Abstract
Eucommia ulmoides staminate flowers (EUF), a newly approved functional food in China, have great potential for hormonal regulation. Herein, we aim to demonstrate the chemical composition and pharmacological activity of EUF in testosterone production and hormonal regulation. EUF extract and its components, kaempferol and geniposidic acid, exhibited a strong stimulating effect by increasing testosterone secretion, reducing ROS production, or promoting viability in Leydig cells. Meanwhile, the increased testosterone production was related to the upregulation of mRNA and protein expression of the steroidogenic pathway, such as steroidogenic acute-regulatory protein (StAR), 3β -hydroxysteroid dehydrogenase type 1 (HSD3B1), 17α-hydroxylase/17,20-lyase (CYP17A1), and nuclear receptor subfamily 5 group A member 1 (NR5A1). However, PKA inhibitor H89 or adenylyl cyclase inhibitor SQ22536 could block their effect. The results of transgenic yeast models showed the androgenic agonistic effects of kaempferol and naringenin and the estrogenic agonistic effects of rutin. These results indicated that the testosterone promotional effect of EUF was related to the activation of the steroidogenic pathway and potential hormonal regulation. Kaempferol and geniposidic acid might be the key active ingredients.
Similar content being viewed by others
Introduction
Eucommia ulmoides Oliver, a well-known and monotypic species of the Eucommia, is widely used as a traditional medicinal plant in Asia with a long history. It has been cultivated in nearly three hundred and fifty thousand hectares in China, accounting for more than 99% of the world's total resources1. As a traditional aphrodisiac, its leaves, stem, bark, and staminate flower can be both exploited2. Studies have shown that the water extract of E. ulmoides can increase the weight of testis and the number of Leydig cells in mice; it can also enhance serum and penile testosterone levels in diabetic rats3. In Korea, the leaves of E. ulmoides have been used as a folk remedy for the treatment of diabetes4. In 2014, the National Health Commission of China added EUF to their novel food list5.
Due to the abundance of nutrients and bioactive components, E. ulmoides has a potential role in hormonal effects6. The male flowers have higher nutritional value and health-promoting impact than the bark and leaves7. It has been reported that flavonoids and triterpenes are the main components of EUF8, and the phytochemical investigation provided 27 compounds, including 11 triterpenes, 7 flavonoids, 4 fatty acids, 1 coumarin, 1 chromone, and 3 cyclopeptide alkaloids9. It exhibited multiple pharmacological activities, including anti-fatigue, anti-hypertensive, anti-hyperlipidemia, and hypnotic effects10,11,12,13,14. However, the research regarding its utilization as an aphrodisiac is still unknown.
Testosterone, a hormone produced via steroidogenesis in testicles, plays an essential role in fetal development and the male reproductive system. Leydig cells are the primary cells of testosterone synthesis and secretion. Although Leydig cells make up only 2% to 4% of the testicular cell population, they can produce 95% of the plasma testosterone content15,16,17. Luteinizing hormone (LH) from the pituitary gland regulates testosterone production in testicular Leydig cells via binding to and activating G protein-coupled receptors. This leads to adenylyl cyclase activation, increased intracellular 3′,5′-cyclic adenosine monophosphate (cAMP) formation, and cAMP-dependent phosphorylation of proteins through PKA. The elevation of cAMP/ PKA stimulates signals of downstream steroidogenic proteins, such as StAR, CYP11A1, HSD3B1 and CYP17A118,19. The present research studied the effects of EUF extract and their nutritional ingredients on Leydig cell testosterone production and steroidogenic signaling pathway using a rat Leydig cells model, suggesting EUF to be a candidate drug for hormonal regulation.
Materials and methods
Experimental animals
SPF Sprague–Dawley male rats (7 weeks, n = 27) were purchased from Hubei Experimental Animal Research Center and housed under conditions with controlled temperature (22 °C ± 1 °C) and 12 h light/12 h dark cycle for 7 days to adapt to the new environment. The experimental scheme of rat treatment has been approved by Hubei Experimental Animal Research Center. The Laboratory animal permission number was SCXK 2020–0018. All methods were carried out following relevant guidelines and regulations, and this study was carried out in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Samples
EUF was harvested between March and April, and EUF and the main compounds of EUF were obtained from Wuhan Yibei Biological Company (Wuhan, China). The EUF was extracted by refluxing with water, and then the solution was filtered and concentrated by vacuum drying to powder.
