Abstract
Endoscopic submucosal dissection is a standard treatment for early esophageal squamous cell carcinoma. However, submucosal or lymphovascular invasion increases the risk of lymph node metastasis. Although 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) parameters are associated with prognosis in patients with advanced esophageal squamous cell carcinoma, the utility of FDG PET/CT in diagnosing superficial esophageal carcinoma remains unclear. This study aimed to investigate the association between FDG PET/CT parameters and histopathological findings in superficial esophageal carcinoma. Fifty-three patients with superficial esophageal cancer who underwent FDG PET/CT scans before undergoing interventions were retrospectively analyzed. The maximal standardized uptake value (SUVmax), metabolic tumor volume, and total lesion glycolysis were significantly higher in the cases with submucosal invasion (T1b) compared with those confined to the muscularis mucosa (T1a). In contrast, classification of intrapapillary capillary loops patterns with magnifying endoscopy did not yield statistical differences between T1a and T1b. Multivariable analysis revealed that SUVmax was the only independent predictor of submucosal and lymphovascular invasion. This study demonstrated that SUVmax may be useful in predicting submucosal and lymphovascular invasion. Thus, the value of SUVmax may guide clinical decision-making in superficial esophageal squamous cell carcinoma.
Similar content being viewed by others
Introduction
Squamous cell carcinoma (SCC), one of the most common types of esophageal carcinoma, has a poor prognosis1,2. However, recent advancements in endoscopic technology permitted its detection at early stages3. Treatment of early-stage esophageal cancer depends on the depth of tumor invasion4. According to the Japanese Esophageal Cancer Society guidelines, lesions within the muscularis mucosae (MM) (Clinical stage T1a) can be endoscopically treated. Surgery or chemoradiotherapy is recommended instead of endoscopic submucosal dissection (ESD) for lesions that strongly suggest invading the submucosal layer (SM) before intervention (clinical stage T1b)5. Additionally, it is recommended to undergo either surgical intervention or chemoradiation therapy for post-ESD where invasion to the submucosa (pT1b) is confirmed or involvement of the lymphovascular system is detected5,6.
Magnified endoscopy with narrow-band imaging (NBI) was developed for tumor depth estimation5. Regardless of tumor depth, lesions with lymphovascular invasions require additional therapy. In addition to the general endoscopic findings including tumor elevation and esophageal wall stiffness, a combination of intrapapillary capillary loop (IPCL)7,8 patterns and avascular area (AVA)7 of the tumor is useful to predict submucosal invasion9. However, these techniques demand technical mastery and knowledge regarding magnifying endoscopy classifications5,7. Additionally, methods for estimating lymphovascular invasion are lacking.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) is used for imaging cancer lesions, identifying tumor size and ___location, distinguishing between benign and malignant tumors, assessing metastasis status and therapeutic effect, and detecting recurrence4. FDG uptake is commonly measured semi-quantitatively using a standardized uptake value (SUV)10. Mantziari et al.11 reported the feasible predictive value of SUV in preoperative cT stage and disease-free survival. Recently, the maximal standard uptake value (SUVmax) of tumors has been reported to be associated with pathological tumor depth. For example, Lin et al.12 has demonstrated that SUVmax is able to differentiate between T2–3 and T1 cancers. Additionally, metabolic tumor volume (MTV), calculated by setting a certain SUV threshold and determining the volume of the region exceeding it, and total lesion glycolysis (TLG), which is obtained by multiplying the MTV by average the SUV, have been suggested to predict the prognosis of esophageal SCC treated with chemoradiotherapy13. Magnified endoscopy, in combination with FDG PET, has been proposed to predict tumor depth4. However, this concept has not been studied in early-stage esophageal cancer with detailed FDG PET values, such as SUVmax, MTV, and TLG.
This study investigated the association between various FDG PET uptake values and histological characteristics of the respective specimens for T1 esophageal squamous cell carcinoma (ESCC). Similarly, the association between various PET uptake values and pathological characteristics, including factors influencing treatment choices such as tumor depth and lymphovascular invasion, was explored.
Results
Patient demographics
The records of 96 consecutive patients with superficial esophageal SCC were initially analyzed. Patients presenting with pT2–3 (n = 16) on histology after surgery and those who underwent chemoradiation therapy or photodynamic therapy (n = 27) were excluded from the analysis due to lack of histological specimen (Fig. 1). The details of 27 patients who underwent CRT, radiation, chemotherapy, or PDT, as well as those who did not undergo any treatment, are presented in Supplemental Table 1.
