Abstract
Gram pod borer, Helicoverpa armigera (Hub.) is the major insect pest of pigeonpea and prediction of number of generations (no. of gen.) and generation time (gen. time) using growing degree days (GDD) approach during three future climate change periods viz., Near (NP), Distant (DP) and Far Distant (FDP) periods at eleven major pigeonpea growing locations of India was attempted. Multi-model ensemble of Maximum (Tmax) and Minimum (Tmin) temperature data of four Representative Concentration Pathways viz., RCP 2.6, 4.5, 6.0 and 8.5 of Coupled Model Inter comparison Project 5 (CMIP5) models was adopted here. The increase in projected Tmax and Tmin are significant during 3 climate change periods (CCPs) viz., the NP, DP and FDP over base line (BL) period under four RCP scenarios at all locations and would be higher (4.7–5.1 °C) in RCP 8.5 and in FDP. More number of annual (10–17) and seasonal (5–8) gens. are expected to occur with greater percent increase in FDP (8 to 38%) over base line followed by DP (7 to 22%) and NP (5to 10%) periods with shortened annual gen. time (4 to 27%) across 4 RCPs. The reduction of crop duration was substantial in short, medium and long duration pigeonpeas at all locations across 4 RCPs and 3 CCPs. The seasonal no.of gen. is expected to increase (5 to 35%) with shortened gen. time (4 to 26%) even with reduced crop duration across DP and FDP climate periods of 6.0 and 8.5 RCPs in LD pigeonpea. More no. of gen. of H. armigera with reduced gen. time are expected to occur at Ludhiana, Coimbatore, Mohanpur, Warangal and Akola locations over BL period in 4 RCPs when normal duration of pigeonpeas is considered. Geographical ___location (66 to 72%), climate period (11 to 19%), RCPs (5–7%) and their interaction (0.04–1%) is vital and together explained more than 90% of the total variation in future pest scenario. The findings indicate that the incidence of H. armigera would be higher on pigeonpea during ensuing CCPs in India under global warming context.
Similar content being viewed by others
Introduction
Climate change is a major threat to sustainable agriculture1 and its impact on incidence of insect pests is an important dimension of overall impact on agriculture. Understanding of the complex, spatially variable and species-specific effects of climate change is essential to develop an appropriate pest management strategy. Complete comprehension and quantification of insect herbivore-climate relationship is the crux of the pest management strategy as insect growth and development is driven by the climate. IPCC2 summary report projected that global mean surface temperatures would rise in the range of 1–3 °C by the end of this century. When averaged over 2081–2100, the global surface temperature is very likely to be higher by 1.0 to 1.8 °C in the very low, 2.1 to 3.5 °C in the intermediate and 3.3 to 5.7 °C in the very high GHG emission scenarios in comparison with the average between 1850 and 1900. The projected temperature rise for India is in the range of 1.7–2.0 °C by 2030s & 3.3–4.8 °C by 2080s over preindustrial period3. Further, the average temperature over India is projected to rise by approximately 4.4 °C relative to the recent past (1951–2014) under RCP 8.5 scenario4.
The variation of temperature is prominent and non-uniform among different areas, provinces, locations and negative impacts have been projected on various crops5. Bale et al. (2002)6 reported that insect development and its distribution is mostly temperature driven. Under warming conditions, early infestation, increased survival of insects and more crop damage would occur. About two to four-fold increase in herbivory7 and severity in pest outbreak8 are reported as a result of climate change. Thermal requirement of insects is species-specific, and the role of temperature is vital and prominent on development of each insect species9. Degree days are the amount of heat required for an organism to develop within certain life stages and growing degree day (GDD) is the summation of heat units that accumulated above base temperature during a 24-h period. Degree days concept is an effective way to explain shifts in phenology, arising from climate change over space and time10 and it is a very strong, integrative measure of the spatio-temporal variation of temperature that imposes the thermal limits within which insect species grow11.
Temperature effects on insect pests are species-specific and elaborative studies were conducted earlier. The effect of temperature on the insects is direct and dependent on the amount of time they are exposed, as that causes physiological changes that aid survival12. The development and distribution of insects are influenced by their thermal thresholds; though thermal requirements vary with species; climate warming can expand the survival limits13 and often may lead to higher number of generations (no. of gen.) in a year or a season. Increased adult moth activity14, increased number of generations15, higher incidence16, higher population growth rate17 were the effects of increased temperature on insect biology.
Temperature is crucial in deciding the insect population growth rate and development and further reflected in number of generations which indicates the number of reproductive events in a given period (calendar year/growing season). It affects the crop-insect interactions, implying the level of incidence indirectly. More generations not only promote the population growth but also hastens the evolutionary process and adaptation to climate18. Increased temperature leads to higher developmental rate and in turn increases the voltinism of the pest. The possibility of number of generations in a year is a function of temperature, precipitation and availability of host19. Insects are sensitive to increased temperature20 and its impact on insect leads to advancement of phenology/ life stages. In case of insect pests, longer developmental duration at low temperatures and a shorter developmental duration at high temperatures was documented earlier21.
Pigeonpea (Cajanus cajan L.), a legume crop belonging to the tribe Phaseoleae, is one of the most important grain legumes. It has 21% of dietary protein22 in seeds and is rich in minerals like phosphorus, magnesium, iron, calcium, sulphur and potassium23.The global area under cultivation of pigeonpea is 5.7 Mha with a productivity of 861.3 kg/ha and overall production of 4.91 Mt per annum over the triennium of 2017–202024. India is the major producer (78%). Myamnar, Malawi, Tanzania, Kenya and Uganda also have significant area under pigeonpea. Among the pigeonpea growing countries, India ranked 1 in area and production representing around 82% of world pigeonpea area and 78% of total production. Pigeonpea is grown in all states of India for various purposes. In the triennium of 2017–2020, pigeonpea was cultivated on an average area of 4.6 Mha with an average production of 3.8 Mt per year and with a productivity of 824 kg per hectare25 in India.
