Abstract
Fibrosing interstitial lung disease (ILD) can cause high mortality and sensitive evaluation of fibrosing ILD could be critical. The aim of this study is to develop a scoring system to predict prognosis of fibrosing ILD. 339 patients with fibrosing ILD were enrolled as a derivation cohort. Cox multiple regression analysis indicated that smoking history (HR = 3.826, p = 0.001), age(HR = 1.043, p = 0.015), CEA(HR = 1.059, p = 0.049),CYFRA21-1(HR = 1.177, p = 0.004) and DLCO% predicted (HR = 0.979, p = 0.032) were independent prognostic factors for fibrosing ILD. The clinical scoring system for fibrosing ILD was established based on the clinical variables (age [A], CEA and CYFRA21-1 [C], DLCO% predicted [D], and smoking history [S]; ACDS). The area under the receiver operating characteristic curve (AUROC) of the scoring system for predicting prognosis of fibrosing ILD was 0.90 (95%CI: 0.87–0.94, p < 0.001). The cutoff value was 2.5 with their corresponding specificity (90.7%) and sensitivity (78.8%). To validate the value of ACDS score levels to predict the survival of patients with fibrosing ILD, 98 additional fibrosing ILD patients were included as a validation cohort. The log-rank test showed a significant difference in survival between the two groups(ACDS score < 2.5 and ACDS score ≥ 2.5) in validation cohort. The independent risk factors for mortality in patients with fibrosing ILD are higher CEA, higher CYFRA21-1, smoking history, lower DLCO%predicted at baseline and older age. ACDS is a simple and feasible clinical model for predicting survival of fibrosing ILD.
Similar content being viewed by others
Introduction
Interstitial lung diseases (ILDs) are a group of heterogeneous lung diseases with pulmonary alveolar unit inflammation or interstitial fibrosis that are associated with substantial morbidity and mortality. The causation of ILD includes idiopathic and specific etiology including autoimmune disease, vasculitis, drugs, tumors and occupational or environmental exposure1,2. Idiopathic pulmonary fibrosis (IPF) is one of a family of idiopathic interstitial pneumonias characterized by usual interstitial pneumonia (UIP) in high-resolution computed tomography (HRCT) and pathology3. IPF has a poor prognosis with median survival from the time of diagnosis approximately 3 years4. Fibrosing ILDs other than IPF, such as connective tissue disease (CTD) associated ILD, including ILD associated with rheumatoid arthritis (RA-ILD), systemic sclerosis (SSc-ILD) and polymyositis/dermatomyositis are also known to have progressive disease behaviors similar to IPF5,6,7. Therefore, it is important to recognize the risk factors associated with poor prognosis in patients with fibrosing interstitial lung disease.
Several biomakers has been reported to be as diagnostic and prognostic biomarkers of fibrosing ILD, including Krebs von den lugen-6 (KL-6), Surfactant proteins A and D (SP-A and SP-D), serum interleukin 6 (IL-6) levels and tumor markers8,9,10. However, the relationship between proportion of each serum marker and fibrosing ILD is not clear. Therefore, in this study, we retrospectively studied the clinical characteristics of patients with fibrosing ILD and established a novel model to better guide personalized therapeutic choices in persons.
Materials and methods
Study subjects
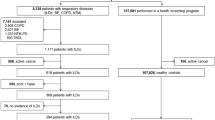
We retrospectively reviewed 647 patients who were diagnosed of fibrosing ILD (IPF and CTD-associated UIP) from inpatient of the department of respiration of Nanjing Drum Tower Hospital from February 2017 to February 2020. Overall, 308 patients were excluded based on exclusion criteria. A total of 339 patients were analyzed as a derivation cohort(Fig. 1A). To validate the value of clinical scoring system to predict the survival of patients with fibrosing ILD, a validation cohort was performed which consisted of 98 patients with fibrosing ILD who were admitted to the department of respiration of Nanjing Drum Tower Hospital between February 2020 and February 2021(Fig. 1B). Patients with incomplete data were excluded. Exclusion criteria for all fibrosing ILD subjects were: (1) subjects had combined pneumonia, lung malignancy, or other pulmonary diseases; (2) subjects lacked of pulmonary function test results; (3) subjects of validation cohort overlapped with derivation cohort. We analyzed demographic features, clinical characteristics, lung function parameters and therapy. Survival status was determined by reviewing the medical records or telephone follow-ups until February 2021.
This study was consented by Ethics Committee of Nanjing Drum Tower Hospital. The Ethics Committee waived the need for informed consent as the study was retrospective and the data were analyzed anonymously.
