Abstract
To compare diffusion-kurtosis imaging (DKI) and diffusion-weighted imaging (DWI) parameters of single-shot echo-planar imaging (ss-EPI) and readout-segmented echo-planar imaging (rs-EPI) in the differentiation of luminal vs. non-luminal breast cancer using histogram analysis. One hundred and sixty women with 111 luminal and 49 non-luminal breast lesions were enrolled in this study. All patients underwent ss-EPI and rs-EPI sequences on a 3.0T scanner. Histogram metrics were derived from mean kurtosis (MK), mean diffusion (MD) and the apparent diffusion coefficient (ADC) maps of two DWI sequences respectively. Student’s t test or Mann–Whitney U test was performed for differentiating luminal subtype from non-luminal subtype. The ROC curves were plotted for evaluating the diagnostic performances of significant histogram metrics in differentiating luminal from non-luminal BC. The histogram metrics MKmean, MK50th, MK75th of luminal BC were significantly higher than those of non-luminal BC for both two DWI sequences (all P<0.05). Histogram metrics from rs-EPI sequence had better diagnostic performance in differentiating luminal from non-Luminal breast cancer compared to those from ss-EPI sequence. MK75th derived from rs-EPI sequence was the most valuable single metric (AUC, 0.891; sensitivity, 78.4%; specificity, 87.8%) for differentiating luminal from non-luminal BC among all the histogram metrics. Histogram metrics of MK derived from rs-EPI yielded better diagnostic performance for distinguishing luminal from non-luminal BC than that from ss-EPI. MK75th was the most valuable metric among all the histogram metrics.
Similar content being viewed by others
Introduction
Breast cancer (BC), a disease of high heterogeneity, is divided into four molecular subtypes (luminal A, luminal B, human epidermal growth factor receptor 2- (HER2-) enriched, and triple negative) according to immunohistochemistry (IHC) markers, such as receptor expression of estrogen (ER) and progesterone (PR), HER-2 expression status, and Ki67 index 1. These subtypes make up an uneven proportion of breast cancer patients and behave differently in therapeutic response, metastatic patterns and prognosis2,3. Generally, luminal subtype (luminal A and B) accounts for the largest proportion (around 70%) of all breast cancers4. Luminal subtype is hormone-receptor positive thus responds best to endocrine therapy (ET) (luminal A) or combined chemotherapy and ET (luminal B)5,6,7. Non-luminal subtype including HER2-enriched and triple negative breast cancer is more aggressive and more likely to suffer from more frequent recurrence and metastasis as well as poorer prognosis when compared with luminal subtype8. HER2-enriched type benefits from targeted antibody therapy such as trastuzumab (Herceptin). Triple-negative breast cancer which is negative for ER/PR/HER2, shows no response to endocrine therapy or trastuzumab (Herceptin) and conventional cytostatic chemotherapy remains the only therapeutic option9.
The heterogeneity of the BC subtypes (luminal vs. non luminal) would affect clinical efforts to manage treatment measures and anticipate risks10. Therefore, there is an urgent need for accurate preoperative differentiation between luminal and non-luminal subtypes. The BC subtype classification before surgery is mainly based on breast biopsy by now. However, there exists a discordance between the biopsies and postoperative pathological diagnoses due to sampling bias11. Currently, MR imaging such as contrast-enhanced imaging and diffusion-weighted imaging (DWI) has been proven to provide the entire tumor information about the molecular subtype characterization12,13.
DWI as a non-contrast MRI technique can provide additional information on tumor characteristics and heterogeneity by calculating quantitative parameters derived from different quantitative models, such as a mono-exponential model and diffusion-kurtosis imaging (DKI)14. Breast DWI images are usually acquired with single-shot echo-planar imaging (ss-EPI) or readout-segmented echo-planar imaging (rs-EPI)15. While the motion-insensitivity and speed of ss-EPI currently preserve its role as the standard clinical DWI technique for breast, it suffers from susceptibility artifacts, geometric distortions, signal-intensity dropout, and T2* induced blurring, primarily due to slow traversal through k-space along the phase-encoding direction16,17. The rs-EPI sequence provides an alternative method for improved speed, resolution, or image quality by sampling a subset of k-space points in the readout direction at each shot as a means of reducing distortion and blurring at the expense of longer scan time and some navigator-correction18,19. However, breast DWI recommended technique has not yet been fully established in clinical application. In recent years, certain studies have attempted to figure out the correlations between DWI or DKI parameters and tumor subtype7,20,21,22. Despite several attempts to standardize imaging markers, there is still diversity in DWI scanning techniques and DWI models, which most likely contributed to DWI assessment's low capacity to distinguish between BC molecular subtypes.