Among the several insect pests (about 150) that attack pigeonpea, gram pod borer Helicoverpa armigera (Hub.) is a major insect pest which causes up to 20–30% yield losses26. Prediction of ensuing pest scenarios using temperature data based on climate projections of Coupled Model Inter comparison Project 3 (CMIP3)27 was attempted by several research workers28,29,30. These climate projections are generally with more accuracy at the global scale than at smaller regional scale31. Recently the Coupled Model Inter-comparison Project phase 5 (CMIP5) models are available with high resolution and are more comprehensive in nature over CMIP332 and these projections for India are far more reliable3. Individual GCM projections have uncertainty and to alleviate the uncertainty, multiple and/or ensemble of GCMs is generally preferred33.
Earlier, many authors33,34 have used the World Climate Research Program’s (WRCP’s) CMIP5 multi-model dataset for generating multi-model ensemble climate change scenarios. The advantage of the CMIP5 multi-model dataset is the accuracy of newly developed representative concentration pathways (RCPs)3. We adopted ensembled mean climate data here which is closer to the observed climate than any individual model. IPCC Fifth Assessment Report’s RCP based climate change projections for 4 RCPs, viz., 2.6, 4.5, P 6.0 and 8.5 are adopted for our analysis.
The present study was attempted to understand the impact of simulated scenario of increased temperatures on no. of gen. and gen. time of H. armigera on pigeonpea for the future CCPs using temperature data of four RCPs across eleven locations of India.
Materials and methods
Currently, climate models are the best tools to simulate future climate change scenarios and the accuracy of temperature projections mainly depends on type of model and scenario adopted.
Projected temperature in RCPs
We followed the IPCC AR 5 climate change projections based on emission scenarios known as RCPs (Representative Concentration Pathways). As explained earlier, ensemble multimodel climate change scenarios were obtained with bias correction and at 0.5 × 0.5° spatially disaggregated climate change projections from WRCPs CMIP5 multi-model data set. In total 32 GCM climate change projections from 23 modeling Centers/Groups were used. As different GCMs have multiple runs, we used 51, 61, 34 and 64 projections (runs) for RCP2.6, RCP4.5, RCP6.0 and RCP8.5, respectively, for simulating climate change scenarios. Srinivasa Rao et al.34, mentioned more about details of climate change projections and advantages of hybrid ensemble data33,35 in their earlier publication.
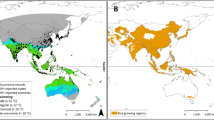
In this study, climate change scenarios for 4 RCPs and 3 CCPs were adopted. Climate projections were studied over three CCPs/time slice periods (TSPs) viz. NP—Near Period (2020–2039), DP—Distant Period (2040–2069) and FDP—Far Distant Period (2070–2099). Two approaches viz., calendar year (total 365 days, 1–52 Standard Weeks) and pigeonpea crop duration (150 days from 26th to 52nd Standard Weeks) were considered for predicting the number of generations (no. of gen.) and generation time (gen. time) of H. armigera. Minimum (Tmin) and maximum (Tmax) temperatures for 11 pigeonpea growing locations of India for the future/ensuing periods under 4 RCPs were compared over baseline period (1976–2005). The details of these eleven pigeonpea growing locations of India are- Akola (20°42’N; 77°2’E), Ananthapur (14°41’N; 77°35’E), Bengaluru (12°58’N; 77°35’E ), Bhubaneshwar (20°16’N; 85°50’E), Coimbatore (10°57’N; 78°58’E ), Gulbarga (17°21’N; 76°51’E ), Jabalpur (24°8’N; 80°58’E ), Kanpur (26°27’N; 80°14’E), Ludhiana (30°90’N; 75°85’E), Mohanpur (22°77’N; 88°39’E) and Warangal (17°96’N; 79°59’E) depicted in a map (Fig. 1) drawn using QGIS software 3.14.16 version (open-source).
Selected pigeonpea locations of India for the study in India map using QGIS software version 3.14.16. The URL is https://download.qgis.org/downloads/QGIS-OSGeo4W-3.14.16-2-Setup-x86.exe.
Annual & seasonal generations of H. armigera
Prediction of no. of gen. of H. armigera was done using ‘ingen’ software36 which is openly accessible (http://www.nicra-icar.in/). The ingen software was earlier15,29,30,34,37 used to obtain output of estimated thermal requirements /growing degree days, insect pest generations & gen. time. Lower threshold temperature of 10 °C was adopted to calculate the Growing Degree Days (GDD) with horizontal cutoff method. Prediction of no. of gen. and gen. time of H. armigera was done separately for annual/ calendar year and crop/growing season.
Estimation of crop duration and generations in RCPs
Advancement of phenological stages of crop leads to reduction of duration for maturity with increased temperature. The degree day requirement of pigeonpea varies with sowing date38 ___location39 and variety40. We calculated the degree day requirement for short-duration pigeonpea (SDP-130 days), medium-duration pigeonpea (MDP-150 days) and long-duration pigeonpea (LDP-180 days) was in the range of 2100 to 3600 DD. Here, the accumulated heat units of 1900–2700, 2100–3000 and 2450–3550 GDD for SD, MD and LD pigeonpeas respectively were considered across 11 locations for the estimation of reduction in duration. Increased temperature influences the duration of crops41 by causing the advancement of phenological events and in the present study, no. of gen. and gen. time of H. armigera was also estimated with reduced duration of pigeonpea across SDP, MDP and LDPs.
Statistical analysis
Change in gen. time (annual and seasonal) and no. of gen. (annual and seasonal) from baseline to near period (NP), distant period (DP) and far distant period (FDP) across 4 RCPs and 11 pigeonpea growing locations were tested for their statistical significance using two sample t test. Usually it is assumed that two populations being compared are homogenous with respect to their variances which may not always be true. In view of this, Levene test was employed to establish equality of variances or otherwise. Corrective measures were taken by employing two sample t test for unequal variances for comparisons with heterogenous variances. Further, two sample t test becomes valid only when the distributions of the two populations being compared are normal. To ascertain this assumption, Shapiro–Wilk test was conducted. The standard Analysis of Variance (ANOVA) was adopted to partition the variation in the study parameters viz., no. of gen. and gen. time of H. armigera into various sources viz., ___location, scenario, climate period and their interactions15,42 and quantified their contribution to total variability. All statistical analyses were conducted using SPSS 16.0 version.
