Abstract
The long-term application of chemical fertilizers has caused to the farmland soil compaction, water pollution, and reduced the quality of vegetable to some extent. So, its become a trend in agriculture to find new bio-fertilizers. Chlorella extract is rich in amino acids, peptides, nucleic acids, growth hormones, potassium, calcium, magnesium, iron, zinc ions, vitamin E, B1, B2, C, B6, folic acid, free biotin and chlorophyll. Chlorella extract can promote biological growth, mainly by stimulating the speed of cell division, thereby accelerating the proliferation rate of cells and playing a role in promoting plant growth. Whether Chlorella extract can be used to improve the growth of pepper (Capsicum annuum), needs to be verified. In current study, a pepper variety 'Chao Tian Jiao' was used as experiment material, by determining the changes of the related characteristics after spraying the seedlings with Chlorella extract, and its effect on growth of Capsicum annuum plants was investigated. The results showed that the Chlorella extract significantly increased plant height of pepper seedlings (treatment: 32.2 ± 0.3 cm; control: 24.2 ± 0.2 cm), stem diameter (treatment: 0.57 ± 0.02 cm; control: 0.41 ± 0.03 cm) and leaf area (treatment: 189.6 ± 3.2 cm2; control: 145.8 ± 2.5 cm2). Particularly, the pepper seedlings treated with Chlorella extract, developed the root system in better way, significantly increased the chlorophyll a, and the activities of SOD, POD and CAT enzymes were also improved significantly. Based on our results, we can speculate that it is possible to improve the growth of Capsicum annuum seedlings and reduce the application of chemical fertilizers in pepper production by using Chlorella extract.
Similar content being viewed by others
Introduction
Pepper is a one-year or limited perennial herb, an important agricultural crop due to the nutritional value of its fruits, and an excellent source of a wide array of phytochemicals with well-known antioxidant properties (carotenoids, capsaicinoids, phenolic compounds, particularly flavonoids, quercetin, and luteolin), which is known as the king of vitamin C in vegetables1,2. The pepper is very popular among those who enjoy spicy foods, and its cultivated area in China is growing year after year3.
In recent decades, the use of chemical fertilizers has increased crop yield, however, the long-term application of chemical fertilizers has caused the soil aggregate structure destroyed, causing soil compaction and soil beneficial bacteria death, and the nitrate content of the vegetables exceeded the standard4,5. Therefore, reducing chemical fertilizer and increasing crop productivity have become the main research direction of agricultural production in China.
One approach to increasing crop productivity is the development of environment-friendly bio-fertilizers. To increase the content of nutritional constituents in plants, many approaches have been studied such as genetic selection, which has included allele selection, gene and genome duplication, and new genotypes creation. However, despite the advantages that these techniques offer, some of them may also pose potential problems for food safety and require special attention in order to ensure consumer health protection6,7. On this account, the use of bio-fertilizers in agricultural practices is proposed as a safe tool to enhance the nutritional properties of food crops. Bio-fertilizers are recognized as environment-friendly compounds with beneficial effects on plants8,9. In particular, they decrease the use of chemical fertilizers by increasing the amount of micro- and macro-nutrients taken up by plants, positively influencing root morphology and plant growth10,11. They also display hormone-like activity and influence plant metabolism by interacting with biochemical processes and physiological mechanisms12,13,14.
Recent studies suggest that active molecules contained in bio-fertilizers can promote nitrogen assimilation8,9,10,11,12. Furthermore, the induction of the metabolic pathway associated with the synthesis of phenylpropanoids in plants treated with bio-fertilizers may explain why these products can help plants to overcome stress situations15,16. In recent studies, the application of products with bio-fertilizers action to pepper plants was found to exert positive effects on plant growth14,17,18. Therefore, searching for bio-fertilizers has recently become a trend in agricultural production.
Chlorella is a single-cell green algae organism, that contains a large amount of various functional compounds such as crude protein 50–60%, carbohydrate 15–20%, crude lipid 12–18%, growth hormones, potassium, calcium, magnesium, iron, zinc, vitamin E, B1, B2, C, B6, folic acid, free biotin and chlorophyll19,20. Chlorella has high photosynthetic efficiency, which is the only plant on the Earth that can grow four times in 20 h, and it is known as the ‘canned sun’. Now, Chlorella extract has been used as bio-fertilizer in many crops, like Chinese chives, spinach19, lettuce21, wheat22 and Hibiscus esculentus23, which can increase the biomass of the above crops.