UPLC-MS chromatogram was applied for examining components in EUF extract, and 24 compounds were identified. The content of pinoresinol diglucoside, geniposidic acid, and chlorogenic acid is 0.03, 0.63, and 0.22 mg/mL. Fifteen compounds, including six flavonoids (kaempferol, naringenin, quercetin, rutin, kaempferol-3-O-rutinoside, astragalin), five iridoids (chlorogenic acid, isochlorogenic acid A, caffeic acid, pinoresinol diglucoside, ferulic acid), and four phenylpropanoids (geniposidic acid, asperulosidic acid, deacetyl asperulosidic acid, aucubin), were selected to detect their effects on testosterone production in Leydig cells20.
Collection and culture of Leydig cells
Purifying rat Leydig cells was carried out according to Klinefelter GR et al. with some modifications21. First, rats were euthanized by cervical dislocation after anesthetizing, and testicles were placed in 0.5 g/L collagenases and digested at 37 °C for 30 min. The cell supernatant containing Leydig cells was filtered through a 70 µm nylon mesh. Cells were washed and resuspended by DMEM/F12 medium. The cells were added to the prefabricated Percoll concentration gradient centrifuge (5%, 30%, 58%, 70%) at 1650 rpm at 4 °C for 30 min, followed by the collection of the second cell band and added with the medium. The cells were then cultured in DMEM/F12 (Hyclone, USA) supplemented with 8% fetal bovine serum (Gibco, USA), 2% equine serum, and 1% penicillin at 37 °C in a 5% CO2 incubator for 24 h before the following experiments.
Leydig cell purity
According to the reported histochemical method of 3β-hydroxysteroid dehydrogenase (3β-HSD) enzyme staining with some modifications22,23. Dyeing reagents were the following: staining solution A (1 mL DMSO with 10 mg DHEA and NBT), staining solution B (1 mL PBS with 10 mg β-NAD), and solution C (1 mL PBS with 1 mg niacinamide). Solutions A, B, C, and PBS were mixed in a ratio of 1:10:10:79. Leydig cells were first digested with trypsin and resuspended with medium (containing serum). After counting with a cell counter, we added 50 μL of the cell suspension and 200 μL of 3β-HSD stain into a 96-well plate, and then the plate was incubated in an incubator for 2 h. The percentage of positively stained cells with distinct blue reaction products was counted under an inverted microscope (Olympus, Tokyo, Japan). Cells with purity greater than 80% after staining with 3β-HSD enzyme were acquired for subsequent experiments.
CCK-8 assay
The cells were seeded in 96-well culture plates at a density of 104 cells per well, then allowed to attach for 24 h before being treated with varying concentrations of EUF extract (12.5, 25, 50,100 μg/mL) and its monomer ingredients (0, 12.5, 25, 50 μM) for 24 h. Subsequently, 100 µL CCK-8 (Sigma, USA) of 1 mg/mL was added to each well for 2 h. The Optical density of each well at 450 nm was measured with a microplate reader (BioTek Instruments, Inc., USA).
Testosterone levels detected by ELISA
The cells were seeded in 24-well culture plates at a density of 4 × 104 cells per well and divided into the positive group (10 μM forskolin), sample groups (EUF extract or monomer ingredients for 24 h), and control group. Testosterone concentrations in the medium were measured following the manufacturer's instructions by a rat testosterone ELISA kit (NanjingJiancheng Institute of Biological Engineering, China) (sensitivity: 0.5–150 nmol/L; inter-assay variation: CV < 10%; co-efficient variation: CV < 12%).
Reactive oxygen species (ROS) measurement
ROS levels were determined by the oxidation of 2′,7′-dichlorofluorescin diacetate into a strong green fluorescent substance (DCF) that could not cross cell membranes. In this experiment, cells were divided into the control group, the Tert-butyl hydroperoxide (TBHP) group (model group), and the TBHP + drug group. ROS was measured following the manufacturer's instructions by a Reactive Oxygen Species Assay Kit (Elabscience, China).
RT-qPCR analysis
Cells were cultured separately in EUF extract, kaempferol, and geniposidic acid for 24 h. RNA was extracted from the cells following the instructions of the RNeasy Plus Mini RNA extraction kit (Tiangen, China). Synthesis of cDNA was performed on a PCR amplifier following the reverse transcription kit manual. Semi-quantitative real-time PCR was performed in a Fast Real-Time PCR system (LightCycler 480II, Roche, Switzerland). The primer sequences used in this study were as Table 1. The electrophoresis results were observed on a gel imaging system (FCQ, ProteinSimple, USA).