Results
The findings of Levene’s test indicate equal variance (with p value > 0.05) in the groups compared in no. of gen. (annual and seasonal) and gen. time (annual and seasonal) of H.armigera for 515 datasets out of 528 (11 locations × 4 RCPs × 3 CCPs with 30 observations in each period × 4 variables) studied showing the homogeneity of the data. The remaining 13 datasets were compared with unequal variances to compare the means. The Shapiro–Wilk test of significance indicated the normality of no. of gen. (annual and seasonal) and gen. time (annual and seasonal).
Rise in Tmax and Tmin
The predicted Tmax and Tmin across 11 locations of India under 4 RCP scenarios when compared over baseline (Fig. 2a,b) indicated significant increase in both the temperatures in 4 RCPs. The increases were markedly higher and more evident in RCP 6.0 and RCP 8.5 during DP and FDP CCPs.
It is expected that projected temperatures (Tmax and Tmin) would follow increasing trend during 3 CCPs at 11 locations. Tmax (0.7 to 4.7 °C) and Tmin (0.8 to 5.1 °C) would increase considerably during NP, DP and FDP of 4 RCP scenarios. This trend was more evident in RCP 8.5 of FDP followed by 6.0 and 4.5 scenarios.
Annual and seasonal generations
Four RCPs
Significant (F3, 3828 = 1768.82, P < 0.001) differences were observed (Table. 1) in annual num. of gen. of H. armigera on pigeonpea with reduced gen. time (F3, 3828 = 1045.33, P < 0.001) among RCPs with RCP 8.5 (16.83 ± 0.45; 21.31 ± 0.30) followed by RCP 6.0 (15.70 ± 0.34; 22.75 ± 0.15), RCP 4.5 (15.48 ± 0.33; 22.98 ± 0.54) over RCP 2.6 (14.79 ± 0.29; 24.25 ± 0.19). Similar trend was observed during the crop season also with significant (F3, 3828 = 943.68, P < 0.001) increase no. of gen. with shortened gen. time (F3, 3828 = 979.94, P < 0.001) in RCP 8.5 (8.49 ± 0.28; 21.21 ± 0.06) followed by RCP 6.0 (7.20 ± 0.18; 23.67 ± 0.42) RCP 4.5 (7.05 ± 0.19; 23.77 ± 0.27) and RCP 2.6 Scenarios (7.02 ± 0.18; 25.12 ± 0.73) (Fig. 3a,b).
Climate change periods
Significant (F2, 3828 = 5700.80, P < 0.001) differences were recorded (Table. 1) in the annual no. of gen. of H. armigera among the CCPs with significant (F2, 3828 = 3467.677, P < 0.001) reduction in gen. time during FDP (16.83 ± 0.45, 21.31 ± 0.30) followed by DP (15.57 ± 0.35, 22.78 ± 0.10) and NP (14.52 ± 0.28, 24.33 ± 0.02) over BL period. During the crop season, (F2, 3828 = 2933.30, P < 0.001) increase in the no. of gen. (8.49 ± 0.28) with significant (F2, 3828 = 3389.99, P < 0.001) reduction in gen. time (21.21 ± 0.06) was noticed in FDP. The comparison of pest incidence between DP and NP CCPs revealed that pest incidence would be higher for DP (7.88 ± 0.21, 23.72 ± 0.37) than NP (7.37 ± 0.16, 25.56 ± 0.72) period over BL period (Fig. 4a or b or both).
(a) Variation in no. of gen. and gen. time of H. armigera in crop season during 3 CCPs over baseline at 11 pigeonpea growing locations of India (RADAR). (b) Variation in no. of gen. and gen. time of H. armigera in crop season during 3 CCPs over baseline at 11 pigeonpea growing locations of India (PANELS).
Locations
Among eleven pigeonpea growing locations, Coimbatore (16.83 ± 0.45, 21.31 ± 0.30) and Warangal (16.22 ± 0.29, 25.25 ± 0.61) would get significantly (F10, 3828 = 4802.96, P < 0.001) (Table 1) more annual no. of gen. of H. armigera with reduction in gen. time (F10, 3828 = 3234.60, P < 0.001) in RCP 8.5. Moderate levels were observed in Bhubaneshwar (14.96 ± 0.44, 23.67 ± 0.79) and Jabalpur (14.71 ± 0.52, 24.09 ± 0.61) locations. Bengaluru (13.19 ± 0.45, 26.83 ± 1.09) and Ludhiana (12.50 ± 0.48, 28.50 ± 1.44) locations experience less no. of gen. in FDP. Similar incidence was followed in these locations in DP and NP.
The interaction between RCPs x ___location; RCPs x time slice; ___location x time slice and RCPs x ___location x time slice were significant with respect to annual gen. time and seasonal gen. time. With annual and seasonal no. of gen., the interaction was significant with RCPs × time slice; ___location x time slice only (Table 1). During crop growing season, Coimbatore (8.49 ± 0.28, 21.21 ± 0.06) and Mohanpur (7.81 ± 0.22, 23.73 ± 0.40) locations would experience more no. of gen. (F10, 3828 = 3655.79, P < 0.001) with reduced gen. time (F10, 3828 = 4498.44, < 0.001). Moderate levels were estimated for Jabalpur (7.23 ± 0.31, 24.26 ± 0.99) and Gulbarga (7.21 ± 0.31, 26.19 ± 1.08). Ananthapur (6.79 ± 0.14, 27.08 ± 0.53) and Bengaluru (6.3 ± 0.28, 30.04 ± 1.46) would have lesser no. of gen.
