Abstract
Systemic Immune Inflammatory Index (SII) is a novel indicator of inflammation. However, no studies have reported the effect of SII on the association between metals and total fat (TOFAT). We aim to investigate the mediated effect of SII on the relationship between urinary metals and TOFAT in a US adult population. This cross-sectional study was conducted among adults with complete information on SII, urine metal concentrations, and TOFAT from the 2011–2018 National Health and Nutrition Examination Survey (NHANES). Multifactorial logistic regression and restricted cubic splines were used to explore the association between urine metal levels and TOFAT. Furthermore, serial mediation analyses were used to investigate the mediating effect of SII on metals and TOFAT. A total of 3324 subjects were included in this study. After adjusting for confounders, arsenic (As), cadmium (Cd), cobalt (Co), cesium (Cs), inorganic mercury (Hg), molybdenum (Mo), manganese (Mn), lead (Pb), antimony (Sb), and thallium(Tl) had negative decreased trends of odds ratios for TOFAT (all P for trend < 0.05). In the total population, we found that Cd, Co, and Tu were positively associated with SII (β = 29.70, 79.37, and 31.08), whereas As and Hg had a negative association with SII. The mediation analysis showed that SII mediated the association of Co with TOFAT, with the β of the mediating effect being 0.9% (95%CI: 0.3%, 1.6%). Our findings suggested that exposure to As, Cd, and Hg would directly decrease the level of TOFAT. However, Co would increase TOFAT, completely mediated by SII, mainly exerted in females rather than males.
Similar content being viewed by others
Introduction
With the improvement of living standards, especially in developed countries, such as the United States, obesity has become increasingly common1. According to data from the World Health Organization, more and more adults are overweight or obese2, with the global prevalence of obesity nearly tripling between 1975 and 2016. Obesity is a metabolic disease that not only causes serious physical health issues but can also increase the incidence of other metabolic diseases such as hypertension and diabetes3. This has made obesity a widespread public health problem worldwide. Although the exact causes of obesity are not fully understood, environmental factors may contribute to its development.
Of particular concern is the exposure to harmful metals, which can disrupt human metabolism and affect fat metabolism functions. Metals such as lead (Pb), cadmium (Cd), arsenic (As), manganese (Mn), and barium (Ba) are widely present in the environment, including in the air, soil, and water4. People can be exposed to these harmful metals through various means, such as consuming contaminated food, drinking contaminated water, and inhaling polluted air. These metals are associated with adverse health effects, including sarcopenia, osteoporosis, kidney damage, etc.5,6,7. Previous studies have reported that exposure to As, Cd, cobalt (Co), Pb, and mercury (Hg) could cause chronic diseases such as diabetes and obesity8,9,10.
Inflammation is the body's response to harmful foreign substances and results from the interaction between the organism and the environment11. Systemic inflammation can be directly measured through blood biochemical markers or derived ratios12. Discovering novel inflammatory indices is crucial for studying various diseases. Many studies have indicated that the Systemic Immunity Inflammation Index (SII) can assess tissue and cell damage caused by injury factors. SII is calculated by multiplying the platelet count by the neutrophil count and dividing by the lymphocyte count13. Research has shown that SII is associated with many chronic diseases. Mahemuti et al.12 found a remarkable correlation between SII and hyperlipidemia. Xu et al.14 showed that SII influenced mortality in cardiovascular disease patients. Chen et al.15 also demonstrated that combining SII and other clinical data could predict the risk of death in senior cardiovascular patients.
Changes in inflammatory indicators may result from the toxic effects of hazardous metals16. For example, Cd exposure can activate inflammatory pathways and cause chronic inflammation17. Epidemiological studies by Zhong et al.18 have shown that SII is associated with exposure to Mn, Cd, and Pb. Additionally, obesity is linked to fat inflammation19, where immune cell infiltration in visceral fat contributes to adverse metabolic complications20. Some studies have revealed that obesity is associated with altered white blood cell counts21.
Building on these studies, we hypothesized that SII might play a role in the relationship between metals and total body fat (TOFAT). However, the specific role of SII in this context has not been reported. Therefore, using the data from the National Health and Nutrition Examination Survey (NHANES), we first investigated the association between metal exposure and TOFAT. We then further examined the mediating effect of SII on the relationship between metals and TOFAT.