Flow chart of the patient selection. Of the 96 patients with superficial esophageal squamous cell carcinoma who underwent positron emission tomography/computed tomography (PET/CT), 80 patients were determined as T1. The patients who underwent either endoscopic submucosal dissection (n = 27) or surgery (n = 26) were included for further analysis.
The remaining 53 patients were included in the analysis. The patient characteristics are shown in Table 1. Of the 53 patients, 36 were males and 17 were females. Their ages were in the range of 40–77 years and the median age was 68 years. The tumor sites were distributed as follows: upper (4/53), middle (35/53), and lower (14/53). The macroscopic tumor types were as follows: 0–I (2/53), 0–IIa (6/53), 0–IIb (10/53), 0–IIc (32/53), and 0–III (3/53). A total of 27 and 26 patients underwent ESD and surgical resection, respectively. All tumors were diagnosed according to their depth within the SM. The distribution of histological depth was as follows: 3 epithelium (EP), 15 lamina propria mucosa (LPM), 11 MM, 7 SM1 (invasion depth ≤ 200 μM), 7 SM2 (200 μM < invasion depth ≤ 800 μM), and 7 SM3 (invasion depth > 800 μM)5. Twelve patients were positive for lymphatic invasion, 7 for venous invasion, and 4 for both. Four patients who underwent surgery had histologically confirmed lymph node metastasis.
PET/CT values and depth of invasion in T1 esophageal SCC
We initially aimed to identify the factors distinguishing between T1a (EP, LPM, and MM) and T1b (SM1–3). A total of 29 and 24 lesions were histologically diagnosed as T1a and T1b, respectively, after interventions. Tumor features such as size, ___location, macroscopic types, and patient characteristics, did not vary between the patients with T1a and T1b tumors (Table 2).
Generally, reports indicate that type B2 vessel and AVA-middle suggest that the tumor depth is MM(T1a) or SM1(T1b), and type B3 vessel and AVA-large suggest lesions deeper than SM2(T1b). Although the vessel type in magnified endoscopy numerically showed a fewer ratio of B1 and more B3 patterns in T1b compared to that in T1a tumors in this study, no statistical difference was observed. In contrast, T1b tumors exhibited larger AVA areas than T1a tumors. Notably, the PET values of SUVmax, MTV, and TLG were also significantly higher in the T1b tumors compared to those in the T1a tumors (SUVmax, 3.49 vs. 1.33; MTV, 1.58 vs. 0.89; and TLG, 6.85 vs. 1.71; Table 2).
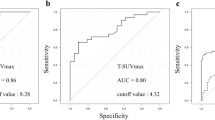
When the ROC curve was drawn to distinguish between T1b and T1a using PET/CT values, the area under the curve (AUC) was 0.673 and the cutoff values were 3.472 for SUVmax, 0.663 for TLG (AUC 0.665), and 0.398 for MTV (AUC 0.657). Of these parameters, SUVmax yielded the highest AUC (Fig. 2). Additionally, the presence of B3 vessels in magnifying endoscopy examination was a significant factor to differentiate between T1b and pT1a. Multivariable analysis revealed that SUVmax ≥ 3.472 (OR 7.82; 95% CI 1.63–37.5; p = 0.010) was an independent significant factor to predict T1b (Table 3).
ROC curve of the PET/CT parameters to detect submucosal invasion in esophageal superficial squamous cell carcinoma. (a) Diagnostic performance of the maximal standardized uptake value (SUVmax). Area under the curve (AUC), 0.673; cutoff level, 3.472; sensitivity, 50.0%; specificity, 86.2%; accuracy, 69.8%. (b) Diagnostic performance of total lesion glycolysis (TLG). AUC, 0.665; cutoff level, 0.663. (c) Diagnostic performance of metabolic tumor volume (MTV). AUC, 0.657; cutoff level, 0.398.
PET/CT values and lymphovascular invasion in esophageal SCC
The existence of lymphatic or vascular invasion is associated with patient prognosis and influences the course of treatment after ESD. Thus, we compared the potential factors contributing to lymphovascular invasion. Compared with the lesions that are histologically diagnosed as negative for lymphovascular invasion, those exhibiting positive lymphovascular invasion had a larger median size. Additionally, the IPCL type in magnified endoscopy tended to include less type B1 and more B3 in lymphovascular invasion-positive tumors compared with those without lymphovascular invasion. This indicates that tumors with lymphovascular invasion present with larger and irregular vessels (Table 4).