Recent studies have suggested that histogram analysis may be able to more accurately assess the intratumor heterogeneity and aggressiveness of BC23,24. The aim of our research was to compare the histogram metrics of rs-EPI and ss-EPI sequences for differentiating luminal from non-Luminal BC.
Materials and methods
Patients
This prospective, unicentric study was approved by the local Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and written informed consent was obtained from all participating subjects. It complies with the ethical principles of the Declaration of Helsinki. From December 2018 to December 2019, 194 women who met the inclusion criteria were selected for breast MRI in our institution database. The following inclusion criteria were used: (1) suspicious lesions that diagnosed as Breast Imaging Reporting and Data System (BI-RADS) category 4 or 5 at mammography or ultrasonography; (2) complete breast MRI scanning; (3) underwent surgery for lesion excision within one week following the MRI.
The exclusion criteria consisted of the following: (1) severe MRI susceptibility or motion artifacts (n = 5); (2) non-mass like lesions (n=11); (3) the largest size of lesions< 1cm (n = 6); (4) biopsy or breast-related treatment such as radiotherapy and chemotherapy for breast lesion before MRI (n = 9); (5) no pathological findings reported invasive ductal carcinoma (IDC) (n = 3).
MR imaging
Breast MR imaging was performed using a 3.0-tesla MR scanner (Skyra, Siemens Healthineers, Erlangen, Germany) with a breast bilateral 16-channel phased-array coil. Images were obtained using the following sequences:
-
Axial T2-weighted MR imaging (T2WI) with fat saturation: repetition time (TR) = 3700ms, echo time (TE) = 101ms, field of view (FOV) = 320 x 320 mm2, matrix = 224 × 320, slice thickness = 4.0 mm, voxel size = 1.5 × 1.5 × 5 mm, acquisition time (TA) = 2min6sec;
-
Axial ss-EPI with fat saturation: TR = 5000 ms, TE = 96 ms, FOV = 169 × 280 mm2, matrix = 114 × 188, slice thickness = 5.0 mm, voxel size = 1.5 × 1.5 × 5 mm, averages = 5; TA =4 min 35 sec, 4 different b factors (0, 50, 1000, and 2000 s/mm2);
-
Axial rs-EPI with fat saturation: TR = 5000 ms, TE = 68 ms, FOV = 169 × 280 mm2, matrix = 114 × 188, slice thickness = 5.0 mm, voxel size = 1.5 × 1.5 × 5 mm, averages = 1, readout segments = 5, TA = 4 min 27 sec, 4 different b factors (0, 50, 1000, and 2000 s/mm2);
-
Axial T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI): TR = 5.40ms, TE = 2.46ms, flip angle = 10°, FOV = 320 x 320 mm2, matrix = 243 × 320, slice thickness = 1.5mm, temporal resolution = 11.2s/phase, TA = 5min57sec. The contrast material (Omniscan, GE Healthcare, Milwaukee, WI, USA) was administrated intravenously using an automated injector with a dose of 0.1mmol/kg of body weight at an rate of 2.5 ml/ sec, which was followed by a 20-ml saline flush.
Image analysis
All DWI imaging data were postprocessed using the prototype software, Body Diffusion Toolbox (version 0.2.2, Siemens Healthcare)25. Two radiologists (Z.C.A. and H.Y.Q. with 5 and 9 years of experience in breast MR imaging, respectively) who recognized the IDC diagnosis but were blinded to DWI image sequence information and the detailed histological results, reviewed the MR images. The processing workflow of DWI images consisted of the following three steps:
-
a.
Images loading. The DWI images were loaded to the software.
-
b.
