at a tertiary-care referral hospital located in Seoul, Korea. We enrolled patients who had pathologically confirmed KFD and underwent 18F-FDG PET/CT in the same admission period.
Exclusion criteria
We excluded patients who did not meet the criteria to assess KFD severity.
Data collections
Data collected included age, sex, clinical manifestations, laboratory test values, histologic findings, treatment methods and durations, fever duration, and outcomes such as relapse. All procedures performed in human studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of Yonsei University Health System Clinical Trial Center (4-2019-0977). Since the study was retrospective and the study subjects were anonymized, the Institutional Review Board Review Board of Yonsei University Health System Clinical Trial Center waived the requirement for written consent from the patients.
Definition of severe KFD
For assessment of disease severity, we established the presence of severe KFD based on the clinical manifestations of advanced KFD disease10,26,27,28. Severe KFD was defined as KFD with at least one of the following manifestations: encephalitis, peripheral neuropathy, HLH, long fever duration (> 7 days), or leukopenia (< 4000/μL).
Imaging technique
All patients fasted for at least 6 h before the PET/CT scans were taken. Serum glucose levels (preferably < 150 mg/dL) were measured followed by intravenous administration of 5.5 MBq/kg 18F-FDG (with a maximum of 400 MBq). PET and combined low-dose CT scans were performed with commercial PET/CT scanners (Discovery STE, Discovery D600, Discovery D710 [GE Healthcare, Chicago, IL, USA], or Biograph TruePoint40 [Siemens Healthineers, Erlangen, Germany]) after 1 h. The PET scan was performed with an acquisition time of 2 min per bed position in the 3-dimensional mode. PET data were reconstructed iteratively using an ordered subset expectation maximization algorithm with the low-dose CT datasets for attenuation correction.
Imaging analysis
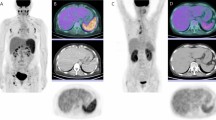
For semi-quantitative and volumetric analysis, various metabolic PET parameters were measured including the maximum SUV (SUVmax), MTV, and TLG in the lymph nodes as well as the mean SUV (SUVmean) and TLG in the spleen using commercially available imaging software (MIM Software, Cleveland, OH, USA). All PET/CT images were read by two experienced nuclear medicine physicians. For background activity, a spherical volume of interest (VOI) with a diameter of 30 mm was drawn at the inferior right lobe of the liver, excluding the main ducts and vessels. The SUVmean of that VOI was adopted as a threshold value to determine the boundaries of the pathologic lymph nodes in each PET/CT study. After SUV thresholding, FDG-avid regions were automatically segmented using the isocontour threshold method. The SUVmax of the total lymph nodes was defined as the highest metabolic foci (SUVmax) in all the metabolically active lymph nodes in the body. The total MTV of lymph nodes was defined as the sum of the MTVs of all individual focal lesions identified in the analysis. The TLG of each focal lesion was calculated by multiplying the SUVmean and voxel number of that lesion. The total lymph node TLG of each patient was defined as the sum of the TLGs for all focal lesions in the analysis. The SUVmean and TLG of the spleen were identified by manually drawing regions of interest on each slice of the attenuation-corrected axial PET images (Fig. 1).
Maximum intensity projection images of representative mild and severe Kikuchi-Fujimoto disease patients with lesion segmentation. (a) A patient with severe Kikuchi-Fujimoto disease. Systemic increase in 18F-FDG uptake in the spleen and lymph nodes is seen. (b) A patient with mild Kikuchi-Fujimoto disease. Lesser FDG-avid lymph nodes are seen.
Statistical analysis
All our statistical analysis were only two variables (mild, severe). Descriptive statistics for continuous variables are presented as medians ± interquartile range (IQR), and categorical variables are shown as numbers (percentage). The Mann–Whitney U test was performed to analyze differences between the mild and severe groups, while the χ2 test or Fisher’s exact test was performed on categorical data using SPSS 25.0 (IBM, Armonk, NY, USA). To determine independent predictors of severity in the KFD group, we performed a multivariable analysis with a logistic regression model including risk factors associated with a p value of less than 0.05 in the univariate analysis. Differences were considered to be statistically significant at a 2-sided p value of less than 0.05.