Conversely, the AVA area was not significantly different between the two groups. In the PET/CT values, the SUVmax and TLG were significantly higher in lymphovascular invasion-positive lesions. Although the MTV was numerically higher in lymphovascular invasion-positive tumors, statistical significance was not achieved. The patient background, tumor size, macroscopic type, and tumor ___location, did not differ significantly between the two groups in this study. Based on these findings, we then examined the potential predictors of lymphovascular invasion.
The SUVmax AUC for the presence of lymphovascular invasion was 0.690, which was the highest value among the three PET parameters that were assessed in our study (Fig. 3).
ROC curve of the PET/CT parameters to detect lymphovascular invasion. (a) Diagnostic performance of the maximal standardized uptake value (SUVmax). Area under the curve (AUC), 0.690; cutoff level, 3.616; sensitivity, 53.3%; specificity, 86.8%; accuracy, 77.3%. (b) Diagnostic performance of total lesion glycolysis (TLG). AUC, 0.665; cutoff level, 0.663. (c) Diagnostic performance of metabolic tumor volume (MTV). AUC, 0.656; cutoff level, 0.199.
When the baseline SUVmax was set at 3.616 based on the Youden index, the sensitivity and specificity values for predicting lymphovascular invasion were 53.3% and 86.8%, respectively. The type B3 vessels associated with lymphovascular invasion in the univariate analysis were further assessed using multivariable analysis. Only SUVmax ≥ 3.616 remained a significant predictor of lymphovascular invasion (p = 0.017; Table 5).
Discussion
Previous reports indicate a significant difference in the metastatic rate and prognosis between T1a (confined within MM) and T1b (invading to SM), as well as the depth of SM invasion (SM1, < 200 μm; SM2, > 200 μm)14. For example, tumor depth within the superficial esophageal SCC affects the metastatic recurrence rate as follows; pT1a-MM, 1.3%; pT1b-SM1, 10.7%; and pT1b-SM2, 6.5%. The five-year overall survival rate varies as follows; pT1a-MM, 84.1%; pT1b-SM1, 71.4%; pT1b-SM2, 67.4% (MM vs. SM1-2 log rank p = 0.018)14. Additionally, the five-year relapse-free survival rates are as follows: pT1a-MM, 82.8%, pT1b-SM1, 64.3%; pT1b-SM2, 65.2% (MM vs. SM1-2 log rank p = 0.008)14. The 2022 edition of the Japan Esophageal Society (JES) esophageal cancer practice guidelines recommends performing either surgery or chemoradiation therapy based on the condition of the patients in clinically suspected T1b cases5. Combination of vessel type and AVA in magnified endoscopy aids in distinguishing MM/SM1 and SM27. For example, type B2 vessel and AVA-middle indicate that the tumor depth is MM or SM1, whereas type B3 vessel and AVA-large indicate SM2. The positive predictive values of these methods are reported as 67% for MM/SM1 and 77% for SM2 with JES classification15. However, it is challenging to distinguish between T1a and T1b using magnifying endoscopy9,15. Additionally, endoscopic examination cannot detect the potential of lymphovascular invasion. Endoscopic ultrasonography (EUS) plays a crucial role in the T staging of esophageal SCC. EUS has been reported to predict tumor invasion with a relatively high sensitivity and specificity. For example, Radlinski et al. and Thosani et al.16,17 reported that the sensitivity and specificity of T1a staging were 0.85 and 0.87, respectively. However, varied results have been reported from different studies due to the ___location, shapes, method, and frequency of EUS probe, and the experience of the endoscopist16. Ishihara et al.18 reported that the EUS did not improve the diagnostic accuracy of cancer invasion depth in their prospective multi-center study.
Toriyama et al.19 have suggested that FDG PET/CT positivity and NBI are associated with depth of superficial ESCC, although PET positivity was not the sole independent predictive factor determining the indication of ESD in their study. Our results showed that the SUV max had a better ability to distinguish between T1a and T1b compared to the NBI findings.