Regions of interest (ROI) drawing. The largest lesion (avoiding obvious calcifications, necrosis, and vessels) was selected by consensus with matched images on T2WI and DCE-MRI as references. ROI was freehand defined around mass on the ss-EPI images with a b value of 1000 sec/mm2 and copied to the corresponding parametric maps (MK, MD and ADC). In the meantime, the ROI was saved as a mask file, which can be loaded onto the rs-EPI image within the software.
-
c.
Histogram analysis. The mathematical quotation of calculating ADC value based on mono-exponential model was as follows: \(\mathit{ln}\left[S\left(b\right)\right]=\mathit{ln}\left[S\left(0\right)\right]-bADC\) 26. Where S(b) and S(0) are the signal intensity at a certain b value of 1000 sec/mm2 and 0 respectively. The mathematical quotation of calculating MK and MD value based on DKI model was as follows:\(\text{ln}\left[S\left(b\right)\right]=\text{ln}\left[S\left(0\right)\right]-bMD+{ b}^{2}M{D}^{2}MK+O\left({b}^{3}\right)\) 27. Where Sb is the signal intensities of 4 b-values (0, 50, 1000, and 2000 sec/mm2). O(b3) is the fit error. Previous studies suggested that more than three b-values including at least two b-values both above and below 1000 s/mm2, to facilitate the successful capture of the non-Gaussian behavior28,29. Histogram metrics were extracted from each ROI of the parametric maps, which included mean, the 25, 50th, 75th and 100th percentiles, skewness and kurtosis values.
-
d.
The readers repeated all quantitative measurements after a time interval of 2 weeks.
Histopathologic analysis
ER and PR positivity were defined by more than 1% of the nuclei stained positive in ten high-power fields. HER2 was considered positive with an IHC score of 3+ or fluorescence in situ hybridization (FISH) amplification with a ratio ≥ 2.0. According to the expression status of ER, PR, HER2, and Ki-67, the tumor was classified as Luminal A (ER and/or PR positive, HER2 negative, and Ki-67 < 14%), Luminal B (ER and/or PR positive, HER2 negative, and Ki-67 ≥ 14% or ER and/or PR positive, HER2 positive), HER2-enriched (ER and PR negative, HER2 positive), and TN (ER negative, PR negative, and HER2 negative)3.
Statistical analysis
Statistical analyses were performed using SPSS (version 20.0; SPSS, Inc, Chicago, IL, USA). The intraclass correlation coefficient (ICC) was calculated for histogram metrics derived from ss-EPI and rs-EPI to evaluate inter and intra-reader agreement. The agreement, with an ICC ranging from 0 to 0.40, was considered poor, 0.40–0.59 was defined as fair, 0.60–0.74 was deemed as good, 0.75–1.00 was indicated excellent30. The measurements of one random radiologist were used for subsequent statistical analyses.
The student’s t test when normally distributed or Mann-Whitney U test when not normally distributed was used for the comparison of each histogram metric between ss-EPI and rs-EPI for luminal and non-luminal BC, respectively, and the differences of each histogram metric between luminal and non-luminal BC in the respective sequence. The ROC curves were constructed to compare differences in diagnostic performance between two sequences for the differentiation of luminal subtype from the other subtype. Then the sensitivity and specificity for histogram metrics were obtained at the threshold values. The area under the receiver operating characteristic (AUC) was calculated and compared between ss-EPI and rs-EPI. For all tests, p value less than 0.05 was considered statistically significant.
Informed consent
Written informed consent was obtained from all participating subjects
Results
Demographics
A total of 160 patients (mean age ± SD, 44.3± 17.2 years; age range, 24-67 years) with 160 lesions (mean diameter, 1.53 ± 0.71 cm) were finally enrolled in our study cohort. There were 35 (21.9%) luminal A, 76 (47.5%) luminal B, 29 (18%) HER2-enriched, and 20 (12.5%) triple negative tumors.