Percentage variation in annual and seasonal generations
Higher percent increase of annual no. of gen. of H. armigera was predicted to happen in FDP period (7.65 to 37.83%) over BL period followed by DP (7.47 to 21.89%) and NP (5.25 to 9.51%) across 4 RCPs (Sup Figure 1 and 2). Similarly, the expected increase of seasonal no. of gen. over the baseline was highest in FDP period (7.07 to 35.00%) followed by DP (7.09 to 20.31%) and NP (5.00 to 8.77%) in four RCPs. Higher reduction of annual gen. time of H. armigera over BL period was observed in RCPs for future TSP/ CCPs. The highest reduction (percentage) in annual gen. time over BL was predicted for FDP (6.86 to 26.83%) followed by DP (6.78 to 17.67%) over NP (3.69 to 8.96%) in four RCPs. The highest percent reduction was noticed with RCP 8.5 over other three scenarios. Similar trend was with seasonal time and the percent reduction was greater in FDP (4.87 to 25.77%) followed by DP (4.70 to 18.37%) and NP (3.78 to 10.42%) over the baseline.
Partitioned variation
Geographical ___location (66.29%) and time period (15.74%) were the key sources of variation in annual no. of gen. of H. armigera, which together explained about 82.03% of the total variation. Rest of the variables viz., scenarios and interactions contributed less than 20% of the variation (17.97%). Similarly, in annual gen. time of H. armigera, geographical ___location (66.33%) and time period (14.22%) explained most of the variation (80.55%) (Fig. 5). Similar trend was with seasonal no. of gen. and seasonal gen. time of H. armigera where geographical ___location and time period together accounted for 82.89% and 82.39% of the total variation respectively.
Trend in generation time (seasonal) under different RCPs
Among four RCPs, the higher reduction of gen. time is noted in RCP 8.5 scenario followed by 6.0 and 4.5 scenarios. Across three CCPs, it is more evident in FDP, DP climate change period than NP period. The trend is more reflected in Coimbatore ___location followed by Kanpur and Ludhiana locations and least reflection was recorded in Bengaluru and Ananthapur locations across 4 RCP scenarios (Fig. 6a, b).
Variability in seasonal generation time across locations, CCPs and RCPs
The changes in mean seasonal gen. time of H. armigera in eleven locations across different CCPs and RCPs were depicted as ‘box plot’ figure capturing the variability in the parameter and here every point indicates the mean of seasonal generation time of the 11 locations studied across 4 CCPs. The seasonal gen. time followed a decreasing trend i.e., lesser gen. time with advancing climate period in 4 RCPs based on comparing the medians. The lowest gen. time recorded was 21 days in FDP of RCP 8.5 as against 39 days in BL (Fig. 7). The Outliers, Ananthapur (31 days) and Bengaluru (32 days) in FDP of RCP 4.5 and RCP 6.0 were higher than the maximum range. Similar reduction in generation time was noted in annual number of generations also.
Generations in altered duration of pigeonpea
Reduction of duration
The reduction of pigeonpea duration was expected to occur across three CCPs based on GDD of 1900–2700 for SDP, 2100–3000 for MDP and 2450–3550 for LDPs at 11 locations. The data depicted in Fig. 8 indicated the reduction of crop duration across 11 locations and might be due to advancement of crop maturity.
The reduction of crop duration (days) in SDP (2–22), MDP (3–35) and LDP (5–57) was substantial at all locations across 4 RCPs and 3 CCPs and was more evident in RCP 6.0 and 8.5 (Fig. 8). The higher reduction of crop duration was in LDP (57 days) and MDP (35 days) in far distant period (FDP) of RCP 8.5 at Mohanpur and Ludhiana locations. Similar reduction of duration was reflected in other locations in DP and NP also in RCPs of 6.0 and 4.5. The reduction of crop duration was moderate at Coimbatore and Akola and least at Ananthapur, Bhubaneshwar and Warangal locations in RCP 6.0 and 8.5 scenarios (Fig. 8). Though the reduction of duration was noted in SDP, it was meagre in comparison with LDP and MDPs.
Number of generations in altered duration
Figure 9a depicts changes in the number of generations of H. armigera on LD pigeonpea possible with RCP 6.0 and 8.5 scenarios. There was an observed increase in the number of insect generations even with the shortened duration of LD pigeonpea during FDP and DP for both RCP 6.0 and 8.5 with the changes being more conspicuous with RCP 8.5-based projections. At most of the locations, it was predicted that even with a reduced duration of LDP, a greater number of generations were expected to occur in both scenarios and the variation was more pronounced at Ludhiana (5.77). However, the changes in the number of generations are relatively low in the case of MD pigeonpea in most locations (Fig. 9b). In the case of SDP, not much difference in no. of gen. was recorded in RCP 2.6 and 4.5 scenarios due to reduction of crop duration meaning that the reduced crop duration may offset the accelerated rate of reproduction.
Discussion
Increase in temperature causes advancement of insect developmental stages which in turn results in reduction of duration of life cycle and thus more generations of insects may occur in shorter period of time. The role of temperature is vital and impactful on insects in completing the life cycles and producing more generations43. Formulation of effective pest control measures needs complete comprehension of population dynamics of insect pests. The mean annual maximum temperature increased at the rate of 0.018 °C per year in the past 20 years and Arora et al.44, noted that mean temperature increased by 0.42 °C in the last 100 years. It is implied that more insect population is function of more generations would occur in a calendar year and growing season. Nationwide rise in temperature (Tmax and Tmin) was predicted across the four RCPs45. The increases in temperature reported by Chaturvedi et al.3 were about 1.7–2.0 °C by 2030s in RCP 4.5 and RCP 6.0, with overall increase in temperature for India by 1–4 °C.
Results of the present study also exhibit a similar rise in temperature (Tmax and Tmin) across eleven pigeonpea locations of India in Tmax (0.7–4.7 °C) and Tmin (0.8 to 5.1 °C) over the baseline during NP, DP and FDP of 4 RCP scenarios. We predicted the pest scenario i.e., no. of gen. of H. armigera using the projected temperature data from four RCP scenarios by adopting standard GDD approach in three future TSPs viz., NP, DP and FDP at eleven pigeonpea growing locations in India. There was a similar increase of temperatures for other parts of Asia46 by 2.06–3.63 °C and for other parts of the world47 by 2 °C and 6 °C.