The presence of venous and lymphatic invasion indicates poor prognosis15,20,21. However, lymphovascular invasion can only be diagnosed using postoperative histopathology, and preoperative prediction is extremely difficult22. A previous report also indicated that enhanced CT could potentially predict lymphovascular invasion, although the study patients were not limited to superficial SCC but also advanced SCC22. The advantage of FDG PET/CT in predicting lymphovascular invasion has been described in lung cancer, hepatocellular carcinoma, and colon cancer23,24,25; however, it has been poorly studied in superficial ESCC. Our results revealed that the value of SUVmax was an independent factor for predicting lymphovascular invasion. Unlike other imaging modalities in oncologic imaging, FDG PET images reflect metabolic tumor activity26. Thus, FDG PET/CT may be advantageous in predicting tumor activity that reflects lymphovascular tumor invasion. Accordingly, our study indicated that PET/CT could serve as an additional parameter in determining whether to proceed with surgery or chemotherapy, thereby minimizing delays associated with ESD5.
Our study has several limitations. This study design was retrospective, and only histologically confirmed cases of ESCC were included; thus, selection bias could have occurred. A prospective study is needed to verify this study findings. Secondly, in our study, the median interval between biopsy and FDG PET/CT was 33 days (range 4–196) days. Although there was no correlation between the biopsy and FDG PET/CT (R = 0.02; p = 0.343) and only two cases had an interval shorter than 7 days in our study, the impact of biopsy could not be completely excluded. Additionally, PET/CT should be always used in combination with CT and endoscopic examination because it is generally poor at describing anatomic detail and may be influenced by the presence of inflammation.
In conclusion, this study suggests that FDG PET/CT might be effective in discriminating between pT1a and pT1b and identifying the presence or absence of lymphovascular invasion preoperatively. Combined with the current evaluation methods such as magnified endoscopy, the PET parameters can be used to support clinical decision-making and predicting metastasis in superficial ESCC.
Methods
Patients
This was a retrospective single-center study of 96 patients who were clinically diagnosed with superficial SCC by endoscopy and CT at Nagasaki University Hospital between June 2010 and December 2019 and underwent PET/CT before treatment. The patients were subjected to PET/CT when the lymph node metastasis was suspected by CT or if there were endoscopic findings that suggest submucosal invasion such as stiffness of the esophageal wall, elevation > 1 mm, Type B3 vessels or AVA-large according to the JES classification4. Patients with adenocarcinoma were not included in the study. The study was conducted in line with the Declaration of Helsinki and adhered to the official disclosure system guidelines of the ethical committee. This study was approved by the Institutional Ethical Committees for Medical Research at Nagasaki University Hospital (#21062120). Informed consent was obtained through the opt-out method on the website. Patients who did not provide the informed consent were excluded from this study: this opt-out consent was also endorsed by the Nagasaki University Hospital Ethics Committee. The clinical and pathological staging of cancers was performed in accordance with the 8th edition of TNM classification by the Union for International Cancer Control27. The PET values as well as the clinical data, including age, tumor size, macroscopic types, and categorization of tumor vascular with magnification endoscopy with NBI types, were reviewed. The IPCL and AVA were analyzed according to previous reports8,9,14.
FDG PET/CT imaging
The patients were required to fast at least six hours prior to FDG injection. Individual FDG doses in the range of 200–300 MBq were intravenously administered, and images were obtained 50 min after injection. PET/CT utilized a PET/CT scanner (Siemens, Germany), which was equipped with PET and CT units. Images were reconstructed using an ordered subset expectation maximization algorithm with two iterations and 24 subsets. The reconstruction parameters were as follows: 200 × 200 matrix, field of view of 815 mm, and 6-mm Gaussian filter. The pixel values in the originally reconstructed images had a unit of radioactivity (MBq/ml) and were converted to the unit of the SUV. Tissue activity concentrations were normalized to body weight and injected FDG activity. SUV was expressed as follows: SUV = activity (MBq/cm3) × body weight (kg)/injected activity (MBq). The images of each patient were individually analyzed using Metavol (https://www.metavol.org/)28, a software for metabolic tumor volume measurement in FDG PET/CT. We employed an SUV of 3.0 as the cut-off value for tumor detection. If the SUV was below this threshold, both TLG and MTV were recorded as zero. The SUV max was automatically calculated using the software when the SUV was above the cut off value. If the SUV did not reach 3.0 and the tumor was visible on PET-CT and corresponded to the area observed in endoscopy, a 1-cm circular region of interest (ROI) was manually positioned over the tumor area, and the SUVmax within this ROI was determined. A cut-off value of 2.0 was applied to the SUVmax, and any value below this threshold value was considered as zero.
