Abstract
The present study investigated the role of a urethral support system to maintain urinary continence after robot-assisted radical prostatectomy (RARP), with a focus on pelvic floor muscles, such as the puboperinealis muscle (PPM) and rectourethralis muscle (RUM). Finally, 323 patients who underwent RARP were analyzed in this study. All patients performed a one-hour pad test 1, 3, 6, 9, and 12 months after RARP to assess urinary incontinence and MRI before and 9 months after RARP to evaluate the pelvic anatomical structure. The preoperative cross-sectional area of PPM (2.21 ± 0.69 cm2) was significantly reduced by 19% after RARP (1.79 ± 0.60 cm2; p < 0.01). Positive correlations were observed between the amount of urinary leakage according to the 1-h pad test 1, 3, 6, 9, and 12 months after RARP and the change in the cross-sectional area of PPM by RARP (p < 0.01, < 0.001, < 0.001, < 0.001, and < 0.001, respectively). A positive correlation was also noted between the amount of urinary leakage 6 and 12 months after RARP and the preoperative RUM diameter (p < 0.05). The amount of urinary leakage 1, 3, 6, 9, and 12 months after RARP negatively correlated with the change in the antero-posterior diameter of the membranous urethra (MU diameter) from the static to dynamic phases during the Valsalva maneuver by cine MRI. Furthermore, the change in the MU diameter negatively correlated with the change in the cross-sectional area of PPM (p < 0.05). PPM and RUM play significant roles as a supportive mechanism to maintain urinary continence by functioning as a urethral support.
Similar content being viewed by others
Introduction
Although robot-assisted radical prostatectomy (RARP) has recently become the gold standard treatment for localized prostate cancer, postoperative urinary incontinence is still a complication that decreases the quality of life (QOL) of patients1. Preoperative risk factors for urinary incontinence after prostatectomy include an advanced age, higher BMI, larger prostate volume, severe preoperative lower urinary tract symptoms, and a history of prostatectomy for benign prostatic hyperplasia2. Preoperative anatomical variables measured on magnetic resonance imaging (MRI), such as the length of the membranous urethra (MU) and periurethral supporting structures, have also been associated with urinary incontinence after RARP3. However, the mechanisms underlying the development of urinary incontinence have not yet been examined in detail.
The levator ani muscle is an important anatomical and functional structure for maintaining urinary continence, and its preservation improves urinary continence4,5,6,7. It comprises the puborectalis, pubococcygeus, and iliococcygeus muscles. The puboperinialis muscle (PPM) is the medial part of the puborectalis, arising from the posterior surface of the pubis on each side and passing posteriorly beside the urethra8. The rectourethralis muscle (RUM) is located at the interface between PPM and the rectum. Right and left PPM muscle edges are located close together in an area between the urethra and rectum, immediately lateral to RUM. Since these structures may form a strong sling and influence the stabilization of the MU, we considered the preoperative status of and surgical damage to these structure to potentially be important factors contributing to urinary incontinence after RARP. In the present study, we conducted an anatomical analysis of the structures around the urethra using MRI before and after RARP, and discussed the mechanisms underlying postoperative urinary incontinence.
Methods
Patients
We conducted a prospective observational cohort study. Patients who underwent RARP in our hospital between 2014 and 2019 were included in the present study. Patients with organ-confined prostate cancer were considered to be candidates for RARP and were included in the study. Inclusion criteria were (1) clinically localized prostate cancer, (2) age < 75 years, and (3) an Eastern Cooperative Oncology Group performance status of 0. During this period, 533 patients who met the inclusion criteria underwent RARP. The Ethics Committee of Fukushima Medical University Hospital approved this study (approval number: 2614) and all patients provided written informed consent. This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Surgical technique
RARP was performed using a four-arm da Vinci Si™ surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) by the transperitoneal posterior approach reported by Guillonneau et al. in conventional laparoscopic procedures9. This approach is initiated with transverse peritoneotomy between the bladder and rectum, followed by retrovesical dissection. After dissection of the bladder neck, the prostatic vascular pedicles are ligated and the prostate is removed. Preservation of the neurovascular bundles is recommended to patients with low or favorable intermediate risk prostate cancer. Posterior reconstruction was performed on all cases in the present study. Vesicourethral anastomosis was conducted using the Van Velthoven procedure. RARP was performed or supervised by a single surgeon (Y.K.).