Western blot analysis
In this experiment, cells were divided into different groups: control group, 10 µM forskolin group, different EUF extract groups (100, 50, and 25 µg/mL), kaempferol or geniposidic acid groups (50, 25, 5 µM) for 24 h. Inhibitor + different drug groups (10 µM H89 or SQ22563 pretreatment for 1 h).
Total protein extracts for western blot analysis were prepared using lysis buffer. Protein concentration was measured by the BCA method. Western blotting was performed as described in our previous study24. Primary antibodies against StAR, HSD3B1, NR5A1, CYP11A1, CYP17A1 and GAPDH (ABclonal, Wuhan, China) were used at a 1:1000–1:2000 dilution in 2 ml per band. Subsequently, the bands were incubated with secondary antibodies (1:2000, HRP-conjugated anti-rabbit IgG, GB23303) for 1 h at room temperature. The results were detected with the ECL reagent and a gel imaging system (FCQ, ProteinSimple, USA).
Yeast estrogen screen (YES) and yeast androgen screen (YAS) assay
The oestrogenic, anti-oestrogenic, androgenic, and anti-androgenic activities were measured with the XenoScreen XL YES/YAS Assay kit (Xenometrix, XenoScreen XL YES/YAS) according to the manufacturer’s protocol25. The DNA sequence of human estrogen receptor (hER) or human androgen receptor (hAR) is transfected into growing yeast cells (Saccharomyces cerevisiae), and the expression of the reporter gene LAC-Z in yeast cells is regulated by estrogen. 17β-estradiol (E2), 4-hydroxytamoxifen (4-HT), 5α-dihydrotestosterone (DHT), and flutamide (FL) were used as the estrogen agonist, estrogen antagonist, androgen agonist, and androgen antagonist controls, respectively. Antagonistic activities were measured by evaluating the β-galactosidase signal reduction in yeast cells in the presence of 0.2 nM E2 (YES) or 1.0 nM DHT (YAS) in the test medium.
Statistical analysis
All data were presented as the means ± standard deviation from experimental triplicates. GraphPad Prism 8 software was applied to analyze the independent Dunnett’s test. P < 0.05 or P < 0.01 was considered a statistically significant difference.
Results
Leydig cells purity and morphology
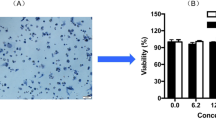
The Leydig cells were fusiform, triangular, or polygonal, with a large nucleus and cell cord arrangement, presenting the "pulling net." The cytoplasm of most of the cells was bluish-black with long antennae, while the cytoplasm of a few cells was pale and greyish-blue. The results showed that the calculated purity reached more than 80% by 3β-HSD enzyme dyeing (Fig. 1A).
Effect of EUF on testosterone secretion in Leydig cells. (A) 3β-HSD staining of Leydig cells (200 ×). (B) Effect of EUF extract on the viability of Leydig cells. (C) Effect of EUF extract on the testosterone content. (D) Effect of monomer on the testosterone content. (E–G) EUF extract, geniposidic acid and kaempferol decreased TBHP-induced intracellular ROS levels. versus the controls: **P < 0.01, *P < 0.05; versus the TBHP group: ## p < 0.01, #p < 0.05. KA, kaempferol; GA, geniposidic acid.
Effect of EUF on the cell viability
As shown in Fig. 1B and Table 2, there was no significant difference in the compound groups except for isochlorogenic acid A, geniposidic acid, and EUF extract, compared with the control group. EUF extract, isochlorogenic acid A, and geniposidic acid had a noticeable effect on promoting cell viability in a dose-dependent and time-dependent manner.
EUF increased testosterone production in Leydig cell
The results showed that forskolin significantly promoted testosterone content (P < 0.01). Compared with the control group, EUF extract, caffeic acid, deacetylcholaginoic acid, naringin, geniposidic acid, rutin, and kaempferol could promote testosterone secretion in Leydig cells at 1 μM and 5 μM. Geniposidic acid and kaempferol showed more substantial effects than others (Fig. 1C,D).