Methods
The diagnosis for IPF was mainly based on the criteria from An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline3. The diagnosis of CTD-ILD referred to the published guideline11. Clinical information at admission was collected including demographics, smoking history. Pulmonary function tests including forced vital capacity (FVC), FVC% predicted, diffusion capacity for carbon monoxide (DLCO), and DLCO% predicted were extracted for analysis. All subjects had UIP pattern on chest HRCT as defined by the guidelines from the American thoracic society and the European respiratory society3,11.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). t-Test or the Mann–Whitney U test was used for continuous variables. Categorical variables were compared by Chi-square test. The independent prognostic role of variables were evaluated by Cox proportional hazard analysis. Receiver operator characteristic (ROC) analyses were performed to calculate area under the ROC curve (AUC) of markers for predicting the prognosis of fibrosing ILD. The Kaplan–Meier method was used to assess survival curves with GraphPad Prism version 7 (Graph Pad Software Inc., La Jolla, CA, USA). The log-rank test was used to evaluate the statistical significance of differences between the higher ACDS score and lower ACDS score groups. Statistical analyses were performed using SPSS18.0 statistical software. Statistical significance was considered at the 0.05 levels.
Ethics approval and consent to participate
The study was conducted according to the Declaration of Helsinki. This study was approved by Ethics Committee of Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School.The Ethics Committee of Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School waived the need for informed consent as the study was retrospective and the data were analyzed anonymously.
Results
Baseline clinical characteristics of fibrosing ILD patients
The baseline clinical features of subjects with IPF (n = 132) and CTD-ILD characterized by UIP on HRCT (n = 207) were summarized in Table 1. Male gender, older age were more common in the IPF group (p < 0.001 and p < 0.001, respectively). Smoking history was similar. Red blood cell distribution width(RDW) levels, serum total bilirubin (TBIL) and direct bilirubin (DBIL) also differed between the two groups (p = 0.008, p < 0.001 and p = 0.001, respectively). Patients with CTD-ILD characterized by UIP on HRCT had a higher DLCO% predicted level compared with the IPF patients.
Constructing a scoring system for predicting prognosis of fibrosing ILD
According to the final follow-up data, 339 fibrosing ILD patients were divided into survivors group (n = 259) and decedents group (n = 80). As was shown in Table 2, there was no difference in the proportion of CTD-UIP and IPF among the survivors group and decedents group (p = 0.072). Cox proportional hazards models were used to examine the influence of variables on the prognosis of patients with fibrosing ILD. The multivariate cox regression analysis showed that smoking history (HR = 3.826, p = 0.001), age (HR = 1.043, p = 0.015), carcinoem-bryonic antigen (CEA) (HR = 1.059, p = 0.049), cytokeratin 21–1(CYFRA21-1) (HR = 1.177, p = 0.004) and DLCO%predicted (HR = 0.979, p = 0.032) were independent prognostic factors for fibrosing ILD (Table 3).
The accuracy of independent prognostic factors for predicting the survival of fibrosing ILD was then evaluated by Receiver Operating Characteristics (ROC) analysis. The area under the ROC curve for CYFRA21-1 in predicting the survival of fibrosing ILD was 0.85 (95% CI, 0.80–0.90; p < 0.001). The prediction ability for smoking history, age, CEA and DLCO%predicted were listed in Table 4. Then, we constructed a simple clinical scoring system for predicting survival of fibrosing ILD with the variables of smoking history, age, CEA, CYFRA21-1 and DLCO%predicted (Table 5).
Association of clinical scoring system with survival of patients with fibrosing ILD in the validation cohort
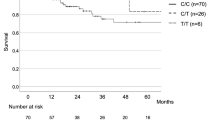
ROC curve was calculated to compare the predictive value of the scoring system in the derivation cohort. The ROC curve was shown in Fig. 2. The area under the curve of the scoring system for predicting survival of fibrosing ILD was 0.90 (95%CI: 0.87–0.94, P < 0.001). The cutoff value was 2.5 with their corresponding specificity (90.7%) and sensitivity (78.8%). In the validation cohort, the patients were divided into a higher ACDS score group (n = 42, ACDS score ≥ 2.5) and a lower ACDS score group (n = 56, ACDS score < 2.5) to analyze the survival using the Kaplan–Meier method (Fig. 3). The log-rank test showed a significant difference in survival between the two groups (p < 0.001).