One-hour pad test to evaluate urinary incontinence
Urinary incontinence was evaluated by the one-hour pad test recommended by the International Continence Society10. We considered it important to perform the assessment of urinary incontinence objectively because the anatomical analysis was objectively conducted by MRI. Patients performed the one-hour pad test 1, 3, 6, 9, and 12 months after RARP to evaluate the amount of urinary leakage. Pad test information was collected by urology nurses.
MRI examinations and imaging techniques
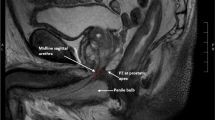
MRI was performed before and 9 months after RARP in all cases because the effects of postoperative inflammation in muscles needed to be excluded. Static and dynamic MRI were performed using a 1.5 T MR scanner (Magnetom Trio; Siemens Healthcare, Erlangen, Germany). Dynamic MRI consisted of a T2-weighted, single slice True Fast Imaging with Steady-state Procession (TrueFISP) sequence in the sagittal, coronal, and transverse planes. The cross-sectional area of PPM and left–right (transverse) diameter of RUM (RUM diameter) were measured on T2-weighted fast spin echo (FSE) sequences. The left and right cross-sectional areas of PPM at the height of RUM on the dorsal side of the urethra were calculated using coronal sections, and the sum of the left and right cross-sectional areas was defined as the cross-sectional area of PPM (Fig. 1A). The tissue between the urethra and rectum was considered to be RUM in the transverse plane, and the left–right diameter was measured (Fig. 1B).
Anatomical analysis of the structure around the urethra using MRI before and after RARP. (A) The left and right cross-sectional areas of PPM (dotted circles) at the height of RUM on the dorsal side of the urethra were calculated using coronal sections. (B) The tissue between the urethra and rectum was considered to be RUM (double arrow) in the transverse plane, and the left–right diameter was measured. PPM puboperinealis muscle, RUM rectourethral muscle, U urethra, R rectum.
Patients also underwent cine MRI to evaluate the antero-posterior MU diameter with a full bladder 9 months after RARP (Fig. 2). Cine MRI in static and dynamic phases was conducted during the Valsalva maneuver and T2-weighed sagittal sections were obtained. Cine acquisition was performed over a 30-s time period during the static and dynamic phases. A urologist provided patients with guidance in the examination room during MRI scanning. Changes in the MU diameter from the static (Fig. 2A) to dynamic phases (Fig. 2B) during the Valsalva maneuver by cine MRI were calculated.
Statistical analysis
All data were analyzed using SPSS statistical package version 24.0 (SPSS Inc., Chicago, IL, USA). The significance of differences was considered when p < 0.05. Parametric comparisons between before and after RARP were performed using the paired t-test. Simple and multiple regression models were used to examine the relationship between two or more variables. Variables with p < 0.05 in the simple regression model were included in the multiple regression model.
Results
Finally, 323 patients (mean age, 67.2 ± 6.0 years; age range, 48–76 years) were enrolled in the present study (Fig. 3). The baseline and perioperative characteristics of patients are shown in Table 1. The average amounts of urinary leakage in the one-hour pad test obtained by weighing the pads were 5.4, 46,1, 22.1, 18.5, 14.3, and 13.0 ml before and 1, 3, 6, 9, and 12 months after RARP, respectively. When urinary continence was defined as total urine leakage of < 2 g in the one-hour pad test10, continence rates were 85.1, 44.6, 75.5, 75.5, 76.5, and 78.0% before and 1, 3, 6, 9, and 12 months after RARP, respectively (Table 2).