Changes in intracellular ROS levels after EUF extract, kaempferol, and geniposidic acid treatment
As shown in Fig. 1E-G, compared with the control group, ROS levels in the TBHP group were significantly increased, indicating that the oxidative stress model was successfully established (P < 0.01). On the other hand, EUF extract, geniposidic acid, and kaempferol groups significantly decreased ROS content compared with the TBHP group (P < 0.05). Thus, results indicated that EUF extract, geniposidic acid and kaempferol could attenuate cellular ROS accumulation.
EUF extract, kaempferol, and geniposidic acid changed mRNA expression of steroidogenic enzyme
We investigated the mRNA expression levels of steroidogenic pathway genes (Star, Nr5a1, Cyp11a1, Cyp17a1, and Hsd3b1) in Leydig cells. The fusion curves of these genes were all single peaks without hetero peaks, indicating that the five target genes and reference genes were all specifically expressed. The results of RT-qPCR showed that the 100 µg/mL EUF extract had the best effect on promoting the mRNA expression of Star, Nr5a1, Cyp11a1, Cyp17a1, and Hsd3b1 in Leydig cells (P < 0.01). Meanwhile, the expression of the Cyp11a1 gene was promoted, but there was no significant difference. (Fig. 2A,B).
Effects of EUF extract (A), geniposidic acid (C), and kaempferol (E) on the mRNA expression of the steroidogenic enzyme in Leydig cells. Agarose gel electrophoresis results of RT-qPCR amplification products (B, D, F). *P < 0.05, **P < 0.01 versus the controls. EUF, E. ulmoides staminate flowers; KA, kaempferol; GA, geniposidic acid.
The results of RT-qPCR showed that Star, Hsd3b1, and Nr5a1 mRNA expression levels were significantly increased (P < 0.05) in the geniposidic acid group in a dose-dependent manner compared with controls. In addition, 50 µM kaempferol could markedly promote the expression of StAR and Nr5a1 genes. It could also significantly promote the expression of Hsd3b1 and Cyp11a1 genes at 25 µM. (Fig. 2C-F).
EUF extract, kaempferol, and geniposidic acid changed protein expression of steroidogenic enzyme
We performed western blot assays to determine the protein levels of StAR, HSD3B1, CYP17A1, CYP11A1, and NR5A1. Compared with the control group, 100 µg/mL EUF extract could significantly promote the protein expression of StAR, HSD3B1, and CYP17A1 (P < 0.01) but had no significant effect on the expression of CYP11A1 (Fig. 3A,B). The protein levels of StAR, HSD3B1, and CYP11A1 were significantly increased in the kaempferol and geniposidic acid group. Meanwhile, geniposidic acid could also promote the protein expression of CYP17A1 and NR5A1 (Fig. 3C-F).
Effects of EUF on the cAMP-PKA signaling pathway
As shown in Fig. 4, PKA inhibitor H89 and adenylyl cyclase inhibitor SQ22536 could inhibit the promotion of EUF extract and kaempferol on StAR, and H89 could also inhibit the promotion effect of EUF extract on the expression of CYP17A1 and NR5A1. Geniposidic acid could up-regulate the expression of StAR protein after the SQ22536 treatment but had no significant impact on the expression of StAR protein after the H89 treatment. Thus, the effect of EUF extract, kaempferol and geniposidic acid on promoting androgen synthesis may be closely related to the steroidogenic pathway.
Effects of EUF extract (A,B), kaempferol, and geniposidic acid (C–H) on protein expression of the steroidogenic pathway in Leydig cells under the treatment of inhibitors. *P < 0.05, **P < 0.01 versus the control group. #P < 0.05, ## P < 0.01 versus the EUF, GA, or KA group. KA, kaempferol; GA, geniposidic acid.
XenoScreen YES/YAS assay
The XenoScreen XL YES/YAS assay25 (Table 3, Fig. 5) revealed that, among the fifteen compounds, only kaempferol (7.88 × 10–4) and naringenin (8.97 × 10–4 ) had androgenic agonistic effects, and rutin had the estrogenic agonistic effect (5.26 × 10–3 ). No compounds showed ER or AR antagonistic activities.
Discussion
Phenylpropanoids, iridoids, and flavonoids are the reported active constituents of E. ulmoides20. The total flavonoid content in EUF was significantly higher than in other parts (leaves, bark, and fruits) of E. ulmoides26. The present study showed that EUF extract and its main compounds, including caffeic acid, deacetylcholaginoic acid, naringin, geniposidic acid, rutin, and kaempferol, could stimulate testosterone production. Geniposidic acid and kaempferol showed similar effects with the positive control forskolin. Considering that geniposidic acid and kaempferol have a higher concentration and more significant activities in EUF, they might be the main active components in EUF.