Comparison of Histogram metrics between ss-EPI and rs-EPI sequence
Good or excellent agreements for inter- and intra-observers were exhibited in most histogram metrics derived from ss-EPI and rs-EPI sequence (Table 1). The mean and 95% CI of each histogram metric for luminal and non-luminal lesions are listed in Tables 1 and 2. The histogram metrics MKmean, MK50th, MK75th, MK100th of luminal BC were significantly higher than those of non-luminal BC in the rs-EPI sequence (P = 0.003, 0.008, 0.002 and 0.001, respectively). The histogram metrics MKmean, MK50th and MK75th of luminal BC were significantly higher than those of non-luminal BC (P = 0.032,0.032 and 0.044, respectively) in the ss-EPI sequence. We did not find any significant differences in the histogram metrics derived from MD and ADC between luminal and non-luminal BC in both two sequences (all P > 0.05).The luminal BC displayed statistical differences in the MKmean, MK50th and MK75th between ss-EPI and rs-EPI sequence (P = 0.014, 0.045 and 0.044, respectively). The non-luminal BC showed significant differences in the MKmean, MK25th, MK50th and MK75th between ss-EPI and rs-EPI sequence (P = 0.000, 0.047, 0.023 and 0.020, respectively). The luminal and non-luminal BC showed no significant differences between two sequences for each histogram metric of MD and ADC (all P > 0.05). Representative images of luminal and non-luminal BC were shown in Figures 1 and 2.
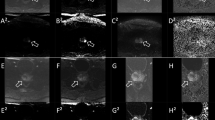
Representative images of a 33-year-old patient with luminal A type breast cancer (a–j). Ss-EPI with DWI image (a); MK image (b) with MKmean: 1.54, MK25th: 1.44, MK50th: 1.56, MK75th:1.66, MK100th: 2.03, MKskewness: −0.50, MKkurtosis: 3.65; MD image (c) with MDmean: 0.72×10-3mm2/s, MD25th: 0.57×10-3mm2/s, MD50th: 0.66×10-3mm2/s, MD75th: 0.79×10-3mm2/s,MD100th: 1.58×10-3mm2/s, MDskewness: 1.75×10-3mm2/s, MDkurtosis: 6.87×10-3mm2/s; and ADC image (d) with ADCmean: 0.49×10-3mm2/s, ADC25th: 0.42×10-3mm2/s, ADC50th: 0.45×10-3mm2/s, ADC75th: 0.52×10-3mm2/s, ADC100th: 0.92×10-3mm2/s, ADCskewness: 1.70×10-3mm2/s, ADCkurtosis: 6.71×10-3mm2/s. Rs-EPI with DWI image (e); MK image (f) with MKmean: 1.28, MK25th: 1.13, MK50th: 1.30, MK75th: 1.43, MK100th: 1.79, MKskewness: −0.36, MKkurtosis: 2.30; MD image (g) with MDmean: 0.70×10-3mm2/s, MD25th: 0.59×10-3mm2/s, MD50th: 0.69×10-3mm2/s, MD75th: 0.93×10-3mm2/s, MD100th: 1.68×10-3mm2/s, MDskewness: 0.99×10-3mm2/s, MDkurtosis: 3.91×10-3mm2/s; and ADC image (h) with ADCmean: 0.53×10-3mm2/s, ADC25th: 0.45×10-3mm2/s, ADC50th: 0.51×10-3mm2/s, ADC75th: 0.61×10-3mm2/s, ADC100th: 0.87×10-3mm2/s, ADCskewness: 0.74×10-3mm2/s, ADCkurtosis: 2.92×10-3mm2/s. Hematoxylin and eosin staining map (×200, i) and Immunohistochemical map (×200, j).