Srinivasa Rao et al.15 predicted the no. of gen. of H. armigera on pigeonpea with temperature data of MarkSim, GCM multimodel (7) data of three scenarios (A1B, A2 and B1) of CMIP3 at eight pigeonpea locations of India. Here, we used RCP-based climate projections of CMIP5 group of climate models with several advantages34,48. We used temperature data of RCP scenarios as they eliminate or reduce the uncertainty (http://www.cawcr.gov.au/projects/Climatechange) in projections over the earlier ones based on SRES49 and RCPs have greater applicability in Indian conditions. Further, we estimated the possible reduction in duration of LDP, MDP and SDP and hypothesized that the no. of gen. would vary with reduction of crop duration among three pigeonpeas, while in our earlier study15, we considered a constant crop duration. In the present study, we attempted the estimation of the number of generations with altered crop duration also. Different degree day models and quantified equations/relationships are often adopted50 to predict the pest scenarios.
Several studies15,16,29,30,34,37,42,51,52 predicted the pest status in different climate change scenarios using GDD and pest modelling approach and mentioned that the pest scenario is a function of emission scenarios, geographical ___location, climate change period and model adopted. The present paper predicted the status of H. armigera on pigeonpea during future CCPs using RCP projected temperature ensemble data across India, as little or no studies have been attempted so far.
Insects are poikilothermic and thermo-sensitive, and their distribution and abundance get influenced by the smaller variations of temperature53. Temperature is the key factor which affects the growth and development of crop plants and insect herbivores remarkably. GDD approach was adopted earlier16,54,55 for prediction of pest status.
More no. of gen. per year in multivoltine species56 was due to the considerable increase in surface temperatures and similar findings were with lepidopteran pests Endopiza viteana57 and Paralobesia viteana58. Our present findings showed that more number of annual generations of H. armigera on pigeonpea (6 to 38%) would be in RCP 8.5 followed by 6.0, 4.5 over 2.6 RCPs and parallel trend is expected in growing season also. Several research workers15,42,43,56,58,59 also found increased number of insect generations with increase in temperature.
Shortened gen. time was noticed in all four RCPs in both the approaches adopted by us viz., calendar year (4–27%) and growing season (4–26%) and the trend was more evident in RCP 8.5 of FDP over BL. Similar reduction of gen. time with increased temperature was in Lymantria dispar60, Brachmia macroscopa61 and Athetis dissimilis on maize62. Our findings using temperature data of seven different models of Marksim indicated the occurrence of 1 to 2 additional generations with reduced gen. time of S. litura29 on peanut and H. armigera15 on pigeonpea. It was mentioned that adaptation of insects would be quicker to climate change due to short gen. time63 and the same is reflected in present findings. Direct influence of increased temperature on metabolic rate and developmental rate of insects64 and multivoltine insects are believed to take advantage of climate warming. Present findings are in tune with Altermatt18 who observed that increased temperature causes advancement of phenology, which in turn lead to more no. of gen. of an insect.
Among the three variables studied viz., emission scenarios, TSPs and locations, the no. of gen. and gen. time varied significantly among the locations. Among the locations, Coimbatore would experience higher seasonal no. of gen. (7.33–8.48) followed by Mohanpur (6.49–7.805) Warangal and Akola locations over the BL period of the ___location across the four RCPs and TSPs. The increment is least in Ludhiana (4.72–5.62) (Fig. 6a). Climate change caused the variation in spatial distribution of different insect pests among different provinces28,65. More crop losses are expected due to higher no. of gen. during the crop season or calendar year. No. of gen. shows the hastening of reproductive events which in turn affect the host-insect herbivore interactions implying the increased level of incidence18. Increased voltinism of insect pest may lead to higher infestation66. Often, voltinism may amplify the outbreak of pest species67 also. Though occurrence of no. of gen. is dependent on several factors, the role of increased temperature is vital and often leads to higher developmental rate. The preference of the host by the insect, the presence of natural enemies and other collateral factors also influence the voltinism which are not covered by the ingen software, however, it takes care of temperature-driven influences. Though the impact is confounding in nature, temperature plays a major and significant role and the same is captured in the present study. The findings of present study indicate higher percent increase in no. of gen. (6–38%) with reduced gen. time (7–27%) of H. armigera in RCP scenario 8.5 during 3 CCPs in both season and calendar year at majority of pigeonpea locations of India which are higher than earlier studies by Srinivasa Rao et al.15, with Marksim model data.
The results on analysis of partitioned variation indicated significant contribution (about 83%) (Fig. 5) of geographical ___location and time period together in the variation in annual and seasonal no. of gen. and annual and seasonal gen. time indicating their vital role in prediction of the same. Pest modeling is most practical and useful method for predicting the weather impacts on insect pests68. Our findings indicated that temperature variation is evident across geographical locations of India among various climate change scenarios meaning that temperature impacts on insects vary with ___location. Pest modelling is the most adopted tool to quantify these impacts which are ___location and species specific. Present findings indicated that 83 percent of the total variation in projected no. of gen. of H. armigera on pigeonpea is explained by geographical ___location and climate period only. Similar findings were stated by Ziter et al.42 and Srinivasa Rao et al.15,29. The contribution of these two variables to total variation in our present studies was lesser than our earlier studies29 which accounted for about 94%. This might be due to the adoption of RCP scenario data which has more reliability for India and had about 7–11% of the total variation. The interactions among three variables also contributed substantially though they are in less percentages.
It is documented that at higher temperatures and warming conditions, advancement of crop phenological stages would take place resulting in reduction of crop duration69,70,71. Earlier authors38,39,40 justified the advancement of phenological stages of crops and maturity with increased temperature causing variation in thermal requirement of pigeonpea. The present findings also indicated the reduction of crop duration based on Growing Degree Days and noted that the advancement of crop maturity was considerable in MDP and LDP and the reduction of crop duration was higher in medium (3–35 days) and long (5–57 days) duration pigeonpeas as against SDP (2–22) at 11 locations across 4 RCPs and 3 CCPs (Fig. 9). Similar reduction of pigeonpea duration by 10 days with 2 °C rise in temperature was found in Gulbarga region72. The results are in conformity with those of Purnamawati et al.41 who also reported the reduction of crop duration under increased temperature conditions. The present findings indicate clearly that reduction of crop duration across three pigeonpeas was considerable at increased temperature conditions in 4 RCP scenarios of 3 CCPs.