Testosterone synthesis is mainly regulated by the classic LH-LHR-cAMP-PKA pathway of testosterone synthesis. It's regulated by LH height and Leydig cell membranes of G protein-coupled receptor (LH receptor-LHR), activation of adenylyl cyclase, promotes the generation of cAMP, catalytic ATP by PKA phosphorylation. Then, testosterone is synthesized under the action of a series of processes such as StAR, CYP11A1, CYP17A1, and HSD3B1. As an inhibitor of adenylyl cyclase, SQ22536 down-regulates the synthesis of cAMP and then inhibits the activities of testosterone biosynthesis-related proteins such as StAR and NR5A127. H89 could inactivate PKA target protein sites and down-regulate the phosphorylation of downstream proteins such as StAR28. The results showed that EUF extract, geniposidic acid, and kaempferol could significantly promote the expression of testosterone synthesis genes or proteins. After pretreatment with SQ22536 and H89, 100 μg/mL EUF extract, 50 μM kaempferol, and 50 μM geniposidic acid could significantly increase testosterone synthetase StAR expression. Therefore, EUF extract, kaempferol, and geniposidic acid raised testosterone synthetase protein expression by activating steroidogenic signaling pathways.
Studies have shown that kaempferol, a phytoestrogen possessing estrogen receptor stress inhibitory activity, could inhibit carcinogenesis and cancer progression29. Besides, it could stimulate estrogen signaling followed by the WNT/β-catenin pathway to induce osteoblasts differentiation30, activate the opening of BK ca channels in human umbilical vein endothelial cells and strengthen insulin secretory capacity of beta cells through the cAMP/PKA pathway31,32. These were similar to our results of the androgenic agonistic effects of kaempferol and naringenin in the XenoScreen XL YES/YAS assay. It has been reported that geniposidic acid showed a protective and promotional effect on primary cultured endothelial cells in an atherosclerotic model33. Eucommiae Cortex fractions had potent induction of growth hormone release. The cortex fractions and its active component, geniposidic acid, can participate in activating osteoblasts to facilitate osteogenesis and suppressing osteoclast activity to inhibit osteolysis34. Similar to the report, we found that geniposidic acid and EUF extract significantly promoted the viability of the Leydig cell.
Here, it could be concluded that EUF extract, kaempferol, and geniposidic acid could stimulate testosterone levels, evidenced by reducing ROS production and promoting Leydig cell viability or raising testosterone secretion. Besides, the active ingredients showed potential hormonal activity on transgenic yeast and upregulation of the steroidogenic pathway. The effect was blocked by PKA inhibitor H89 or adenylyl cyclase inhibitor SQ22536 in Leydig cells, suggesting that their stimulatory mechanism was associated with activating the steroidogenic pathway. It is the first time to elucidate the effect, pharmacodynamic material basis, and mechanism of EUF in testicular testosterone production.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AR:
-
Androgenic receptor
- CYP11A1:
-
Cytochrome P450 cholesterol side-chain cleavage
- CYP17A1:
-
17α-Hydroxylase/17,20 lyase
- cAMP:
-
3′,5′-Cyclic adenosine monophosphate
- DHT:
-
5α-Dihydrotestosterone
- E2:
-
17β-Estradiol
- ER:
-
Estrogenic receptor
- EUF:
-
Eucommia ulmoides staminate flowers
- FL:
-
Flutamide
- GA:
-
Geniposidic acid
- hAR:
-
Human androgen receptor
- hER:
-
Human estrogen receptor
- HSD3B1:
-
3β-Hydroxysteroid dehydrogenase type 1
- 4-HT:
-
4-Hydroxytamoxifen
- KA:
-
Kaempferol
- LH:
-
Luteinizing hormone
- NA:
-
Naringenin
- NR5A1:
-
Nuclear receptor subfamily 5 group A member 1
- PKA:
-
Protein kinase A
- RT-PCR:
-
Real-time reverse transcription polymerase chain reaction
- ROS:
-
Reactive oxygen species
- StAR:
-
Steroidogenic acute-regulatory protein
- TBHP:
-
Tert-butyl hydroperoxide
- YAS:
-
Yeast androgen screen
- YES:
-
Yeast estrogen screen
References
Wang, Y. et al. Comparative evaluation of hydrothermal carbonization and low temperature pyrolysis of Eucommia ulmoides oliver for the production of solid biofuel. Sci. Rep. https://doi.org/10.1038/s41598-019-38849-4 (2019).