Magnifying endoscopic classification
According to the magnifying endoscopic classification reported in previous studies7,9, the vessel type was classified into two types: (1) type A: normal IPCL or mild abnormal microvessels without severe irregularity; and (2) type B: abnormal microvessels with severe irregularity. Additionally, the type B vessels were subclassified into B1, B2, and B3. If the AVA in the tumor was detectable, the AVA was classified based on size (< 0.5 mm, small AVA; 0.5–3 mm, middle AVA; > 3 mm, large AVA).
Statistical analysis
Fisher’s exact test and Wilcoxon rank-sum test were used to compare the qualitative and quantitative variables between the groups. To identify the predictive factors that are closely related to invasion depth or lymphovascular invasion, receiver operating characteristic (ROC) curves were plotted for the FDG PET/CT parameters and other clinicopathological factors. Subsequently, the AUC was calculated, and the point with the largest AUC was defined as the point with the greatest association with invasion depth or lymphovascular invasion. The best cut-off values for the predictive factors had a minimum distance from the upper-left corner to the point on the ROC curve. Single-variable and multivariable logistic regression analyses were then performed based on the cut-off values to determine the factors contributing to invasion depth and lymphovascular invasion. Variables with p < 0.2 in the univariate analysis were selected for multivariate logistic regression, and variables with p < 0.05 in the multivariate logistic analysis were selected as predictive factors that are closely related to invasion depth or lymphovascular invasion. We excluded the variables which are highly correlated (|r|> 0.7, Pearson’s correlation coefficient).For instance, SUVmax, TLG, and MTV were not assessed together to avoid multicollinearity. All the statistical tests were two-sided, and the results were considered statistically significant at p < 0.05. All data analyses were performed using the JMP Pro software (version 17.0.0; SAS Institute, Cary, NC, USA).
Data availability
The data that support the findings of this study are available from the corresponding author, Y.A., on reasonable request.
References
Ferlay, J. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019).
Pennathur, A., Gibson, M. K., Jobe, B. A. & Luketich, J. D. Oesophageal carcinoma. Lancet 381, 400–412 (2013).
Uedo, N. et al. Role of narrow band imaging for diagnosis of early-stage esophagogastric cancer: Current consensus of experienced endoscopists in Asia-Pacific region. Dig. Endosc. 23(Suppl 1), 58–71 (2011).
Ishihara, R. Endoscopic diagnosis and treatment of superficial esophageal squamous cell cancer: Present status and future perspectives. Curr. Oncol. 29, 534–543 (2022).
Kitagawa, Y. et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 20, 343–372 (2023).
Minashi, K. et al. Efficacy of endoscopic resection and selective chemoradiotherapy for stage I esophageal squamous cell carcinoma. Gastroenterology 157, 382-390.e3 (2019).
Oyama, T. et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: Magnifying endoscopic classification of the Japan esophageal society. Esophagus 14, 105–112 (2017).
Inoue, H. Endoscopic diagnosis of tissue atypism (EA) in the pharyngeal and esophageal squamous epithelium; IPCL pattern classification and ECA classification. Kyobu Geka 60, 768–775 (2007).
Yamaguchi, H. et al. Prediction of depth of invasion and lymph node metastasis in superficial pharyngeal cancer by magnifying endoscopy using the Japan esophageal society classification. DEN Open 3, e151 (2023).
Thie, J. A. Understanding the standardized uptake value, its methods, and implications for usage. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 45, 1431–1434 (2004).
Mantziari, S. et al. 18F- FDG PET/CT-derived parameters predict clinical stage and prognosis of esophageal cancer. BMC Med. Imaging 20, 7 (2020).
Lin, D. et al. The role of primary tumor SUVmax in the diagnosis of invasion depth: A step toward clinical T2N0 esophageal cancer. Ann. Transl. Med. 9, 112 (2021).
Yildirim, B. A., Torun, N., Guler, O. C. & Onal, C. Prognostic value of metabolic tumor volume and total lesion glycolysis in esophageal carcinoma patients treated with definitive chemoradiotherapy. Nucl. Med. Commun. 39, 553–563 (2018).