Even with reduced crop duration, higher no. of gen. with shortened gen. time was expected in LDP and MDP in far distant period (5.48 to 5.86; 22.17 to 22.76) than distant and near periods and was more evident in RCP 8.5 scenario. In case of SDP, no much difference in no. of gen. and gen. time was noted in RCP 2.6 and 4.5 scenarios and in NP period. An increase in the number of generations of H. armigera was predicted to occur even with the reduced duration of LD pigeonpea during FDP and DP for both 6.0 and 8.5 RCP scenarios and was more apparent with later scenario. Similar trend was with most of the locations and more evident at Ludhiana ___location. Whereas in MD pigeonpea, the change in the number of generations was found to be relatively low. Present findings indicated that the reduction of crop duration may offset the accelerated rate of reproduction of H. armigera in MD and SD pigeonpeas. Thus, the adoption of LDP has to factor in the higher need for pest management. The phenology of various ecological processes was potentially sensitive to climate change as influenced by temperature20 and advances the phenology of the H. armigera73 and similar trend was noted in the present findings.
Conclusions
Increase in mean atmospheric temperature can often influence insect generations by causing the reduction in gen. time. The present findings predict more no. of gen. of H. armigera at eleven pigeonpea growing locations during future TSPs across four RCPs. Similar trend is expected with altered crop duration of pigeonpea also. Pest scenario is not only driven by temperature but also other factors like elevated CO2, differential rainfall, crop phenology and different trophic interactions. The present findings captured the significance of increased temperature and its impact on pest scenario only and the availability of complete dataset on other parameters may give the clear-cut comprehension of impact of climate change.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
FAO. Climate change and food security: A Framework Document. FAO Inter-Departmental Working Group on Climate Change. Rome, Italy: Food and Agriculture Organization of the United Nations, p. 93 (2008)
IPCC: Summary for policymakers. In: Climate Change. The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. In Press. pp. 18–19 (2021).
Chaturvedi, R. K., Jaideep, J., Mathangi, J., Bala, G. & Ravindranath, N. H. Multi-model climate change projections for India under representative concentration pathways. Cur. Sci. 103, 791–802 (2012).
Krishnan, R. et al. Introduction to climate change over the Indian region. In Assessment of Climate Change over the Indian Region (eds Krishnan, R. et al.) (Springer, 2020). https://doi.org/10.1007/978-981-15-4327-2_1.
Rama Rao, C. A. et al. Atlas on Vulnerability of Indian Agriculture to Climate Change 116 (Central Research Institute for Dryland Agriculture, 2013).
Bale, J. S. et al. Herbivory in global climate change research: Direct effects of rising temperatures on insect herbivores. Glob. Change Biol. 8, 1–16 (2002).
Coley, P. D. Possible effects of climate change on plant/herbivore interactions in moist tropical forests. Clim. Change. 39, 455–472 (1998).
Kurz, W. A. et al. Mountain pine beetle and forest carbon feedback to climate change. Nature 452, 987–990 (2008).
Campbell, A., Frazer, B. D., Gilbert, N., Gutierrez, A. P. & Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 11, 431–438 (1974).
Hodgson, J. A. et al. Predicting insect phenology across space and time. Glob. Change Biol. 17, 1289–1300. https://doi.org/10.1111/j.1365-2486.2010.02308.x (2010).
Cayton, H. L., Haddad, N. M., Gross, K., Diamond, S. E. & Ries, L. Do growing degree days predict phenology across butterfly species?. Ecology 96(6), 1473–1479 (2015).
Bale, J. S. & Hayward, S. A. L. Insect overwintering in a changing climate. J. Exp. Biol. 213, 980–994 (2010).
Franco, A. M. A. et al. Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Global. Change Biol. 12(8), 1545–1553 (2006).
Maelzer, D. A. & Zalucki, M. P. Analysis of long-term light-trap data for Helicoverpa spp. (Lepidoptera: Noctuidae) in Australia: The effect of climate and crop host plants. Bull. Entomol. Res. 89(5), 455–463 (1999).
Mathukumalli, S. R. et al. Prediction of Helicoverpa armigera Hubner on pigeonpea during future climate change periods using MarkSim multimodel data. Agric. Forest Meteorol. 228, 130–138 (2016).
Gandhi, B. K. et al. Forecasting Helicoverpa armigera (Lepidoptera: Noctuidae) larval phenology in pigeonpea and chickpea crops using growing degree days. J. Agrometerol. 22(3), 320–331 (2020).
Porter, J. H., Parry, M. L. & Carter, T. R. The potential effects of climatic change on agricultural insect pests. Agric. For. Meteorol. 57, 221–240. https://doi.org/10.1016/0168-1923(91)90088-8 (1991).
Altermatt, F. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. Lond. B. 277, 1281–1287 (2010).
Fitt, G. P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 34, 17–52 (1989).
Bartomeus, I. et al. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Nat. Acd. Sci. 108(51), 20645–60649 (2011).
Juan, C. et al. Effect of temperature on the development, survival, and fecundity of Heliothis viriplaca (Lepidoptera: Noctuidae). J. Econ. Entomol. 111(4), 1940–1946 (2018).
Sharma, H. C., Srivastava, C. P., Durairaj, C. & Gowda, C. L. L. Pest management in grain legumes and climate change. In Climate Change and Management of Cool Season Grain Legume Crops (eds Yadav, S. & Redden, R.) 115–139 (Springer, 2010). https://doi.org/10.1007/978-90-481-3709-1_7.
Kunyanga, C., Imungi, J. & Vellingiri, V. Nutritional evaluation of indigenous foods with potential food-based solution to alleviate hunger and malnutrition in Kenya. J. App. Biosci. 67, 5277–5288 (2013).
FAO. World food and agriculture—Statistical Yearbook 2021. ISBN: 978–92–5–134332–6 (2021) https://doi.org/10.4060/cb4477en.
Directorate of economics and statistics, latest APY state data. Five year series data from 2015–16 to 2019–20: Food grains. Accessed on: 23–3–2022 (2021) https://eands.dacnet.nic.in/APY_96_To_06.html.