Discussion
The present study retrospectively compared the clinical and follow-up data between 259 survivors and 80 decedents with fibrosing ILD in the derivation cohort. In this study, we demonstrated that smoking history, age, CEA, CYFRA21-1 and DLCO% predicted could predict the survival of fibrosing ILD patients independently. A new predictive scoring system namely ACDS (age [A], CEA and CYFRA21-1 [C], DLCO% predicted [D], and smoking history [S]) was proposed. Importantly, we found that scoring system level was closely associated with the prognosis of fibrosing ILD patients. Furthermore, we demonstrated that patients with relatively low ACDS score had significantly longer overall survival than patients with relatively high ACDS score in the validation cohort.
Fibrosing ILD had similar biological and clinical behaviours which was characterised by progressive deterioration in lung function, progressive deterioration in lung function and high mortality rate12,13. Investigating the prognostic value of markers across fibrosing ILD was of great importance to clinical evaluation and make continues to elucidate the approach to fibrosing ILD management. In the past few years, several serum markers were identified as simple and readily accessible biomarkers to predict the survival and severity of fibrosing ILD. There were researches studying tumor markers such as CEA , carbohydrate antigen 19–9 (CA 19–9) and CYFRA21-1 that might reflect the severity and prognosis of fibrosing ILD14,15,16. One retrospective study by Fahim A et al., which included 41 non-smoking patients with idiopathic pulmonary fibrosis(IPF), reported that serum CEA concentration was elevated in approximately half of patients with IPF and was correlated with disease severity17. These results were consistent with the finding of our study of CEA as a biomarker in fibrosing ILD patients. In our study, CEA was identified as an independent prognostic factor for fibrosing ILD. CEA is a glycoprotein involved in cell adhesion and is produced by colonic epithelium. It has reported that CEA localizes in metaplastic epithelium lining honeycombed bronchioles by immunohistochemical staining. As cuboidal pneumocytes are the predominant source of epithelial renewal in severe lung damage and fibrosis, these cells are the most likely source of CEA release18.
In this study, elevated serum levels of CYFRA21-1 were observed in decedents group with fibrosing ILD. In a study by Vercauteren et al., higher level of CYFRA 21–1 in BAL of IPF patients resulted in worse survival in comparison with the CYFRA 21–1 low counterpart19. The expression of CYFRA21-1 in the lung has been identified in bronchiolar epithelial cells and pneumocytes. Elevation of serum CYFRA21-1 concentration might be associated with lysis or regeneration of these cells15. Furthermore, we demonstrated that serum CEA and CYFRA21-1 were significantly correlated with decreased DLCO%predicted in this study. The severity of ILD is usually based on pulmonary function test results such as DLCO%predicted20. Thus, serum CEA and CYFRA21-1 levels might be useful for reflecting the severity of fibrosing ILD.
A large amount of studies reported that smoking was closely associated with the onset and progress of pulmonary fibrosis21,22. A possible explanation may be that cigarettes contain the cytotoxic, mutagenic and proinflammatory substances. According to previous reports, these substances caused cellular oxidative stress, increased epithelial cell apoptosis, and dysregulation of immune responses, which was responsible for the progress of pulmonary fibrosis23,24. In addition, smoking affects the function of macrophages. It induced macrophage polarization to M2 phenotype that enhance the regression of inflammation and tissue remodeling25. Therefore, smoking cessation could be a good way to slow down the development of pulmonary fibrosis in the patients with ILD.
In the past years, few models has been proposed to predict the severity and prognosis of IPF. Glasgow prognostic score (GPS) has been reported to play an important role in predicting mortality in patients with acute exacerbation of IPF26. In our study, smoking history, age, CEA, CYFRA21-1 and DLCO% predicted were identified as independent factors for predicting the prognosis of fibrosing ILD. Moreover, based on these variables, a new predictive scoring system namely ACDS (age [A], CEA and CYFRA21-1 [C], DLCO% predicted [D], and smoking history [S]) was proposed. The scoring system was demonstrated to be as a predictive value for the survival of fibrosing ILD. However, it still needs further perspective study to verify the power of this scoring system based on multicenter and large population of fibrosing ILD patients.
Some limitations of this study should be noted. First, this was a retrospective and observational study of data obtained from a single center. In addition, the mechanism underlying the association of each biomarker with fibrosing ILD remains to be clarified in further in vivo and in vitro studies.