Wu, D. et al. Metabolite profiles, bioactivity, and HPLC fingerprint of different varieties of Eucommia ulmoides Oliv.: Towards the utilization of medicinal and commercial Chinese endemic tree. Molecules 23, 1898–1914. https://doi.org/10.3390/molecules23081898 (2018).
Zhang, W. H., Liu, Z. L., Qi, Y. C. & Dong, H. S. Effect of aqueous extract from Eucommia ulmoides on levels of testosterone in serum and penile tissues of diabetic rats. Chin. J. Hum. Sex. 11, 7–8 (2004).
Kim, H. Y., Moon, B. H., Lee, H. J. & Choi, D. H. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J. Ethnopharmacol. 93, 227–230. https://doi.org/10.1016/j.jep.2004.03.047 (2004).
NHC National Health Commission. National Health Commission Announcement on the approval of six new food raw materials including chitooligosaccharide etc (2014).
Wei, Y., Wen, X. & Yu, H. Quality evaluation of Eucommia ulmoides male flower tea from different producing areas. J. Green Sci. Technol. 22, 200–202 (2018).
Xing, Y. Y. et al. Inhibition of rheumatoid arthritis using bark, leaf, and male flower extracts of Eucommia ulmoides. Evid. Based Complement. Altern. Med. 2020, 3260278. https://doi.org/10.1155/2020/3260278 (2020).
He, X. et al. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 151, 78–92. https://doi.org/10.1016/j.jep.2013.11.023 (2014).
Li, R. et al. Phytochemical constituents, chemotaxonomic significance and anti-arthritic effect of Eucommia ulmoides Oliver staminate flowers. Nat. Product Res. https://doi.org/10.1080/14786419.2020.1858411 (2020).
Shaoyang, L., Xin, L., Wenxue, Z., Jinling, F., Haifang, X. & Lin, D. Study on the sedative and hypnotic effects of water extracts of Eucommia male flowers. In Academic Periodical of Farm Products Processing, Vol. 29 31–34 (2010).
Ding, Z. J. et al. Antihypertensive activity of Eucommia ulmoides Oliv: Male flower extract in spontaneously hypertensive rats. Evid. Based Complement. Altern. Med. 2020, 6432173. https://doi.org/10.1155/2020/6432173 (2020).
Wang, J. Y. et al. Anti-inflammatory effects of Eucommia ulmoides Oliv. male flower extract on lipopolysaccharide-induced inflammation. Chin. Med. J. 132, 319–328. https://doi.org/10.1097/CM9.0000000000000066 (2019).
Jin, X. L., Du, H. Y. & Li, Q. Study on anti-fatigue effects of Eucommia ulmoides Oliv. male flower tea on mice. Food Sci. 7, 432–434 (2008).
Lou, L. et al. Regulation effects of Eucommia ulmoidesmale flower tea on lipid parameters of mice fed with high fat emul. HenanUniv. (Med. Sci.) 28, 273–275 (2009).
Stanley, E. et al. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology 153, 5002. https://doi.org/10.1210/en.2012-1417 (2012).
Svechnikov, K. et al. Origin, development and regulation of human Leydig cells. Horm. Res. Paediatr. 73, 93–101. https://doi.org/10.1159/000277141 (2010).
Xing, X. Y. & Sun, J. Generation and regulation of Leydig cells. Natl. J. Androl. 20, 273–276. https://doi.org/10.13263/j.cnki.nja.2014.03.016 (2014).
Ho, H. J., Shirakawa, H., Giriwono, P. E., Ito, A. & Komai, M. A novel function of geranylgeraniol in regulating testosterone production. Biosci. Biotechnol. Biochem. 82, 956–962. https://doi.org/10.1080/09168451.2017.1415129 (2018).
Wang, Y., Chen, F., Ye, L., Zirkin, B. & Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction (Camb. Engl.) 154, R111–R122 (2017).
Li, X., et al. UPLC-MS analysis and network pharmacology-based investigation into the active ingredients and molecular mechanisms of anti-fatigue of male flowers with Eucommia ulmoides Oliv. Fundam. Clin. Pharmacol. 1–16 (2022). https://doi.org/10.1111/fcp.12798
Sharma, R. S., Pal, P. C. & Rajalakshmi, M. Isolation and culture of Leydig cells from adult rats. Indian J. Clin. Biochem. 21, 27–33. https://doi.org/10.1007/BF02913063 (2006).