We compared the cross-sectional area of PPM and RUM diameter using MRI before and after RARP (Table 3). The preoperative cross-sectional area of PPM (2.21 ± 0.69 cm2) was significantly reduced by 19% after RARP (1.79 ± 0.60 cm2; p < 0.01). No significant change was observed in the RUM diameter between before and after RARP (1.33 ± 0.28 and 1.36 ± 0.29 cm, respectively). There was no significant correlation between the change in the cross-sectional area of PPM and preoperative anti-androgen administration in the simple regression model (p = 0.49). There was also no significant correlation between the change in the cross-sectional area of PPM and neurovascular bundle (NVB) preservation in the simple regression model (p = 0.17). In addition, this correlation was not found regardless of unilateral or bilateral neurovascular bundle (NVB) preservation (p = 0.26 and 0.13, respectively).
We performed a correlation analysis between the amount of urinary leakage according to the one-hour pad test after RARP and BMI, neurovascular bundle (NVB) preservation during RARP or prostate volume, which are considered to be factors which may affect urinary continence11. In the simple regression model, the amount of urinary leakage 1 or 3 months after RARP positively correlated with BMI (p < 0.01 and < 0.05, respectively). The amount of urinary leakage 1, 6, or 9 months after RARP negatively correlated with NVB preservation during RARP (p < 0.05, respectively). The amount of urinary leakage 6 months after RARP positively correlated with prostate volume (p < 0.05; Table 4). The multiple regression model revealed a correlation between the amount of urinary leakage 1 months after RARP and BMI or NVB preservation (p < 0.01 and p = 0.05, respectively; Table 5).
We performed a correlation analysis between the amount of urinary leakage according to the one-hour pad test after RARP and MRI parameters, such as the cross-sectional area of PPM or RUM diameter (Table 4). In the simple regression model, the amount of urinary leakage 1, 3, 6, 9, or 12 months after RARP negatively correlated with the postoperative PPM cross-sectional area (p < 0.05, < 0.001, < 0.001, < 0.001, and < 0.001, respectively). The amount of urinary leakage 1, 3, 6, 9, or 12 months after RARP positively correlated with the change in the cross-sectional area of PPM by RARP (p < 0.001) and the postoperative RUM diameter (p < 0.001, < 0.001, < 0.001, < 0.01, and < 0.001, respectively). Furthermore, positive correlations were observed between the amount of urinary leakage 3, 6, 9, or 12 months after RARP and the preoperative RUM diameter (p < 0.01, < 0.01, < 0.05, and < 0.001, respectively) as well as between the amount of urinary leakage 1 month after RARP and the change in the RUM diameter by RARP (p < 0.05).
The multiple regression model revealed a negative correlation between the amount of urinary leakage 3, 9, or 12 months after RARP and the postoperative cross-sectional area of PPM (p < 0.01; Table 5). The amount of urinary leakage 1, 3, 6, 9, or 12 months after RARP positively correlated with the change in the cross-sectional area of PPM by RARP (p < 0.01, < 0.001, < 0.001, < 0.001, and < 0.001, respectively). These results indicate that a decrease in the cross-sectional area of PPM by RARP induced urinary incontinence after RARP. Representative distribution chart to show the correlation between the amount of urine leakage 12 months after RARP and the change in the cross-sectional area of PPM is shown in Fig. 4. A positive correlation was noted between the amount of urinary leakage 6 or 12 months after RARP and the preoperative RUM diameter (p < 0.05). Furthermore, the amount of urinary leakage 1 month after RARP positively correlated with the postoperative RUM diameter (p < 0.01). These results indicate that a large RUM diameter, which reflects a large distance between the left and right PPM, is a risk factor for the development of urinary incontinence after RARP.