Representative images of a 41-year-old patient with HER2-enriched type breast cancer (a–j). Ss-EPI with DWI image (a); MK image (b) with MKmean: 0.92, MK25th: 0.86, MK50th: 0.96, MK75th: 1.10, MK100th: 1.12, MKskewness: −1.07, MKkurtosis: 3.47; MD image (c) with MDmean: 1.30×10-3mm2/s, MD25th: 1.19×10-3mm2/s, MD50th: 1.30×10-3mm2/s, MD75th: 1.41×10-3mm2/s, MD100th: 1.73×10-3mm2/s, MDskewness: −0.15×10-3mm2/s, MDkurtosis: 6.46×10-3mm2/s; and ADC image (d) with ADCmean: 0.70×10-3mm2/s, ADC25th: 0.63×10-3mm2/s, ADC50th: 0.66×10-3mm2/s, ADC75th: 0.75×10-3mm2/s, ADC100th: 0.92×10-3mm2/s, ADCskewness: 0.82×10-3mm2/s, ADCkurtosis: 2.64×10-3mm2/s. Rs-EPI with DWI image (e); MK image (f) with MKmean: 0.67, MK25th: 0.60, MK50th: 0.67, MK75th: 0.72, MK100th: 0.85, MKskewness: −0.01, MKkurtosis: 2.59; MD image (g) with MDmean: 1.38, MD25th: 1.24, MD50th: 1.33, MD75th: 1.47, MD100th: 2.15, MDskewness: 1.43, MDkurtosis: 5.15; and ADC image (h) with ADCmean: 0.77×10-3mm2/s, ADC25th: 0.72×10-3mm2/s, ADC50th: 0.74×10-3mm2/s, ADC75th: 0.79×10-3mm2/s, ADC100th: 1.24×10-3mm2/s, ADCskewness: 2.31×10-3mm2/s, ADCkurtosis: 9.85×10-3mm2/s. Hematoxylin and eosin staining map (×200, i) and Immunohistochemical map (×200, j).
Diagnostic accuracy of Histogram metrics between ss-EPI and rs-EPI sequence
The ROC curve analysis for significant histogram metrics of both sequences in distinguishing luminal from non-luminal BC is displayed in Table 3 and Figure 3. Analyses for ROC curves showed that MKmean, MK50th, MK75th and MK100th derived from rs-EPI yielded AUCs of 0.857, 0.847, 0.891, and 0.778. MKmean, MK50th and MK75th derived from ss-EPI yielded AUCs of 0.733, 0.714, and 0.740. Figure 3 showed the AUC comparisons of significant histogram metrics in differentiation of luminal vs. non-luminal BC. MK75th derived from rs-EPI yielded the highest AUC of 0.891, with the sensitivity of 78.4%, the specificity of 87.8%, and a cutoff value of 0.966 × 10-3 for distinguishing luminal from non-luminal BC.
Discussion
In our study, histogram metrics of DKI and DWI parameters for distinguishing between luminal and non-luminal BC were compared between ss-EPI and rs-EPI sequence. The findings of this study showed that mean values, 50th/75th percentiles of MK derived from both sequences and 100 percentiles of MK derived from rs-EPI were able to distinguish luminal and non-luminal BC. Moreover, MK histogram parameters derived from rs-EPI significantly improved diagnostic performance for characterization of luminal versus non-luminal BC in comparison with that derived from ss-EPI. MK75th derived from rs-EPI had the largest AUC in discriminating luminal from non-luminal BC. To our knowledge, there was no previous study to compare the diagnostic performances of DKI and DWI histogram metrics between ss-EPI and rs-EPI for differentiating luminal from non-luminal BC.
Luminal BC are more commonly observed in patients diagnosed with invasive BC and have more favorable prognosis compared with non-luminal BC2. Previous studies have attempted to investigate the value of DWI in differentiating luminal and non-luminal BC, but the highest AUC of DWI quantitative parameters varied from 0.608 to 0.71020,22,31. The imaging protocols for DWI acquisition and DWI models for quantitative parameters calculation adapted in these studies are not consistent. Thus, we explored the baseline techniques for DWI acquisition including single-shot and multi-shot echo-planar imaging (EPI) (ss-EPI vs. rs-EPI) in the differentiation of luminal vs. non-luminal BC. This research showed that the luminal subtype showed a higher MK value (MKmean, MK50th and MK75th) compared to non-luminal subtype in both DWI sequences. The findings were consistent with previous findings21,22. Hormone receptor (ER/PR) overexpression could inhibit tumors angiogenic pathways and therefore results in reduced blood perfusion and increase cellularity to restrict water diffusion31,32. In addition, the more characteristic morphological feature of luminal BC is having spiculated margin, which might be correlated with tumor infiltration and microstructural heterogeneity33,34. Thus, higher kurtosis values in luminal BC may be partially explained by the above reasons. However, all histogram metrics of MD and ADC failed to provoke significant differences between the luminal and non-luminal BC in our study. These results were in line with a study investigated by Kang et al.22. The reason for it is that diffusion value (MD and ADC) measurements might be greatly affected by partial-volume effects from uneven fat suppression under high b value (1000 s/mm2) imaging25,35. What’s more, MK75th showed the highest AUC among all histogram metrics of MK for differentiating luminal from non-luminal BC in both ss-EPI and rs-EPI. Due to the histologic heterogeneity of BC, the mean value of MK may underestimate the the deviation of tissue diffusion and the higher percentiles of MK may reflect the most aggressive components of BC except MK100th14. Besides, MK100th did not exhibit the highest AUC because the maximum MK value might be more susceptible to noise and adjacent structures29,36.