At present, the application of Chlorella extract in pepper plants has not been seen. To examine whether Chlorella extract can stimulate the growth of pepper seedlings as a bio-fertilizer, further research is needed. The purpose of this study is to investigate the effects of Chlorella extract as bio-fertilizer on the growth of pepper seedlings.
Results
Effect of Chlorella extract on plant height of pepper seedlings
The leaf surface of the pepper was sprayed with Chlorella extract, and the growth status of plants were analyzed at 0, 7th, 14th and 21st day post treatment. After 21 days of Chlorella extract treatment, the plant height of the Chlorella extract-treated plants were significantly higher than that of the control pepper plants (Fig. 1).
Effects of Chlorella extracts on the plant height of pepper. Plant height was measured on the 0 and 21th day after Chlorella extract treatment. Control: no Chlorella treatment; Treatment: plants were treated by Chlorella extracts; SAS analysis at 5% is shown in lowercase letters to indicate significant differences. Vertical bars in the figures represent ± SD of five independent biological replicates.
Effects of Chlorella extract on the stem diameter, leaf area and fruit growth of pepper
After 21 days of treatment with Chlorella extract, the stem diameter, leaf area and fruits growth of the control and treated plants were measured. The stem diameter of the treated plants were slightly thicker than the control (treatment: 0.57 ± 0.02 cm; control: 0.41 ± 0.03 cm), while leaf area (treatment: 189.6 ± 3.2 cm2; control: 145.8 ± 2.5 cm2) and fruits weight (treatment: 5.12 ± 0.02 g; control: 3.74 ± 0.05 g) of the treated plants were significantly increased as compared to the control plants (Fig. 2). Chlorella extract post-treatment, not only the leaf area of the pepper plants increased (Fig. 2b and c), but number of leaves of the pepper plant also increased significantly (Fig. 3). On 14th and 21st day post Chlorella extract treatment, the number of leaves plant-1 was much higher than the control group (Fig. 3).
Effects of Chlorella extracts on the stem diameter, leaf area and fruit growth of pepper. The stem diameter, leaf area and fruit weight were measured on the 21th day after Chlorella extracts treatment. Control: no Chlorella extracts treatment; Treatment: plants were treated by Chlorella extracts; “C” means “Control group”, and “T” means “Treatment group”. SAS analysis at 5% is shown in lowercase letters to indicate significant differences. Vertical bars in the figures represent ± SD of five independent biological replicates.
Effects of Chlorella extracts on number of leaves plant-1. The number of leaves plant-1 (Control group and Treatment group) were counted from the 0 to 21th day after Chlorella extracts treatment. Control: no Chlorella extracts treatment; Treatment: plants were treated by Chlorella extracts; SAS analysis at 5% is shown in lowercase letters to indicate significant differences. Vertical bars in the figures represent ± SD of five independent biological replicates.
Effect of Chlorella extract on the chlorophyll content of pepper plants
Data regarding Chlorella extract effect on the chlorophyll content of pepper plants leaves is shown in Fig. 4. After 21 days of Chlorella extract treatment, the SPAD value of chlorophyll a and chlorophyll b were measured in the treated and the control group. It was noted that chlorophyll a was increased significantly in the Chlorella extract treated plants, while chlorophyll b did not; indicating that Chlorella extract has a greater impact on chlorophyll a (Fig. 4).
Effect of Chlorella extracts on the Chlorophyll content of pepper plants. Chlorophyll a and chlorophyll b were measured on 21st day after Chlorella extracts treatment. Control: no Chlorella extracts treatment; Treatment: plants were treated with Chlorella extracts; SAS analysis at 5% is shown in lowercase letters to indicate significant differences. Vertical bars in the figures represent were presented as mean ± SD, all experiments were carried out in triplicate.
Effect of Chlorella extract on root growth of pepper
In order to investigate the effect of Chlorella extract on root growth characteristics of pepper plants, the plants were pulled out after 21 days of spraying with Chlorella extract, the soil was washed away, and the root growth of the treated and the untreated control plants was compared. The results showed that there was a significant difference in the root growth of the treated and control plants (Fig. 5). Specifically, the fibrous roots of treated plants were larger, denser and the root length as well as dry weight were significantly increased as compared to the untreated control plants (Fig. 5).