Receiver operating characteristic (ROC) analysis was used to describe the relationship between FDG uptake and disease severity. MedCalc software (version 19.1, Ostend, Belgium) was used to analyze the data. Results of 18F-FDG PET/CT in the severe group were compared with those in the mild group to assess the diagnostic performance of 18F-FDG PET/CT in evaluating the degree of severity of KFD. The diagnostic performance was expressed in terms of sensitivity, specificity, Youden index, positive predictive value (PPV), and negative predictive value (NPV).
Results
Patient characteristics
The baseline characteristics of the 31 patients with KFD who underwent 18F-FDG PET/CT are divided into two groups—mild and severe KFD—and summarized in Table 1. The p values shown are the result from just two variables (mild, severe). All patients had been confirmed with KFD based on the pathological findings of a biopsy. The median age was 27.5 years (IQR, 28 years), and 13 patients were male (41.9%). The most commonly affected site of lymphadenopathy was the neck (n = 18; 58.1%), followed by the axilla (n = 7; 22.6%). In terms of systemic symptoms, almost every patient in our study presented with a fever (n = 30; 96.8%). Among the study patients, 8 (25.8%) patients were categorized into the mild and 23 (74.2%) into the severe KFD group. There were significant differences in age (38.0 ± 29 vs. 26.0 ± 18 years, p = 0.038) and lactate dehydrogenase (LDH) levels (310.5 ± 298 vs. 612.5 ± 672, p = 0.033). However, there were no significant differences in sex (57.1% vs. 37.5%, p = 0.354), sites of lymphadenopathy, or systemic symptoms between the mild and severe groups.
Comparison of PET/CT parameters according to the severity of KFD
We investigated the locations, metabolic activity, and size of hypermetabolic lymph nodes on 18F-FDG PET/CT images. The findings from the 18F-FDG PET/CT examinations are presented in Table 1. We identified hypermetabolic lymph nodes in 31 patients with SUVmax values from the neck, axilla, mediastinum, and the abdominopelvic area. Hypermetabolic lymph nodes were observed in the necks of 18 patients, axillae of 7, mediastina of 4, and abdomens and pelvis of two patients. The median values of SUVmax, MTV, and TLG of 18F-FDG uptake in affected lymph nodes were 10.65 ± 7.64, 97.95 ± 112.29, and 398.73 ± 464.08, respectively. The 18F-FDG uptake in the spleen (SUVmean, 2.19 ± 1.14; TLG, 539.36 ± 395.69) was calculated for all patients.
The median 18F-FDG PET/CT parameters involving the lymph nodes, liver, and spleen were identified in the mild and severe groups. The spleen SUVmean was higher in patients with severe KFD (1.79 ± 0.99 vs. 2.38 ± 1.18, p = 0.058). The median values of total lymph node SUVmax (8.19 ± 7.10 vs. 11.68 ± 7.33, p = 0.214), MTV (61.85 ± 118.32 vs. 99.36 ± 120.21, p = 0.104), and total lymph node TLG (201.57 ± 363.45 vs. 473.52 ± 504.44, p = 0.024) were higher in the severe group than in the mild group, indicating a higher 18F-FDG uptake in the severe group.
Diagnostic performance of 18F-FDG PET/CT in the prediction of KFD severity
Severity predictions were made by analyzing the area under the curve (AUC) of the ROC (Fig. 2), and the corresponding statistics are shown in Table 2. Using the definition of severe KFD as a diagnostic criterion to separate the severe group from the mild group, ROC curve analysis determined the most sensitive and specific cutoff values for total lymph node TLG, spleen SUVmean, total lymph node MTV, spleen TLG, and total lymph node SUVmax as 429.99, 1.79, 34.72, 296.06, and 9.27, respectively. With these cutoff values, total lymph node TLG (77.2%) and spleen SUVmean (72.8%) were found to be more accurate than the other parameters.