There existed significant differences in some histogram metrics of MK between two sequence for luminal and non-luminal BC. The non-luminal BC showed statistically significant differences in the MKmean, MK25th, MK50th and MK75th between ss-EPI and rs-EPI sequence. The ROC analyses also suggest that the diagnostic performance of each histogram metric of MK in rs-EPI sequence was better than the respective histogram metric in ss-EPI sequence. As is known to all, ss-EPI is widely applied for breast DWI. However, the main challenges for ss-EPI are gradient nonlinearities, susceptibility-induced geometric distortion and motion artifacts due to phase error accumulation during the long EPI readout37. These defects lead to inaccuracies in quantitative parameters calculation and degrade the performance of DWI quantitative parameters38. The rs-EPI sequence can shorten echo train lengths with parallel imaging techniques and improve the deficiencies faced with ss-EPI sequence, particularly at 3.0-T39.
Limitations
Our study presents some limitations. First, this was a prospective study based in a single center, with a relatively small sample size, especially in non-luminal BC. Multicenter cohort studies would also be needed to further validate our findings. Second, the lesions included in our study were mass lesions. It would be helpful to explore whether there exist some differences between mass and non-mass like lesions and we will explore it in the future. Third, all MRI acquisitions were performed on a single MRI unit and analyzed using one prototype software, the generalizability of our findings may be limited. What’s more, the two-dimensional ROIs were manually defined, which may be insufficient to reveal the whole-tumor heterogeneity. Whole-tumor histogram analysis of quantitative parameters may better reflect the intratumoral heterogeneity of BC.
Conclusion
The rs-EPI sequence improves the diagnostic accuracy of the differentiation between luminal and non-Luminal breast cancer. Histogram analysis may be useful for differentiating luminal from non-luminal BC. The 75th percentile of MK derived from the rs-EPI sequence was the most valuable metric among all the histogram metrics.
Data availability
The datasets used during the current study is available from the corresponding author on reasonable request.
References
Mazurowski, M. A., Zhang, J., Grimm, L. J., Yoon, S. C. & Silber, J. I. Radiogenomic analysis of breast cancer: luminal B molecular subtype is associated with enhancement dynamics at MR imaging. Radiology 273, 365–372. https://doi.org/10.1148/radiol.14132641 (2014).
Carey, L. A. et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295, 2492–2502. https://doi.org/10.1001/jama.295.21.2492 (2006).
Xie, T. et al. Differentiation of triple-negative breast cancer from other subtypes through whole-tumor histogram analysis on multiparametric MR imaging. Eur. Radiol. 29, 2535–2544. https://doi.org/10.1007/s00330-018-5804-5 (2019).
Pellegrino, B. et al. Luminal breast cancer: Risk of recurrence and Tumor-associated immune suppression. Mol. Diagn. Ther. 25, 409–424. https://doi.org/10.1007/s40291-021-00525-7 (2021).
Tamimi, R. M. et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res. Treat 131, 159–167. https://doi.org/10.1007/s10549-011-1702-0 (2012).
Schnitt, S. J. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod. Pathol. 23(Suppl 2), S60-64. https://doi.org/10.1038/modpathol.2010.33 (2010).
Song, S. E. et al. Machine learning with multiparametric breast MRI for prediction of Ki-67 and histologic grade in early-stage luminal breast cancer. Eur. Radiol. 32, 853–863. https://doi.org/10.1007/s00330-021-08127-x (2022).
Widodo, I. et al. Prognostic value of lymphangiogenesis determinants in luminal and non-luminal breast carcinomas. Asian Pac. J. Cancer Prev. 19, 2461–2467 (2018).