Effect of Chlorella extracts on the growth of pepper roots. Shoot length and shoot dry weight of the pepper plants (Control group and Treatment group) were measured on 21th day after Chlorella extracts treatment. Control: no Chlorella extracts treatment; Treatment: plants were treated by Chlorella extracts; SAS analysis at 5% is shown in lowercase letters to indicate significant differences. Vertical bars in the figures represent ± SD of five independent biological replicates.
Changes of activity of SOD, POD and CAT enzymes
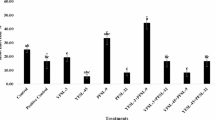
Data reported in Fig. 6. show that Chlorella extract significantly increased the activity of SOD (control 8.5 ± 0.2 U.mg-1.pr; treatment 14.6 ± 0.4 U.mg-1.pr), POD (control 9.0 ± 0.4 U.mg-1.pr; treatment 14.1 ± 0.5 U.mg-1.pr), and CAT (control 16.0 ± 0.4 U.mg-1.pr; treatment 22.0 ± 0.5 U.mg-1.pr). By and large, compared to the control, the activity of SOD, POD and CAT enzymes in the leaves of Chlorella extract treated peppers were significantly higher (Fig. 6).
Changes in related enzyme activities. Activities of the SOD, POD and CAT enzymes in pepper leaves (Control group and Treatment group) were measured on 21st day after Chlorella extracts treatment. Control: no Chlorella extracts treatment; Treatment: plants were treated by Chlorella extracts; SAS analysis at 5% is shown in lowercase letters to indicate significant differences. Vertical bars in the figures represent ± SD of five independent biological replicates.
Discussion
Chlorella extract has been used as bio-fertilizer in many crops, like Chinese chives, spinach19, lettuce19, wheat22 and Hibiscus esculentus23, which can increase the biomass of the above crops. The average height of Chinese chives treated with the Chlorella was 3.7 cm smaller than that of the untreated. The leaf width and fresh weight of Chinese chives treated with the chlorella was 0.5 mm wider and 30.3 g heavier than that of the untreated. The commercialization and yield of Chinese chives treated with the chlorella was 11.9% and 18.3%, respectively higher than that of the untreated. The thickness and number of spinach leaves treated with chlorella was 27.9% and 41.8%, respectively higher than that of the untreated. The fresh weight and yield of the spinach treated with the chlorella was 63.6% and 31.5%, respectively higher than that of the untreated19. The combined seed and soil inoculation of Chlorella vulgaris speed up growing of the Hibiscus esculentus, increase pod yield23. A recent study on wheat discussed about the importance of Chlorella extract in stimulating plant growth26. It was found that as compared to control, the Chlorella extracts increased the wheat plant height by 30%, and total dry matter biomass of the above- and below-ground parts were increased by 22% and 51%, respectively26. Sugar beet (Beta vulgaris subsp. vulgaris) is a commercially important biennial root crop, providing about 20% of the world’s annual sugar production. Chlorella extracts had a positive effect on sugar beet germination by increasing efficiency and regularity of this critical process for B. vulgaris seeds. Further studies have shown that Chlorella extracts contain cytokinin24,27, that could be the possible reason for increase in photosynthetic biomass28. The effect of the application of Chlorella extracts, foliar spray and root drenching, was evaluated in lettuce seedlings by monitoring their morpho-biometric parameters and chlorophyll, carotenoid, and total protein contents. The results show that Chlorella extract positively affected the growth of lettuce seedlings, increasing the dry matter, chlorophyll, carotenoid, and protein contents in the edible portion of the plant22.
In our research, we found that Chlorella extract positively affected the morphological characteristics (such as plant height, stem diameter, total number of leaves) and the chlorophyll content in pepper leaves; as well as significantly improved the root development. Secondly, by spraying Chlorella extract, the pepper seedlings could keep a well-developed root system (Fig. 5) and the related antioxidant enzymes activity were also improved (Fig. 6).
Some researchers found that the influence of Chlorella extract on cell metabolisms was discovered to be mostly due to the physiological action of major and minor nutrients, amino acids, vitamins, and plant growth regulators on cellular metabolism in treated plants, resulting in increased growth and crop yield24,25.