Gaur, P. M. et al. Chickpea Seed Production Manual 28 (International Crops Research Institute for the Semi-Arid Tropics, 2010).
Krishna Kumar, K. et al. Stimulated projections for summer monsoon climate over India by a high-resolution regional climatic model (PRECIS). Curr. Sci. 3, 312–326 (2011).
Tshiala, M. F., Botai, J. O. & Olwoch, J. M. Leafminer agromyzid pest distributionover Limpopo province under changing climate. Afr. J. Agric. Res. 7(48), 6515–6522 (2012).
Srinivasa Rao, M. et al. Model and scenario variations in predicted number of generations of Spodoptera litura fab. on peanut during future climate change scenario. PLoS ONE 10(2), e0116762. https://doi.org/10.1371/journal.pone.0116762 (2015).
Choudhary, J. S. et al. Potential changes in number of generations of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) on mango in India in response to climate change scenarios. J. Agrometeorol. 19(3), 200–206 (2017).
Taylor, K. E., Stouffer, R. J. & Meehl, G. A. An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 93, 7183–1792. https://doi.org/10.1175/BAMS-D-11-00094.1 (2012).
Stouffer, R. J., Taylor, K. E. & Meehl, G. A. CMIPF long term experiment design. CLIVAR Exch. 16, 5–7 (2011).
Islam, A. et al. Modeling the impact of climate change on irrigated corn production in the Central Great Plains. Agric. Water Manag. 110, 94–108 (2012).
Srinivasa Rao, M. et al. Pest scenario of Spodoptera litura (Fab.) on groundnut under representative concentration pathways (RCPs) based climate change scenarios. J. Therm. Biol. 94, 102749. https://doi.org/10.1016/j.jtherbio.2020.102749 (2020).
Islam, Adlul, Ahuja, L. R., Garcia, L. A., Ma, L. & Sasiendran, A. S. Modeling the effect of elevated CO2 and climate change on potential evapotranspiration in the semi-arid Central Great plains. Trans. Asabe. 55(6), 2135–2146 (2012).
Srinivasa Rao, M., Rama Rao, C. A., Raju, B. M. K., Manimanjari, D., Srinivas, K., Maheswari, M. & Ramamohan, I. ingen. National Initiative on Climate Resilient Agriculture (NICRA). ICAR-Central Research Institute for Dryland Agriculture (CRIDA), p. 15 (2014).
Choudhary, J. S. et al. Spatio and temporal variations in population abundance and distribution of peach fruit fly, Bactrocera zonata (Saunders) during future climate change scenarios based on temperature driven phenology model. Clim. Risk Manag. 32, 100277. https://doi.org/10.1016/j.crm.2021.100277 (2021).
Balaji Naik, B., Sreenivas, G., Indudhar Reddy, K. & Devkumar, D. Crop weather relationship studies in pigeonpea during monsoon season. In Virtual National Conf. on 'Strategic Reorientation for Climate Smart Agriculture' V-agmet. pp. 113–119 (2021).
Singh, G. et al. Thermal requirements, growth and yield of pigeonpea [Cajanus cajan (L.) Millsp.] genotypes under different agroclimatic zones of Punjab. J. Nat. Appl. Sci. 9, 2377–2384 (2017).
Rajbongshi, R. et al. Thermal indices in relation to crop phenology and seed yield of pigeon pea (Cajanus cajan L. Millsp.) grown in the north bank plains zone of Assam. MAUSAM 67(2), 397–404 (2016).
Purnamawati, H., Santoso, Y. S. & Wahyu, Y. Determination of Harvesting Time of Three Peanut Varieties Based on Heat Unit Accumulation. International Seminar on Sciences 2013 "Perspectives on Innovative Sciences". Bogor. 388–390 (2013).
Ziter, C., Robinson, E. A. & Jonathan, N. A. Climate change and voltinism in California insect pest species: sensitivity to ___location, scenario and climate model choice. Glob. Change Biol. 18, 2771–2780. https://doi.org/10.1111/j.1365-2486.2012.02748 (2012).
Zheng, F. S., Du, Y. Z., Wang, Z. J. & Xu, J. J. Effect of temperature on the demography of Galerucella birmanica (Coleoptera: Chrysomelidae). Insect Sci. 15, 375–380 (2008).
Arora, M., Goel, N. K. & Singh, P. Evaluation of temperature trends over India. Hydrol. Sci. J. 50, 81–83 (2005).
Rama Rao, C. A. et al. Risk and Vulnerability Assessment of Indian Agriculture to Climate Change 124 (ICAR—Central Research Institute for Dryland Agriculture, 2019).
Rajib, M. A., Rahman, M. M. & Mcbean, E. A. Global warming in Bangladesh perspective: Temperature projections up to 2100. Proc. of the Global Conf. on Global warming. Lisbon, pp. 43–48 (2011).
Riebeek, H. Global warming (2010) http://earthobservatory.nasa.gov/Features/GlobalWarming.
Meinshausen, M. et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Change. 109, 213. https://doi.org/10.1007/s10584-011-0156-z (2011).
Van Vuuren, D. P., Lucas, P. L. & Hilderink, H. Downscaling drivers of global environmental change: Enabling use of global SRES scenarios at the national and grid levels. Glob. Environ. Change 17, 114–130 (2007).
Tonnang, H. E. Z. et al. Advances in crop insect modeling methods—Towards a whole system approach. Ecol. Modell. 354, 88–103 (2017).
Mironidis, G. K. & Savopoulou-Soultani, M. Development, survivorship, and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under constant and alternating temperatures. Environ. Entomol. 37(1), 16–28. https://doi.org/10.1603/0046-225X(2008)37[16:DSAROH]2.0.CO;2 (2008).
Zidon, R., Tsueda, H., Morin, E. & Morin, S. Projecting pest population dynamics under global warming: The combined effect of inter- and intra-annual variations. Ecol. Appl. 26(4), 1198–1210 (2016).
Denlinger, D. & Yocum, G. Physiology of heat sensitivity. In Temperature Sensitivity in Insects and Application in Integrated Pest Management (eds Hallman, G. & Denlinger, D.) 7–57 (Westview Press, 1998).