A correlation analysis of the amount of urinary leakage according to the one-hour pad test after RARP and the change in the MU diameter from the static to dynamic phases during the Valsalva maneuver was also performed (Table 6). The simple regression model revealed a negative correlation between the amount of urinary leakage 1, 3, 6, 9, or 12 months after RARP and the change in the MU diameter (p < 0.001). The multiple regression model also showed that the amount of urinary leakage 1, 3, 6, 9, or 12 months after RARP negatively correlated with the change in the MU diameter (p < 0.001). Although the amount of urinary leakage 1, 3, 6, 9 or 12 months after RARP negatively correlated with preoperative membranous urethral length (p < 0.05, p < 0.05, p < 0.05, p < 0.05 and p < 0.01, respectively) in the simple model, this correlation did not find in the multiple regression model (p = 0.78, p = 0.27, p = 0.33, p = 0.07 and p = 0.30, respectively). It did not correlate with postoperative membranous urethral length in the simple regression model, (p = 0.11, 0.13, 0.33, 0.30 and 0.30, respectively).
We also examined the relationship between the change in the MU diameter from the static to dynamic phases during the Valsalva maneuver and the cross-sectional area of PPM or the RUM diameter (Table 7). The simple regression model showed that the change in the MU diameter from the static to dynamic phases negatively correlated with the change in the cross-sectional area of PPM (p < 0.01) and the preoperative (p < 0.05) or postoperative RUM diameter (p < 0.05). The multiple regression model revealed a negative correlation between the change in the MU diameter and the change in the cross-sectional area of PPM (p < 0.05).
Discussion
The present study investigated the relationship between the anatomical pelvic structure around the urethra by MRI and urinary incontinence after RARP, and suggested that a decrease in the postoperative cross-sectional area of PPM after RARP and a large RUM diameter affected the postoperative urethral support system and consequently induced urinary incontinence.
The levator ani muscle plays a significant role in maintaining urinary continence and its preservation improves urinary continence4,5,6,7. Retzius-sparing RARP, which preserves the levator ani and puboprostatic ligaments, has a higher continence rate than standard RARP12,13,14,15. PPM, which has components of the levator ani muscle, is responsible for the quick stop phenomenon of urination in males, and, thus, the weakening of PPM by transection, traction injury, or denervation may affect urinary continence after radical prostatectomy16. In other words, preserving the integrity and innervation of PPM is crucial for the maintenance of urinary continence16. The right and left PPM course posteriorly from the pubis to flank the urethra, and converge in the midline behind the urethra, anterior to the rectum16. RUM occupies a space between the right and left PPM17. The left and right PPM edges are consistently located in close proximity in an area immediately lateral to RUM17, suggesting that PPM and RUM function as a urethral sling to maintain urinary continence17,18 (Fig. 5A). This urethral support mechanism by the connection between PPM and RUM is important for urinary continence in women19,20. In women, urethral hypermobility and a low urethral closure pressure due to damage to the urethral support mechanism causes stress urinary incontinence21. Mid-urethral sling surgery has become the gold standard treatment for stress urinary incontinence in women22, and the effectiveness of sling surgery depends on adequate postoperative urethral mobility and the urethral closure pressure23. The loss of the prostate may cause instability in the urethral support mechanism in men after RARP, as in women11. Therefore, we considered the urethral support mechanism by the connection between PPM and RUM to be important for urinary continence in men after RARP, and that the low ability or weak strength of inherent PPM and RUM and surgical damage to PPM and RUM after RARP may induce urinary incontinence after RARP.
Anatomical structure around the urethra before and after RARP and the mechanism underlying postoperative urinary incontinence. (A) PPM and RUM function as a urethral sling to maintain urinary continence. (B) Surgical interventions during RARP damage and may lead to the atrophy of PPM and the loss of its function as a urethral sling. (C) The longer preoperative distance between left and right PPM indicate looser function as a urethral sling, and these patients may originally have had weak urethral support. PPM puboperinealis muscle, RUM rectourethral muscle, U urethra, PB; pubic body, PPL puboprostatic ligament, DVC dorsal vein complex.