The photosynthesis of Chlorella is very strong, which is dozens of times that of other plants. The main reason why Chlorella can grow rapidly is that it contains Chlorella Growth Factor (CGF), which is quite rich in nucleoprotein, Nucleic acid, ribonucleic acid (RNA), deoxyribonucleic acid (DNA), various vitamins, amino acids, polysaccharides, complex protein bodies, enzymes, glycoproteins, various plant hormones, etc., it may be the reason for its ability to promote plant growth.
Conclusion
Chlorella extract significantly increased plant height of pepper seedlings, stem diameter and leaf area. Particularly, pepper seedlings treated with Chlorella extracts, its root system was developed in better way, chlorophyll a increased significantly, and the activities of SOD, POD and CAT enzymes were also improved significantly. Based on the above results that Chlorella extracts contributes to the growth and development of pepper plants, we can conclude that with Chlorella extracts treatment it is possible to reduce the application of chemical fertilizers in pepper production.
Methods
Materials
Plant materials Capsicum annuum conoides ‘Chao Tian Jiao’ and Chlorella vulgaris strain were used in the experiment, and the cultural operations were performed according to the relevant guidelines29.
The plant material Capsicum annuum conoides 'Chao Tian Jiao' was provided by the innovation team of pepper core germplasm resources creation and molecular breeding of Henan province while the Chlorella strain was provided by the Zhumadian International Joint Laboratory of Pepper Modern Breeding.
Chlorella cultivation
The Chlorella vulgaris was separately cultivated in Bold Basal culture medium (BBM) according to the method of Kim et al.30 Briefly, the strain of Chlorella vulgaris was placed in a control room at constant temperature (30 °C) for 6 days in a shaking incubator at the speed of 180 rpm, while the pH of the culture medium was 6.0. After seven days, the Chlorella was harvested when its dry biomass concentration was about 180 g/L in 7.5 L bench scale fermenter29.
Preparation of Chlorella extracts
500 g of fresh Chlorella was separately immersed (w/v; 1:10) in 5000 mL ethanol for 72 h, and stirred from time to time. The process was repeated until the extracted solvent became colorless. The Chlorella extracts were filtered separately through a medium flow filter paper (Whatman 40,8 μm). The extract was concentrated in vacuo using a rotary evaporator at 50 °C while lyophilizing the extract. The condensed ethanol extract was dried at ambient temperature before being placed in an airtight amber vial and kept at 4 °C in a desiccator. While using the extract, it was first solubilized with dimethyl sulfoxide (DMSO) and then Chlorella extracts were diluted with distilled water to a concentration of 0.4 mg/L30. The leaves of pepper seedlings were sprayed with extracts solution, whereas the untreated control plants were sprayed with distilled water. Each treatment consists of five plants, and each plant was sprayed with 100 mL solution of Chlorella extracts. The nozzle was about 10 cm away from the pepper leaves during spraying.
Pepper seedlings cultivation
The experiments were carried out in greenhouse. Seeds were sown in a cold frame, and seedlings were transplanted to the pots (Pot specifications: 20 cm × 14 cm, Color: White) when it had developed 8–10 true leaves, the growth medium of these pots: sterile mixture of peat: sand: perlite (1:1:1); Hoagland solution was used to provide nutrients for potted pepper growth. There were 10 replicates for each treatment, one per pot, and the pots were arranged randomly in the greenhouse. Two weeks later, the seedlings of treatment groups were sprayed with the Chlorella extracts at 18:00 on the same day, whereas the mock control plants were sprayed with distilled water instead of Chlorella extracts19. Plants were sprayed with Chlorella extracts, the amount of Chlorella extracts were 100 mL per time, once per 10 min, and three times in total.
Determining the relevant indicators of plant morphology
The height of the pepper plants were measured with a measuring tape, the stem diameter was measured using vernier calipers, and the leaf area was measured by a number grid method. The shape of the leaf is drawn on a transparent coordinate sheet, and then the grids are counted. When calculating the grid, the blade edge is calculated as 1 if it exceeds half a grid; and the blade edge will be not counted if it is less than half grid. The leaf area is calculated based on the number of grids counted. All above-mentioned indexes were measured three times, and the results are expressed as mean ± standard deviation31.
Sample collection
Pepper leaves from the control and Chlorella extracts treated plants were collected on 0th day and 21st day separately, placed in ice boxes, and immediately taken to the laboratory for assay.