Conclusions
In conclusion, smoking history, age, CEA, CYFRA21-1 and DLCO% predicted were independent predictors of the prognosis of fibrosing ILD patients that offers the advantages of convenience, ease of accessibility and low cost. A new predictive scoring system namely ACDS may help predict prognosis in patients with fibrosing ILD.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Antoniou, K. M. et al. Interstitial lung disease. Eur. Respir. Rev. 23, 40–54 (2014).
Ito, Y. et al. Serological and morphological prognostic factors in patients with interstitial pneumonia with autoimmune features. BMC Pulm. Med. 17, 111 (2017).
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, 44–68 (2018).
Nicholson, A. C. et al. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 162, 2213–2217 (2000).
Zamora-Legoff, J. A. et al. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthrit. Rheumatol. 69, 542–549 (2017).
Guler, S. A. et al. Does systemic sclerosis-associated interstitial lung disease burn out? Specific phenotypes of disease progression. Ann. Am. Thorac. Soc. 15, 1427–1433 (2018).
Marie, I. et al. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthrit. Rheum. 63, 439–3447 (2011).
Hamai, K. et al. Comparative study of circulating MMP-7, CCL18, KL-6, SP-A, and SP-D as disease markers of idiopathic pulmonary fibrosis. Dis. Markers 2016, 4759040 (2016).
De Lauretis, A. et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 40, 435–446 (2013).
Maher, T. M. et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: An analysis from the multicentre PROFILE cohort study. Lancet Respir Med 5, 946–955 (2017).
Travis, W. D. et al. An official American thoracic society/European respiratory society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188, 733–748 (2013).
Flaherty, K. R. et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir. Res. 4, e000212 (2017).
Wells, A. U. et al. What’s in a name? That which we call IPF, by any other name would act the same. Eur. Respir. J. 51, 1800692 (2018).
Fujita, J. et al. Marked elevation of CA19-9 in a patient with idiopathic pulmonary fibrosis: CA19-9 as a bad prognostic factor. Respirology 3, 211–214 (1998).
Kanazawa, H. et al. CYFRA 21-1, a cytokeratin subunit 19 fragment, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Clin. Sci. 94, 531–535 (1998).
Bergamaschi, S. et al. Tumor markers are elevated in patients with rheumatoid arthritis and do not indicate presence of cancer. Int. J. Rheum. Dis. 15, 179–182 (2012).
Fahim, A. et al. Serum carcinoembryonic antigen correlates with severity of idiopathic pulmonary fibrosis. Respirology 17, 1247–1252 (2012).
Kawanami, O., Ferrans, V. J. & Crystal, R. G. Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest. 46, 39–53 (1982).
Vercauteren, I. M. et al. CYFRA 211 in bronchoalveolar lavage of idiopathic pulmonary fibrosis patients. Exp. Lung Res. 41, 459–465 (2015).
Tashkin, D. P. et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann. Rheum. Dis. 75, 374–381 (2016).
Marten, K. et al. Nonspecific interstitial pneumonia in cigarette smokers: A CT study. Eur. Radiol. 19, 1679–1685 (2009).
Schwartz, D. A. et al. The influence of cigarette smoking on lung function in patients with idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 144, 504–506 (1991).
Rahman, I., Biswas, S. K. & Kode, A. Oxidant and antioxidant balance in the airways and airway diseases. Eur. J. Pharmacol. 533, 222–239 (2006).
Van der Vaart, H., Postma, D. S., Timens, W. & ten Hacken, N. H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 59, 713–721 (2004).
Hodge, S. et al. Smoking alters alveolar macrophage recognition and phagocytic ability: Implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 37, 748–755 (2007).
Kang, H. S., Cho, K. W., Kwon, S. S. & Kim, Y. H. Prognostic significance of Glasgow prognostic score in patients with acute exacerbation of idiopathic pulmonary fibrosis. Respirology 23, 206–212 (2018).
Funding
This study was supported by the National Natural Science Foundation of China (Grant NO. 82000071 to Dr.Liu).
Author information
Authors and Affiliations
Contributions
S.-Y.S., L.-L.C., X.-Q.L., C.W., M.Y and Y.-L.X. were involved in conception and design. S.-Y.S., L.-L.C., X.-Q.L. and M.Y. were involved in analysis and interpretation. S.-Y.S., and L.-L.C. were involved in acquisition of data. S.-.Y.S., X.-.Q.L., M.Y. and Y.-L.X. were involved in writing and revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, S., Chen, L., Liu, X. et al. Development of a scoring system with multidimensional markers for fibrosing interstitial lung disease. Sci Rep 12, 14217 (2022). https://doi.org/10.1038/s41598-022-16382-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16382-1