We herein focused on morphological changes in PPM and RUM before and after RARP. Sohn et al. examined the anatomy of men preoperatively with MRI, and found that men with a thicker puborectalis muscle at the anorectal angle regained continence earlier after prostatectomy24. We demonstrated that the cross-sectional area of PPM decreased by 19% after RARP. Moreover, the decrease in the cross-sectional area of PPM after RARP correlated with postoperative urinary incontinence and induced inadequate urethral compression. These results suggest that surgical interventions during RARP may damage PPM itself and nerve and/or blood supply around PPM, leading to the atrophy of PPM, the loss of its function as a urethral sling (Fig. 5B), and, as a result, urinary incontinence after RARP. Therefore, to avoid postoperative urinary incontinence by loss of PPM muscle volume, it may be important to minimize damage to nerves and blood supply by refraining from using electrocautery around PPM and minimize mechanical damage to PPM itself from surgical manipulation during RARP. However, NVB preservation may not be able to avoid loss of PPM muscle volume.
RUM also may play a significant role as a urethral support system by its connection with left and right PPM to maintain urinary continence. Individual variations have been reported in the development of RUM17,18. Soga et al. showed that RUM influenced MU stabilization with the rhabdosphincter, and also that it was one of the important structures forming a urethral sphincteric complex18. In the present study, pre- and postoperative RUM diameters positively correlated with urinary incontinence; however, the change in the MU diameter from the static to dynamic phases during the Valsalva maneuver did not correlate with the pre- or postoperative RUM diameter. Therefore, although the longer pre- and postoperative distances between left and right PPM were not directly proven to indicate weaker pre- and postoperative function as a urethral sling in the present study, these patients may originally have had weak urethral support from before RARP, which may easily induce postoperative urinary incontinence (Fig. 5C). Therefore, the preoperative measurement of RUM by MRI may predict urinary incontinence after RARP.
Preoperative pelvic floor muscle training (PFMT) enhances the postoperative function of pelvic muscles, including the levator ani muscle, reduces urinary incontinence after prostatectomy, and improves QOL outcomes related to urinary continence25,26,27. PFMT with a strength training protocol to hypertrophy the levator ani muscle may yield higher continence rates25. PFMT recuperates strength and lost tone in the sling mechanism after prostatectomy, and potentially enhances the pelvic floor support of vesicourethral anastomosis and the bladder base by strengthening PPM16. Additionally, the development of surgical procedures to reinforce the urethral sling system may promote urinary continence after RARP. Vladimir et al. reported that the advanced reconstruction of the vesicourethral support technique using the levator ani muscle attenuated urinary incontinence after RARP28. Previous studies demonstrated the effectiveness of urethral sling or bladder neck suspension techniques during RARP for urinary continence29,30,31. We showed that bladder neck sling suspension during RARP promoted the early return of urinary continence29. Cestari et al. found that a proper sling procedure during RARP allowed for the restoration of the sphincteric apparatus capability to its presurgical status; however, its capability decreased after prostate removal and subsequent ureterovesical anastomosis30. The efficacy of the male sling to treat post-prostatectomy incontinence has been reported32. Our anatomical data on the relationship between pelvic muscles and urinary incontinence after RARP appear to support the effectiveness of PFMT, surgical procedures, such as the urethral sling or bladder neck suspension techniques, and postoperative male sling procedures to maintain urinary continence after RARP.
There are several limitations that need to be addressed. First, this was a single-center analysis. Furthermore, postoperative MRI was only performed 9 months after RARP in all cases because the effects of postoperative inflammation in muscles need to be excluded. Second, we conducted pre- and postoperative anatomical analyses of the structure around the urethra using MRI, but not a functional analysis, such as the urethral pressure profile. Third, Valsalva effort is variable and subjective. The degree of dynamic movement is influenced by this effort. Therefore, further detailed studies are warranted.
Conclusions
PPM and RUM play significant roles in the supportive mechanism to maintain urinary continence as a urethral support. Surgical interventions during RARP lead to the atrophy of PPM, the loss of its function as a urethral sling, and, ultimately, urinary incontinence after RARP. The preoperative measurement of PPM by MRI may predict urinary incontinence after RARP. The further development of surgical procedures to prevent or minimize damage to and reinforce this urethral support system may contribute to the mitigation of urinary incontinence after RARP.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RARP:
-
Robot-assisted radical prostatectomy
- PPM:
-
Puboperinealis muscle
- RUM:
-
Rectourethral muscle
- UI:
-
Urinary incontinence
- MU:
-
Membranous urethra
References
Litwin, M. S. et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 273(2), 129–135 (1995).