Determination of chlorophyll content
SPAD value of chlorophyll a and chlorophyll b were measured by a chlorophyll analyzer (SPAD502 Plus Chlorophyll Meter, Spectrum Technologies, Inc.); each sample was measured three times and their average value was calculated32.
Determination of enzyme activities
Superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) activities of the control and treated plants leaves were measured by nitrogen blue tetrazolium (NBT) method, spectrophotometry and ultraviolet absorption33. All the specific activities of the enzyme fractions were calculated based on the amount of protein in the fraction34.
Data processing
SAS 6.12 software (SAS Institute, Gary, North Carolina) was used for data analysis. All measured values were presented as mean ± standard deviation of the means. Duncan’s multiple-range test was chosen, and least significant ranges (LSR) analysis at 5% significantly differences were shown; and figures were drawn using Sigma Plot 10.0.
References
Deli, J., Matus, Z. & Tóth, G. Carotenoid composition in the fruits of asparagus officinalis. J. Agric. Food Chem. 48, 2793–2796 (2000).
Howard, L. R., Talcott, S. T., Brenes, C. H. & Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 48, 1713–1720 (2000).
Odgerel, B. & Tserendulam, D. Effect of Chlorella as a biofertilizer on germination of wheat and barley grains. P. Mongolian Acad. Sci. 56(4), 26–31 (2016).
Sun, R. B., Guo, X. S., Wang, D. Z. & Chua, H. Y. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 95(6), 171–178 (2015).
Yu, Y. Q., Luo, Z. B., Fu, H. & Jin, Y. Effect of balanced nutrient fertilizer: A case study in Pinggu District, Beijing China. Sci. Total Environ. 754, 1–8 (2021).
Ahmad, P. et al. Role of transgenic plants in agriculture and biopharming. Biotech. Adv. 30, 524–540 (2012).
Sherlock, R. & Morrey, J. D. Ethical Issues in Biotechnology (Rowman and Littlefield. Publishers, Inc., 2002).
Schiavon, M., Ertani, A. & Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of TCA cycle and N metabolism in Zea mays L. J. Agric. Food Chem. 56, 11800–11808 (2008).
Muscolo, A., Sidari, M. & Nardi, S. Humic substance: relationship between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 129, 57–63 (2013).
Nardi, S., Carletti, P., Pizzeghello, D., Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems. PART I. Fundamentals and Impact of Mineral-Organic-Biota Interactions on the Formation, Transformation, Turnover, and Storage of Natural Nonliving Organic Matter (NOM). (Ed. Senesi, N., Xing, B., Huang, P.M.) 301–335 (John Wiley and Sons, Hoboken, 2009).
Ertani, A., Nardi, S. & Altissimo, A. Review: long-term research activity on the biostimulant properties of natural origin compounds. Acta Hort. 1009, 181–188 (2013).
Ertani, A. et al. Biostimulant activity of two protein hydrolysates on the growth and nitrogen metabolism in maize seedlings. J. Plant Nutr. Soil Sci. 172, 237–244 (2009).
Vaccaro, S. et al. Effect of a compost and its water-soluble fractions on key enzymes of nitrogen metabolism in maize seedlings. J. Agric. Food Chem. 57, 11267–11276 (2009).
Azcona, I. et al. Growth and development of pepper are affected by humic substances derived from composted sludge. J. Plant Nutr. Soil Sci. 174, 916–924 (2011).
Schiavon, M. et al. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 36, 662–669 (2010).
Ertani, A., Schiavon, M., Muscolo, A. & Nardi, S. Alfalfa plant-derived biostimulant stimulates short-term growth of salt stressed Zea mays L. plants. Plant Soil 364, 145–158 (2013).
Pascual, I., Azcona, I., Morales, F., Aguirreolea, J. & Sanchez-Diaz, M. Growth, yield and physiology of verticillium-inoculated pepper plants treated with ATAD and composted sewage sludge. Plant Soil 319, 291–306 (2009).
Pascual, I. et al. Growth, yield and fruit quality of pepper plants amended with two sanitized sewage sludges. J. Agric. Food Chem. 58, 6951–6959 (2010).
Kim, M. J., Shim, C. K., Kim, Y. K., Ko, B. G. & Kim, B. H. Effect of Biostimulator Chlorella fusca on improving growth and qualities of Chinese Chives and Spinach in organic farm. Plant Pathol. J. 34(6), 567–574 (2018).
Pulz, O. & Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biot. 65, 635–648 (2004).