Naito, S. et al. Long-term outcomes of esophageal squamous cell carcinoma with invasion depth of pathological T1a-muscularis mucosae and T1b-submucosa by endoscopic resection followed by appropriate additional treatment. Dig. Endosc. 34, 793–804 (2022).
Katada, C. et al. Retrospective assessment of the diagnostic accuracy of the depth of invasion by narrow band imaging magnifying endoscopy in patients with superficial esophageal squamous cell carcinoma. J. Gastrointest. Cancer 50, 292–297 (2019).
Radlinski, M. & Shami, V. M. Role of endoscopic ultrasound in esophageal cancer. World J. Gastrointest. Endosc. 14, 205–214 (2022).
Thosani, N. et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: A systematic review and meta-analysis. Gastrointest. Endosc. 75, 242–253 (2012).
Ishihara, R. et al. Assessment of the diagnostic performance of endoscopic ultrasonography after conventional endoscopy for the evaluation of esophageal squamous cell carcinoma invasion depth. JAMA Netw. Open 4, e2125317 (2021).
Toriyama, K. et al. Clinical relevance of fluorodeoxyglucose positron emission tomography/computed tomography and magnifying endoscopy with narrow band imaging in decision-making regarding the treatment strategy for esophageal squamous cell carcinoma. World J. Gastroenterol. 25, 6767–6780 (2019).
Jeon, J. H. et al. Prognostic significance of venous invasion and maximum standardized uptake value of 18F-FDG PET/CT in surgically resected T1N0 esophageal squamous cell carcinoma. Eur. J. Surg. Oncol. 43, 471–477 (2017).
Gu, Y.-M. et al. Prognostic value of lymphovascular invasion in patients with esophageal squamous cell carcinoma. Ann. Transl. Med. 7, 256 (2019).
Li, Y. et al. Contrast-enhanced CT-based radiomics analysis in predicting lymphovascular invasion in esophageal squamous cell carcinoma. Front. Oncol. 11, 644165 (2021).
Lin, C.-Y. et al. Predictive value of 18F-FDG PET/CT for vascular invasion in patients with hepatocellular carcinoma before liver transplantation. Clin. Nucl. Med. 42, e183–e187 (2017).
Yang, Y. et al. Preoperative prediction of lymphovascular invasion of colorectal cancer by radiomics based on 18F-FDG PET-CT and clinical factors. Front. Radiol. 3, 1212382 (2023).
Li, C., Tian, Y., Shen, Y., Wen, B. & He, Y. Utility of volumetric metabolic parameters on preoperative FDG PET/CT for predicting tumor lymphovascular invasion in non-small cell lung cancer. AJR Am. J. Roentgenol. 217, 1433–1443 (2021).
Griffeth, L. K. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc. (Bayl. Univ. Med. Cent.) 18, 321–330 (2005).
Rice, T. W., Patil, D. T. & Blackstone, E. H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 6, 119–130 (2017).
Hirata, K. et al. A semi-automated technique determining the liver standardized uptake value reference for tumor delineation in FDG PET-CT. PLoS One 9, e105682 (2014).
Acknowledgements
We thank Hitomi Minami, Daisuke Fukuda, and Daisuke Norimura for their support in conducting this research. We also thank Tae Kitano and Takahiro Motoyama for their excellent technical support.
Funding
This study was supported by Network-type Joint Usage/Research Center for Radiation Disaster Medical Science.
Author information
Authors and Affiliations
Contributions
T.H., H.K., and M.K. conducted the research and wrote the manuscript. N.A. participated in the data collection of the PET values. A.T., S.J., K.M., and M.K. gathered clinicopathological data. N.Y., K.T., A.J., and K.K. contributed for the final version of the manuscript. M.N. and K.N. gave critical input on important intellectual content. Y.A. designed the study and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tanaka, H., Hashiguchi, K., Tabuchi, M. et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography parameters are associated with histological outcomes in superficial esophageal squamous cell carcinoma. Sci Rep 14, 17493 (2024). https://doi.org/10.1038/s41598-024-65066-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65066-5
This article is cited by
-
The usefulness of combining narrow-band imaging with magnifying endoscopy and 18F-fluorodeoxyglucose positron emission tomography for predicting the depth of invasion in superficial esophageal squamous cell carcinoma
Esophagus (2025)
-
The 13th World Federation of Nuclear Medicine and Biology congress (WFNMB 2022): summarize the past half century and discuss the next half century of WFNMB-
Annals of Nuclear Medicine (2025)