Diffenbaugh, N. S., Christian, H. K., Michael, A. W. & Corinne, E. A. Global warming presents new challenges for maize pest management. Environ. Res. Lett. 3(4), 44007–9 (2008).
Chen, S., Shelby Fleischer, J., Micheal Saunders, C. & Matthew Thomas, B. The influence of diurnal temperature variation on degree-day accumulation and insect life history. PLoS ONE 10(3), e0120772 (2015).
Lastuka, Z. Climate change and its possible influence on the occurrence and importance of insect pests. Plant Protect. Sci. 45, S53–S62 (2008).
Tobin, P. C., Sudha, N. & Saunders, M. C. Phenology of grape berry moth (Lepidoptera: Tortricidae) in cultivated grape at selected geographic locations. Environ. Entomol. 32(2), 340–346 (2003).
Tobin, P. C., Sudha, N., Greg, L. & Saunders, M. C. Historical and projected interactions between climate change and insect voltinism in a multivoltine species. Glob. Change Biol. 14(5), 951–957. https://doi.org/10.1111/j.1365-2486.2008.01561.x (2008).
Kocmankova, E. et al. Estimating the impact of climate change on the occurrence of selected pests in the Central European region. Clim. Res. 44(22), 95–105 (2010).
Karolewski, P., Grzebyta, J., Oleksyn, J. & Giertych, M. J. Effects of temperature on larval survival rate and duration of development of Lymantria monacha (L.) on needles of Pinus silvestris (L.) and of L. dispar (L.) on leaves of Quercus robur (L.). Polish J. Ecol. 55(3), 595–600 (2007).
Ma, L., Wang, X., Liu, Y., Su, M.-Z. & Huang, G.-H. Temperature effects on development and fecundity of Brachmia macroscopa (Lepidoptera: Gelechiidae). PLoS ONE 12(3), e0173065. https://doi.org/10.1371/journal.pone.0173065 (2017).
Guo, T. T. et al. Impact of temperature on the growth and development of Athetis dissimilis (Lepidoptera: Notuidae). J. Econ. Entomol. 110(1), 274–281. https://doi.org/10.1093/jee/tow229 (2017).
Slade, E. & Roslin, T. Dung beetle species interactions and multifunctionality are affected by an experimentally warmed climate. Oikos https://doi.org/10.1111/oik.03207 (2016).
Morecroft, M. D. & Speakman, L. Biodiversity Climate Change Impacts Summary Report. Living with Environmental Change. (2015) ISBN 978–0–9928679–6–6.
Jhonson, A. M., Appelberg, G., Harding, S. & Barring, L. Spatio temporal impact of climate change on the activity and voltinism of the spruce bark beetle Ips typographus. Glob. Change Biol. 15, 489–499 (2009).
Choudhary, J. S. et al. Spatio-temporal temperature variations in MarkSim multimodel data and their impact on voltinism of fruit fly Bactrocera species on mango. Sci. Rep. 9, 9708. https://doi.org/10.1038/s41598-019-45801-z (2019).
Steinbauer, M. J., Kriticos, D. J., Lukacs, Z. & Clarke, A. R. Modelling a forest lepidopteran: Phenological plasticity determines voltinism which influences population dynamics. Forest Ecol. Manag. 198, 117–131 (2004).
Olfert, O. & Weiss, R. M. Impact of climate change on potential distributions and relative abundances of Oulema melanopus, Meligethes viridescens and Ceutorhyncus obstrictus in Canada. Agric. Ecosyst. Environ. 113, 295–301 (2006).
George, T., Bartholomew, D. P. & Singleton, P. W. Effect of temperature and maturity group on phenology of field grown nodulating and non-nodulating soybean isolines. Biotronics 19, 49–59 (1990).
Song, Y. L., Chen, D. L., Liu, Y. J. & Xu, Y. The influence of climate change on winter wheat during 2012–2100 under A2 and A1B scenarios in China. Adv. Clim. Change Res. 3(3), 138–146. https://doi.org/10.3724/SP.J.1248.2012.00138 (2012).
Parthasarathi, T., Velu, G. & Jeyakumar, P. Impact of crop heat units on growth and developmental physiology of future crop production: A review. Res. Rev. J. Crop. Sci. Technol. 2(1), 2319–3395. https://doi.org/10.37591/rrjocst.v2i1.2185 (2013).
Kesava Rao, A. V. R. et al. Assessing impacts of projected climate on pigeonpea crop at Gulbarga. J. Agrometeorol. 15(2), 32–37 (2013).
Huang, J. & Hao, H. Detecting mismatches in the phenology of cotton bollworm larvae and cotton flowering in response to climate change. Int. J. Biometeorol. 62, 1507–1520 (2018).
Acknowledgements
The work was supported by grants from the Indian Council of Agricultural Research (ICAR) in the form of National Innovations on Climate Resilient Agriculture (NICRA) and authors are thankful to project staff for their help in downloading the data and in use of ingen software.
Author information
Authors and Affiliations
Contributions
Conceived and designed the studies—M.S.R., C.A.R., B.M.K.; downloading, organizing climate change projection and generating multi-model ensemble climate change scenarios—A.V.M., A.I., K.S., I.S.; Analyzed the data—M.S.R., G.P., D.L.A.G., B.M.K., N.M.; Contributed reagents/materials/analysis tools—M.P., S.K.Y., S.B., V.K.S., S.K.C.; Wrote the paper—M.S.R., T.V.P., D.L.A.G., C.A.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srinivasa Rao, M., Rama Rao, C.A., Raju, B.M.K. et al. Pest scenario of Helicoverpa armigera (Hub.) on pigeonpea during future climate change periods under RCP based projections in India. Sci Rep 13, 6788 (2023). https://doi.org/10.1038/s41598-023-32188-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32188-1
This article is cited by
-
Host range expansion of Helicoverpa armigera to apple orchards in the Himalayan region
Scientific Reports (2025)
-
Enhancing the bioconversion rate and end products of black soldier fly (BSF) treatment – A comprehensive review
Environment, Development and Sustainability (2024)