Receiver operating characteristic curve of metabolic parameters in Kikuchi-Fujimoto disease. The areas under the curve (AUC) for total lymph nodes, TLG, and spleen SUVmean are higher than that of total lymph nodes MTV. SUVmean, mean standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis.
Predictive factors for KFD severity
We evaluated the predictive factors for severe KFD. A univariate and multivariable analysis of severity in KFD was performed and is presented in Table 3 and Table S1. In the univariate logistic regression, myalgia (odds ratio [OR] 0.150; 95% confidence interval [CI] 0.024–0.946; p = 0.044), total lymph node SUVmax (cutoff 9.27) (OR 8.500; 95% CI 1.335–54.127; p = 0.023), total lymph node MTV (cutoff 34.72) (OR 6.667; 95% CI 1.057–42.065; p = 0.044), total lymph node TLG (cutoff 429.99) (OR 10.889; 95% CI 1.140–103.977; p = 0.038), spleen SUVmean (cutoff 1.79) (OR 11.111; 95% CI 1.701–72.564; p = 0.012), and spleen TLG (cutoff 296.06) (OR 10.500; 95% CI 1.412–78.059; p = 0.022) were statistically significant. The multivariate logistic regression model showed that myalgia (OR 0.035; 95% CI 0.001–0.792; p = 0.035), total lymph node SUVmax (cutoff 9.27) (OR 24.734; 95% CI 1.323–462.407; p = 0.032), and spleen SUVmean (cutoff 1.79) (OR 37.770; 95% CI 1.769–806.583; p = 0.020) were significantly associated with severe KFD.
Discussion
18F-FDG PET/CT can be used to investigate various inflammatory and infectious diseases and benign disorders29. Due to the advantages of 18F-FDG PET/CT in the systematic evaluation of fever of unknown origin30, 18F-FDG uptake has often been assessed in the diagnostic workup of KFD. Alshammari et al. reported that 18F-FDG uptake can be detected not only in the generalized lymph nodes but also in the spleen in patients with KFD24. Another study reported that the spleen showed increased 18F-FDG uptake in patients with febrile autoimmune disease and is associated with an increased risk of all-cause in-hospital mortality22.
In this study, myalgia was found to be correlated with mild KFD. This may be because patients with mild KFD often present with myalgia at the time of diagnosis. Furthermore, patients presenting with myalgia as a systemic symptom are usually evaluated for the disease earlier than those who do not present with myalgia. We investigated the values of 18F-FDG PET/CT in patients with severe KFD to determine whether they can be used as predictive factors for disease severity. Among the various 18F-FDG PET/CT parameters, total lymph node SUVmax and spleen SUVmean were significantly associated with severe KFD. 18F-FDG uptake was significantly higher not only in the affected lymph nodes but also in the spleen in severe KFD. In multivariate logistic regression analysis, total lymph node SUVmax with a cutoff value higher than 9.27 and spleen SUVmean with a cutoff value higher than 1.79 were independent predictors of KFD severity. Increased total lymph node SUVmax and spleen SUVmean might be useful for predicting the disease course when clinical or laboratory data are not available or are not confirmed. We have shown, using multiple multivariable models, that not only the intensity of inflammatory response in lymph nodes (SUVmax), but also the amount of activated lymph nodes (MTV, TLG) is correlated with KFD severity. Similarly, we have shown that spleen intensity (SUVmax) as well as splenic metabolic size (MTV) is also correlated with KFD severity.
The spleen is the largest lymphoid organ in the human body that regulates blood flow and filters microorganisms19. As a specialized immune organ, the spleen has various functions, such as clearance of microorganisms, the site of development for lymphocytes (both T and B), release of immunoglobulins, and production of immune mediators31. Generally, 18F-FDG uptake is related to tissue metabolism, which may explain why an increased 18F-FDG uptake in the spleen may reflect increased glucose consumption in the spleen in the event of an infection21. A recent study demonstrated that current inflammation could result in diffuse splenic 18F-FDG uptake32. Therefore, we presume that increased diffuse 18F-FDG uptake in the spleen can be noted in many inflammatory diseases reflecting the activation of the immune system in the spleen. This relationship between splenic glucose metabolism and inflammation may help explain our results.