Gandhi, N. & Das, G. M. Metabolic reprogramming in breast cancer and its therapeutic implications. Cells https://doi.org/10.3390/cells8020089 (2019).
Holm, J. et al. Assessment of breast cancer risk factors reveals subtype heterogeneity. Cancer Res. 77, 3708–3717. https://doi.org/10.1158/0008-5472.CAN-16-2574 (2017).
Horvat, J. V. et al. Histogram analysis and visual heterogeneity of diffusion-weighted imaging with apparent diffusion coefficient mapping in the prediction of molecular subtypes of invasive breast cancers. Contrast Media Mol. Imaging 2019, 2972189. https://doi.org/10.1155/2019/2972189 (2019).
Devalapalli, A., Thomas, S., Mazurowski, M. A., Saha, A. & Grimm, L. J. Performance of preoperative breast MRI based on breast cancer molecular subtype. Clin. Imaging 67, 130–135. https://doi.org/10.1016/j.clinimag.2020.05.017 (2020).
Szep, M. et al. Multiparametric MRI features of breast cancer molecular subtypes. Medicina (Kaunas) https://doi.org/10.3390/medicina58121716 (2022).
He, M. et al. Histogram analysis comparison of monoexponential, advanced diffusion-weighted imaging, and dynamic contrast-enhanced MRI for differentiating borderline from malignant epithelial ovarian Tumors. J. Magn. Reson. Imaging 52, 257–268. https://doi.org/10.1002/jmri.27037 (2020).
Hu, Y., Hu, Q., Zhan, C., Yin, T. & Ai, T. Intraobserver and interobserver reproducibility of breast diffusion-weighted imaging quantitative parameters: Readout-segmented vs. single-shot echo-planar imaging. J. Magn. Reson. Imaging 58, 1725–1736. https://doi.org/10.1002/jmri.28655 (2023).
Baltzer, P. et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 30, 1436–1450. https://doi.org/10.1007/s00330-019-06510-3 (2020).
Bogner, W. et al. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology 263, 64–76. https://doi.org/10.1148/radiol.12111494 (2012).
Kim, Y. J. et al. Readout-segmented echo-planar imaging in diffusion-weighted mr imaging in breast cancer: Comparison with single-shot echo-planar imaging in image quality. Korean J. Radiol. 15, 403–410. https://doi.org/10.3348/kjr.2014.15.4.403 (2014).
Kishimoto, A. O. et al. Evaluation of malignant breast lesions using high-resolution readout-segmented diffusion-weighted echo-planar imaging: Comparison with pathology. Magn. Reson. Med. Sci. 20, 204–215. https://doi.org/10.2463/mrms.mp.2020-0021 (2021).
Wang, W. et al. Prediction of prognostic factors and genotypes in patients with breast cancer using multiple mathematical models of MR diffusion imaging. Front. Oncol. 12, 825264. https://doi.org/10.3389/fonc.2022.825264 (2022).
Qin, Y. et al. Histogram analysis of multi-model high-resolution diffusion-weighted MRI in breast cancer: Correlations with molecular prognostic factors and subtypes. Front. Oncol. 13, 1139189. https://doi.org/10.3389/fonc.2023.1139189 (2023).
Kang, H. S. et al. Diffusion kurtosis MR imaging of invasive breast cancer: Correlations with prognostic factors and molecular subtypes. J. Magn. Reson. Imaging 56, 110–120. https://doi.org/10.1002/jmri.27999 (2022).
Kim, J. Y. et al. Diffusion-weighted MRI of estrogen receptor-positive, HER2-negative, node-negative breast cancer: Association between intratumoral heterogeneity and recurrence risk. Eur. Radiol. 30, 66–76. https://doi.org/10.1007/s00330-019-06383-6 (2020).
Sun, K. et al. Whole-lesion histogram and texture analyses of breast lesions on inline quantitative DCE mapping with CAIPIRINHA-Dixon-TWIST-VIBE. Eur. Radiol. 30, 57–65. https://doi.org/10.1007/s00330-019-06365-8 (2020).