Qin, H. et al. Predictors for immediate recovery of continence following Retzius-sparing robot-assisted radical prostatectomy: A case–control study. Int. Urol. Nephrol. 51(5), 825–830 (2019).
Mungovan, S. F. et al. Preoperative membranous urethral length measurement and continence recovery following radical prostatectomy: A systematic review and meta-analysis. Eur. Urol. 71(3), 368–378 (2017).
Laucirica, O. et al. Complete puborectalis, puboperinealis muscle and urethral rhabdomyosphincter preservation in laparoscopic radical prostatectomy: Anatomical landmarks to achieve early urinary continence. Int. J. Urol. 27(6), 525–536 (2020).
Song, C. et al. Relationship between the integrity of the pelvic floor muscles and early recovery of continence after radical prostatectomy. J. Urol. 178(1), 208–211 (2007).
Neumann, P. B. & O’Callaghan, M. The role of preoperative puborectal muscle function assessed by transperineal ultrasound in urinary continence outcomes at 3, 6, and 12 months after robotic-assisted radical prostatectomy. Int. Neurourol. J. 22(2), 114–122 (2018).
Muñoz-Calahorro, C. et al. Anatomical predictors of long-term urinary incontinence after robot-assisted laparoscopic prostatectomy: A systematic review. Neurourol. Urodyn. 40(5), 1089–1097 (2021).
Walz, J. et al. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: An update. Eur. Urol. 70(2), 301–311 (2016).
Guillonneau, B. & Vallancien, G. Laparoscopic radical prostatectomy: The Montsouris experience. J. Urol. 163(2), 418–422 (2000).
Abrams, P. et al. Standardisation sub-committee of the international continence society. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 21(2), 167–178 (2002).
Kojima, Y. et al. Urinary incontinence after robot-assisted radical prostatectomy: Pathophysiology and intraoperative techniques to improve surgical outcome. Int. J. Urol. 20(11), 1052–1063 (2013).
Lee, J. et al. Retzius sparing robot-assisted radical prostatectomy conveys early regain of continence over conventional robot-assisted radical prostatectomy: A propensity score matched analysis of 1863 patients. J. Urol. 203(1), 137–144 (2020).
Dalela, D. et al. A pragmatic randomized controlled trial examining the impact of the Retzius-sparing approach on early urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 72(5), 677–685 (2017).
Egan, J. et al. Retzius-sparing robot-assisted radical prostatectomy leads to durable improvement in urinary function and quality of life versus standard robot-assisted radical prostatectomy without compromise on oncologic efficacy: Single-surgeon series and step-by-step guide. Eur. Urol. 79(6), 839–857 (2020).
Checcucci, E. et al. Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: A systematic review and analysis of comparative outcomes. BJU Int. 125(1), 8–16 (2020).
Myers, R. P. et al. Puboperineales: Muscular boundaries of the male urogenital hiatus in 3D from magnetic resonance imaging. J. Urol. 164(4), 1412–1415 (2000).
Matsubara, A. et al. Topographic anatomy of the male perineal structures with special reference to perineal approaches for radical prostatectomy. Int. J. Urol. 10(3), 141–148 (2003).
Soga, H. et al. Topographical relationship between urethral rhabdosphincter and rectourethralis muscle: A better understanding of the apical dissection and the posterior stitches in radical prostatectomy. Int. J. Urol. 15(8), 729–732 (2008).
Wilson, P. D. et al. Posterior pubo-urethral ligaments in normal and genuine stress incontinent women. J. Urol. 130(4), 802–805 (1983).