Kim, S. J., Ko, E. J., Hong, J. K. & Jeun, Y. C. Ultrastructures of Colletotrichum orbiculare in cucumber leaves expressing systemic acquired resistance mediated by Chlorella fusca. Plant Pathol. J. 34(2), 113–120 (2018).
Faheed, F. A. & Abd-El Fattah, Z. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of Lettuce Plant. J. Agri. Soc. Sci. 4, 165–169 (2008).
Agwa, O. K., Ogugbue, C. J. & Williams, E. E. Field evidence of Chlorella vulgaris potentials as a biofertilizer for Hibiscus esculentus. Int. J. Agric. Res. 12(4), 181–189 (2017).
Ördög, V. et al. Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J. Appl. Physicol. 16, 309–401 (2004).
Stirk, W. A., Novák, O., Strnad, M. & van Staden, J. Cytokinins in macroalgae. Plant Growth Regul. 41, 13–24 (2003).
Kholssi, R., Marks, E. A. N., Montero, J. M. O., Debdoubi, A. & Rad, C. Biofertilizing effect of Chlorella sorokiniana suspensions on wheat growth. J. Plant Growth Regul. 38, 644–649 (2019).
Stirk, W. A., Ördög, V., Van Staden, J. & Jäger, K. Cytokinin-and auxin-like activity in Cyanophyta and microalgae. J. Appl. Phycol. 14, 215–221 (2002).
Park, E. R., Jo, J. O., Kim, S. M., Lee, M. Y. & Kim, K. S. Volatile flavor component of leek (Allium tuberosum Rotter). J. Korean Soc. Food Sci. Nutr. 27, 563–567 (1998) ((in Korean)).
Jin, H. et al. Ultrahigh-cell-density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production. Biotechnol. Bioeng. 117, 96–108 (2020).
Kim, M. J., Shim, C. K., Kim, Y. K., Hong, S. J. & Kim, S. C. Isolation and morphological identification of fresh water green algae from organic farming habitats in Korea. Korean J. Org. Agric. 22, 743–760 (2014).
Li, L., Tian, S. L., Jiang, J. & Wang, Y. Regulation of nitric oxide to Capsicum under lower light intensities. S. Afr. J. Bot. 132, 268–276 (2020).
Cho, Y. Y., Oh, S. B., Oh, M. M. & Son, J. E. Estimation of individual leaf area, fresh weight, and dry weight of hydroponically grown cucumbers (Cucumis sativus L.) using leaf length, width, and SPAD value. Sci. Hortic-Amst. 111, 330–334 (2007).
Oster, U., Tanaka, R., Tanaka, A. & Rudiger, W. Cloning and functional expression of gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J. 21(3), 305–310 (2000).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254 (1976).
Acknowledgements
The work was supported by Henan Province Natural Science Foundation (202300410282); The Innovation Scientists and Technicians Troop Construction Projects of Henan Province (C20150054); The Zhumadian International Joint Laboratory of Pepper Modern Breeding.
Author information
Authors and Affiliations
Contributions
S.L.T. and L.L.: conceived and designed the study. L.S. and J.H.L.: performed antioxidant enzyme activity analyses. W.N.Z.: Chlorella and pepper seedlings cultivation. L.S.: performed chlorophyll content analysis. L.L., K.A. and S.L.T.: wrote, edited and revised the final manuscript. K.A. spent a lot of time to polish the language of the manuscript, and he put forward to a good suggestion for modification. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, SL., Khan, A., Zheng, WN. et al. Effects of Chlorella extracts on growth of Capsicum annuum L. seedlings. Sci Rep 12, 15455 (2022). https://doi.org/10.1038/s41598-022-19846-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19846-6
This article is cited by
-
Impact of organic liquid fertilizer on plant growth of Chinese cabbage and soil bacterial communities
Scientific Reports (2025)
-
The screening, identification, and biocontrol potential of antagonistic endophytes against ginseng rust rot disease
Journal of Plant Diseases and Protection (2025)
-
Enterobacter hormaechei Wu15-loaded biochar enhances the ice plant growth by improving saline soil quality
Plant and Soil (2025)
-
Methyl jasmonate and selenium synergistically mitigative cadmium toxicity in hot pepper (Capsicum annuum L.) plants by improving antioxidase activities and reducing Cd accumulation
Environmental Science and Pollution Research (2023)