There are several limitations of this study. First, this was a retrospective study. Second, our study population was small due to the low prevalence of KFD and the high cost of 18F-FDG PET/CT. Finally, we defined severe KFD arbitrarily. Since the severity criteria of KFD have not been previously defined, we defined severe KFD based on previous reports assessed factors associated with a severe clinical course and fetal complications of KFD3,10,26,27,28,33 and our clinical experience. Despite these limitations, our study is the first, to the best of our knowledge, to evaluate the potential association between 18F-FDG PET/CT parameters and KFD severity.
Our study suggests that 18F-FDG PET/CT can be a useful tool to assess disease severity in patients with KFD as a complement to laboratory and clinical findings. Further studies with larger populations are warranted to validate our results regarding the role of 18F-FDG PET/CT in determining KFD severity.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen, C. K., Low, Y., Akhilesh, M. & Jacobsen, A. S. Kikuchi disease in Asian children. J. Paediatr. Child Health 42, 104–107 (2006).
Seo, J. H. et al. A clinical study of histiocytic necrotizing lymphadenitis (Kikuchi’s disease) in children. Int. J. Pediatr. Otorhinolaryngol. 72, 1637–1642 (2008).
Lin, D. Y., Villegas, M. S., Tan, P. L., Wang, S. & Shek, L. P. Severe Kikuchi’s disease responsive to immune modulation. Singapore Med. J. 51, e18-21 (2010).
Dorfman, R. F. & Berry, G. J. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin. Diagn. Pathol. 5, 329–345 (1988).
Chuang, C. H. et al. Clinical and laboratory manifestations of Kikuchi’s disease in children and differences between patients with and without prolonged fever. Pediatr. Infect. Dis. J. 24, 551–554 (2005).
Chen, J. S., Chang, K. C., Cheng, C. N., Tsai, W. H. & Su, I. J. Childhood hemophagocytic syndrome associated with Kikuchi’s disease. Haematologica 85, 998–1000 (2000).
Kim, Y. M. et al. Hemophagocytic syndrome associated with Kikuchi’s disease. J. Korean Med. Sci. 18, 592–594 (2003).
Kim, T. Y. et al. Characteristics of Kikuchi-Fujimoto disease in children compared with adults. Eur. J. Pediatr. 173, 111–116 (2014).
Kucukardali, Y. et al. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin. Rheumatol. 26, 50–54 (2007).
Kang, H. M. et al. Clinical characteristics of severe histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto Disease) in children. J. Pediatr. 171, 208-212 e201 (2016).
Yoshioka, K., Miyashita, T., Nakamura, T., Inoue, T. & Yamagami, K. Treatment of histiocytic necrotizing lymphadenitis (Kikuchi’s disease) with prolonged fever by a single course of methylprednisolone pulse therapy without maintenance therapy: experience with 13 cases. Intern. Med. 49, 2267–2270 (2010).
Jang, Y. J., Park, K. H. & Seok, H. J. Management of Kikuchi’s disease using glucocorticoid. J. Laryngol. Otol. 114, 709–711 (2000).
Kouijzer, I. J. E. et al. The value of 18F-FDG PET/CT in diagnosis and during follow-up in 273 patients with chronic Q fever. J. Nucl. Med. 59, 127–133 (2018).
Jimenez-Ballve, A. et al. Assessment of the diagnostic accuracy of 18F-FDG PET/CT in prosthetic infective endocarditis and cardiac implantable electronic device infection: comparison of different interpretation criteria. Eur. J. Nucl. Med. Mol. Imaging 43, 2401–2412 (2016).
Sah, B. R. et al. Diagnostic performance of 18F-FDG-PET/CT in vascular graft infections. Eur. J. Vasc. Endovasc. Surg. 49, 455–464 (2015).
Toriihara, A. et al. FDG-PET/CT findings of chronic active Epstein–Barr virus infection. Leuk. Lymphoma 59, 1470–1473 (2018).