Petros, P. E. & Ulmsten, U. Urethral pressure increase on effort originates from within the urethra, and continence from musculovaginal closure. Neurourol. Urodyn. 14(4), 337–346 (1995).
Petros, P. E. & Ulmsten, U. I. An integral theory of female urinary incontinence: Experimental and clinical considerations. Acta. Obstet. Gynecol. Scand. Suppl. 153, 7–31 (1990).
Ulmsten, U. et al. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int. Urogynecol. J. Pelvic. Floor. Dysfunct. 7(2), 81–85 (1996).
Viereck, V. et al. Role of bladder neck mobility and urethral closure pressure in predicting outcome of tension-free vaginal tape (TVT) procedure. Ultrasound. Obstet. Gynecol. 28(2), 214–220 (2006).
Sohn, D. W. et al. Pelvic floor musculature and bladder neck changes before and after continence recovery after radical prostatectomy in pelvic MRI. J. Magn. Reson. Imaging. 39(6), 1431–1435 (2014).
Centemero, A. et al. Preoperative pelvic floor muscle exercise for early continence after radical prostatectomy: A randomised controlled study. Eur. Urol. 57(6), 1039–1043 (2010).
Milios, J. E., Ackland, T. R. & Green, D. J. Pelvic floor muscle training in radical prostatectomy: A randomized controlled trial of the impacts on pelvic floor muscle function and urinary incontinence. BMC Urol. 19(1), 116 (2019).
Chang, J. I. et al. Preoperative pelvic floor muscle exercise and postprostatectomy incontinence: A systematic review and meta-analysis. Eur. Urol. 69(3), 460–467 (2016).
Student, V. Jr. et al. Advanced reconstruction of vesicourethral support (ARVUS) during robot-assisted radical prostatectomy: One-year functional outcomes in a two-group randomised controlled trial. Eur. Urol. 71(5), 822–830 (2017).
Kojima, Y. et al. Bladder neck sling suspension during robot-assisted radical prostatectomy to improve early return of urinary continence: A comparative analysis. Urology 83(3), 632–639 (2014).
Cestari, A. et al. Intraoperative retrograde perfusion sphincterometry to evaluate efficacy of autologous vas deferens 6-branch suburethral sling to properly restore sphincteric apparatus during robot-assisted radical prostatectomy. J. Endourol. 31(9), 878–885 (2017).
Canvasser, N. E. et al. Posterior urethral suspension during robot-assisted radical prostatectomy improves early urinary control: A prospective cohort study. J. Endourol. 30(10), 1089–1094 (2016).
Chen, Y. C., Lin, P. H., Jou, Y. Y. & Lin, V. C. Surgical treatment for urinary incontinence after prostatectomy: A meta-analysis and systematic review. PLoS ONE 12(5), e0130867 (2017).
Acknowledgements
We gratefully acknowledge the contributions of the members of the Department of Urology, Fukushima Medical University of School of Medicine.
Author information
Authors and Affiliations
Contributions
M.K. conceptualized the research and manuscript together with S.O., M.U. and Y.K. S.M., R.T., A.O., K.M., R.H.-T., S.H., Y.S. and H.A. had discussions regarding the methodology. The analysis was performed by M.K. and was supervised by Y.K. Data was collected and curated by M.K., S.H. and J.H. The manuscript was written by M.K. under supervision of Y.K. M.K. prepared all figures and tables. All authors reviewed the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kataoka, M., Meguro, S., Tanji, R. et al. Role of puboperinealis and rectourethralis muscles as a urethral support system to maintain urinary continence after robot-assisted radical prostatectomy. Sci Rep 13, 14126 (2023). https://doi.org/10.1038/s41598-023-41083-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41083-8
This article is cited by
-
Cystopexy following anterior-approach robot-assisted radical prostatectomy enhances early continence recovery
Journal of Robotic Surgery (2025)
-
Operative Techniken zur Verbesserung der Kontinenz nach laparoskopischer roboterassistierter Prostatektomie anhand von videoanatomischen Strukturen – eine Übersichtsarbeit
Die Urologie (2025)