Hu, Y. et al. Accelerating acquisition of readout-segmented echo planar imaging with a simultaneous multi-slice (SMS) technique for diagnosing breast lesions. Eur. Radiol. 31, 2667–2676. https://doi.org/10.1007/s00330-020-07393-5 (2021).
Guo, Y. et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J. Magn. Reson. Imaging 16, 172–178. https://doi.org/10.1002/jmri.10140 (2002).
Nogueira, L. et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur. Radiol. 24, 1197–1203. https://doi.org/10.1007/s00330-014-3146-5 (2014).
Rosenkrantz, A. B. et al. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J. Magn. Reson. Imaging 42, 1190–1202. https://doi.org/10.1002/jmri.24985 (2015).
Huang, C. et al. Histogram analysis of breast diffusion kurtosis imaging: a comparison between readout-segmented and single-shot echo-planar imaging sequence. Quant. Imaging Med. Surg. 13, 735–746 (2023).
Hallgren, K. A. Computing inter-rater reliability for observational data: An overview and tutorial. Tutor. Quant. Methods Psychol. 8, 23–34 (2012).
Suo, S. et al. Added value of mean and entropy of apparent diffusion coefficient values for evaluating histologic phenotypes of invasive ductal breast cancer with MR imaging. Eur. Radiol. 29, 1425–1434. https://doi.org/10.1007/s00330-018-5667-9 (2019).
Yang, Z. L. et al. Evaluation of suspicious breast lesions with diffusion kurtosis MR imaging and connection with prognostic factors. Eur. J. Radiol. 145, 110014. https://doi.org/10.1016/j.ejrad.2021.110014 (2021).
Wu, T. et al. Identification of a correlation between the sonographic appearance and molecular subtype of invasive breast cancer: A review of 311 cases. Clin. Imaging 53, 179–185. https://doi.org/10.1016/j.clinimag.2018.10.020 (2019).
Franquet, T., De Miguel, C., Cozcolluela, R. & Donoso, L. Spiculated lesions of the breast: Mammographic-pathologic correlation. Radiographics 13, 841–852. https://doi.org/10.1148/radiographics.13.4.8356272 (1993).
Baron, P., Dorrius, M. D., Kappert, P., Oudkerk, M. & Sijens, P. E. Diffusion-weighted imaging of normal fibroglandular breast tissue: Influence of microperfusion and fat suppression technique on the apparent diffusion coefficient. NMR Biomed. 23, 399–405. https://doi.org/10.1002/nbm.1475 (2010).
Liu, W., Wei, C., Bai, J., Gao, X. & Zhou, L. Histogram analysis of diffusion kurtosis imaging in the differentiation of malignant from benign breast lesions. Eur. J. Radiol. 117, 156–163. https://doi.org/10.1016/j.ejrad.2019.06.008 (2019).
Newitt, D. C. et al. Gradient nonlinearity correction to improve apparent diffusion coefficient accuracy and standardization in the american college of radiology imaging network 6698 breast cancer trial. J. Magn. Reson. Imaging 42, 908–919. https://doi.org/10.1002/jmri.24883 (2015).
Partridge, S. C., Nissan, N., Rahbar, H., Kitsch, A. E. & Sigmund, E. E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging 45, 337–355. https://doi.org/10.1002/jmri.25479 (2017).
Kuhl, C. K. et al. Sensitivity encoding for diffusion-weighted MR imaging at 3.0 T: intraindividual comparative study. Radiology 234, 517–526. https://doi.org/10.1148/radiol.2342031626 (2005).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.H. Data curation: Y.H., Q.H. and Z.L. Formal analysis Y.H., Q.H. and Z.L. Investigation: Y.H., Q.H. and Z.L. Methodology Y.H., Q.H. and Z.L. Project administration: C.H., L.X. Software: Z.L. Supervision: C.H., L.X. Visualization: Y.H., Q.H. and Z.L. Writing—original draft: Y.H. Writing—review and editing: C.H. and L.X. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, Y., Hu, Q., Liu, Z. et al. Histogram analysis comparison of readout-segmented and single-shot echo-planar imaging for differentiating luminal from non-luminal breast cancer. Sci Rep 14, 12135 (2024). https://doi.org/10.1038/s41598-024-62514-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62514-0