Leroy-Freschini, B. et al. 18F-FDG PET/CT for invasive fungal infection in immunocompromised patients. QJM 111, 613–622 (2018).
Hyun, S. H. et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann. Surg. Oncol. 17, 115–122 (2010).
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
Lustberg, M. B., Aras, O. & Meisenberg, B. R. FDG PET/CT findings in acute adult mononucleosis mimicking malignant lymphoma. Eur. J. Haematol. 81, 154–156 (2008).
Kim, K. et al. Diffuse increased splenic F-18 fluorodeoxyglucose uptake may be an indirect sign of acute pyogenic cause rather than tuberculous in patients with infectious spondylitis. Nucl. Med. Commun. 32, 1155–1161 (2011).
Ahn, S. S. et al. Evaluation of spleen glucose metabolism using 18F-FDG PET/CT in patients with febrile autoimmune disease. J. Nucl. Med. 58, 507–513 (2017).
Lefebvre, N. et al. Clinical usefulness of 18F-FDG PET/CT for initial staging and assessment of treatment efficacy in patients with lymph node tuberculosis. Nucl. Med. Biol. 50, 17–24 (2017).
Alshammari, A., Skoura, E., Kazem, N. & Ashkanani, R. Kikuchi disease with generalized lymph node, spleen and subcutaneous involvement detected by fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography. Mol. Imaging Radionucl. Therapy 25, 102–106 (2016).
Ito, K., Morooka, M. & Kubota, K. Kikuchi disease: 18F-FDG positron emission tomography/computed tomography of lymph node uptake. Jpn. J. Radiol. 28, 15–19 (2010).
Jasti, D. B., Naveen Prasad, S. V., Naveen, T. & Vengamma, B. Kikuchi-Fujimoto disease presenting as brainstem encephalitis with secondary blepharospasm. J. Neurosci. Rural Pract. 7, 157–160 (2016).
Longaretti, P., Savasta, S., Caimmi, D., Possenti, I. & Marseglia, G. L. Kikuchi-Fujimoto disease complicated by peripheral neuropathy. Pediatr. Neurol. 46, 319–321 (2012).
Duan, W., Xiao, Z. H., Yang, L. G. & Luo, H. Y. Kikuchi’s disease with hemophagocytic lymphohistiocytosis: a case report and literature review. Medicine (Baltimore) 99, e23500 (2020).
Zhuang, H., Yu, J. Q. & Alavi, A. Applications of fluorodeoxyglucose-PET imaging in the detection of infection and inflammation and other benign disorders. Radiol. Clin. North Am. 43, 121–134 (2005).
Meller, J. et al. Fever of unknown origin: prospective comparison of [18F]FDG imaging with a double-head coincidence camera and gallium-67 citrate SPET. Eur J Nucl Med 27, 1617–1625 (2000).
de Porto, A. P. et al. Assessment of splenic function. Eur. J. Clin. Microbial. Infectious Dis. 29, 1465–1473 (2010).
Nam, H. Y. et al. The clinical implication and prediction of diffuse splenic FDG uptake during cancer surveillance. Clin. Nucl. Med. 35, 759–763 (2010).
Sharma, V. & Rankin, R. Fatal Kikuchi-like lymphadenitis associated with connective tissue disease: a report of two cases and review of the literature. Springerplus 4, 167 (2015).
Acknowledgements
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2016-0108).
Author information
Authors and Affiliations
Contributions
All authors participated either to the following: (1) study conception and design or analysis and interpretation of the data, or both (H.S., Y.J., W.J., Jun Hyoung Kim, Jung Ho Kim, J.A., S.J., J.C., Y.P., J.Y., Y.S., N.K., A.C.); (2) the drafting of the manuscript or its critical revision for important intellectual content (H.S., Y.J., N.K., A.C.); or (3) final approval of the submitted manuscript (N.K., A.C.). The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seong, H., Jeong, Y.H., Lee, W.J. et al. Splenic uptake on FDG PET/CT correlates with Kikuchi-Fujimoto disease severity. Sci Rep 11, 10836 (2021). https://doi.org/10.1038/s41598-021-90350-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90350-z