Mendelson, C., Dufau, M. & Catt, K. Gonadotropin binding and stimulation of cyclic adenosine 3′:5′-monophosphate and testosterone production in isolated Leydig cells. J. Biol. Chem. 250, 8818–8823. https://doi.org/10.1016/S0021-9258(19)40746-1 (1975).
El-Alfy, M. et al. Localization of type 5 17beta-hydroxysteroid dehydrogenase, 3beta-hydroxysteroid dehydrogenase, and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinology 140, 1481–1491. https://doi.org/10.1210/endo.140.3.6585 (1999).
He, H. et al. Via doxorubicin induces endotheliotoxicity and mitochondrial dysfunction ROS/eNOS/NO pathway. Front. Pharmacol. 10, 1531. https://doi.org/10.3389/fphar.2019.01531 (2019).
Routledge, E. J. & Sumpter, J. P. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. 15, 241–248 (2020).
Liu, C. et al. Research advances in chemical constituents and pharmacological activities of different parts of Eucommia ulmoides. China J. Chin. Mater. Med. 45, 497–512. https://doi.org/10.19540/j.cnki.cjcmm.20191108.201 (2020).
Gao, Y. S. & Raj, J. U. Effects of SQ 22536, an adenylyl cyclase inhibitor, on isoproterenol-induced cyclic AMP elevation and relaxation in newborn ovine pulmonary veins. Eur. J. Pharmacol. 436, 227–233. https://doi.org/10.1016/S0014-2999(02)01261-X (2002).
Chijiwa, T. et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 265, 5267–5272. https://doi.org/10.1016/S0021-9258(19)34116-X (1990).
Kim, S. H. & Choi, K. C. Anti-cancer effect and underlying mechanism(s) of Kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 29, 229–234. https://doi.org/10.5487/TR.2013.29.4.229 (2013).
Sharma, A. R. & Nam, J.-S. Kaempferol stimulates WNT/β-catenin signaling pathway to induce differentiation of osteoblasts. J. Nutr. Biochem. 74, 108228. https://doi.org/10.1016/j.jnutbio.2019.108228 (2019).
Xu, Y. C., Leung, G. P. H., Wong, P. Y. D., Vanhoutte, P. M. & Man, R. Y. K. Kaempferol stimulates large conductance Ca2+-activated K+ (BKCa) channels in human umbilical vein endothelial cells via a cAMP/PKA-dependent pathway. Br. J. Pharmacol. 154, 1247–1253. https://doi.org/10.1038/bjp.2008.194 (2008).
Ghorbani, A., Rashidi, R. & Shafiee-Nick, R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed. Pharmacother. 111, 947–957. https://doi.org/10.1016/j.biopha.2018.12.127 (2019).
Gao, Y., Zy, C., Liang, X., Xie, C. & Yf, C. Anti-atherosclerotic effect of geniposidic acid in a rabbit model and related cellular mechanisms. Pharm. Biol. 53, 280–285. https://doi.org/10.3109/13880209.2014.916310 (2015).
Ha, H. et al. Effects of eucommiae cortex on osteoblast-like cell proliferation and osteoclast inhibition. Arch. Pharmacal Res. 26, 929–936. https://doi.org/10.1007/BF02980202 (2003).
Acknowledgements
This work was supported by Key project at central government level (grant number 2060302); China Postdoctoral Science Foundation (grant number 2019M651055); and Hubei Provincial Key Laboratory of Occurrence and Intervention of Rheumatic Diseases (grant number PT022002).
Author information
Authors and Affiliations
Contributions
R.Y. and J.L. conceived and designed the experiment. P.Y. and Z.L. performed the experiment. P.Y., Z.L., S.Y., X.S., and L.Y. analyzed the data and wrote the manuscript. D.T. and J.L. revised the manuscript. All authors have read and approved the final manuscript. §Z.L. and §P.Y. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Z., Yang, P., Xue, S. et al. Testosterone promotion effect of Eucommia ulmoides staminate flower via the steroidogenic pathway and potential hormonal mechanism. Sci Rep 12, 18765 (2022). https://doi.org/10.1038/s41598-022-23578-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23578-y