Methods
Study population
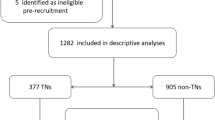
The NHANES is a multistage stratified composite design survey, which started in the 1980s, and the biological samples collected in this study contain serum, plasma, and urine from participants, covering a wide range of measures. The study also contains a large amount of data from questionnaires covering demographic, dietary, and health-related issues, and a physical examination section that includes a variety of laboratory testing data, radiologic testing data, etc., to assess the health status of people in the United States22. The NHANES collects data on about 5000 persons each year23. We combined 4 consecutive NHANES surveys, 2011–2012, 2013–2014 2015–2016, and 2017–2018, into a single analytic sample. A total of 6,986 subjects aged 18 years or older and with complete information on urinary metals and SII were included. There were 3232 participants without TOFAT excluded in this study. Then, the participants who had no data on education level, body mass index (BMI), alcohol use, smoking behavior, physical activity, sedentary and nutrient intakes (n = 430) were also excluded, and the final study population of this study was 3,324. The process of recruiting was shown in Fig. 1.
Urinary metal detections
13 metals were detected in urine [As (total arsenic), Ba, Cd, Co, cesium(Cs), Hg (inorganic), molybdenum(Mo), Mn, Pb, antimony(Sb), tin(Sn), thallium(Tl), and tungsten(Tu)]. All metals were analyzed by inductively coupled plasma dynamic reaction cell mass spectrometry, Laboratory Sciences Division, National Center for Environmental Health, Atlanta, Georgia. Metal concentrations below the limit of detection were calculated by dividing the limit of detection by the root sign 224. We used these estimates in our analyses, consistent with the Centers for Disease Control and Prevention’s National Report on Human Exposure to Environmental Chemicals. All urinary metals were corrected for urinary creatinine (Cr) and reported as μg/g Cr25.
TOFAT and SII assessment
TOFAT was measured by dual-energy x-ray absorptiometry (DXA), a commonly utilized method of measuring body composition because of its rapid detection, simplicity of operation, and low radiation exposure. The use of DXA allowed for the detection of data on bone density and soft tissue counts throughout the body. In NHANES, DXA was used to measure the total fat of the body in participants between the ages of 8–59. Based on previous research, the final analysis included possible confounding factors associated with TOFAT26. Blood samples were transported to the NHANES Mobile Examination Centers for final determination. Detailed descriptions of the sample collection, transportation, and processing were presented in the NHANES Laboratory/Medical Technician Procedures Manual. Automated blood analysis equipment was used to measure lymphocyte, neutrophil, and platelet counts, and finally, the SII level was calculated by multiplying the platelet count by the neutrophil count/lymphocyte count12.
Covariates assessment
According to a statement on the NHANES website, demographic and lifestyle information about the population was collected by specialized personnel. The covariates we included age, gender, BMI, race, education level, drinking, smoking, physical activity, sedentary, and nutrient intake. Age was divided into < 45 years and ≥ 45 years. BMI was divided into normal (18.5–24.9 kg/m2) and abnormal (< 18.5 kg/m2 or ≥ 25.0 kg/m2). The race was divided into Hispanic, non-Hispanic-White, non-Hispanic-Blac, and non-Hispanic other. Education level divided into was less than high school degree, high school degree, and more than high school degree. Alcohol use was divided into never drinker, ever drinker, and current drinker. Smoking was divided into never smoker, ever smoker, and current smoker27. Daily exercise was divided into < 100 min MVPA (moderate to vigorous physical activity) and ≥ 100 min MVPA28. Sedentary was divided into < 360 min and ≥ 360 min. In addition, according to the recommended range of energy intake29, energy was divided into < 2050 kcal, 2050–3050 kcal, and ≥ 3051 kcal. The ratios of carbohydrates, fats, and proteins for supplying energy to the human body are as follows: carbohydrates should account for 55–60% of the total energy, fats should account for 25–30% of the total energy, and proteins should account for 10–15% of the total energy, and so we stratify the three substances according to the ratios. In addition, the results of the current study found a co-linearity between BMI and TOFAT (VIF = 6.370) so BMI was excluded for adjustment30.
Statistical analysis
We followed NHANES guidelines and accounted for complex survey design factors, including sample weights, clustering, and stratification. All statistical analyses were conducted by standards from the Centers for Disease Control and Prevention. Categorical parameters were reported as proportions, and continuous variables were expressed as inter-quartile range (IQR). Demographic characteristics of subjects were evaluated using chi-square, rank sum tests, and correlation analysis. Nonparametric tests were used to compare differences in metal concentrations between groups. Multivariable logistic regression was conducted to explore the associations of metals and SII with TOFAT, obtaining odds ratios (OR) and corresponding 95% confidence interval (CI). Trend tests were conducted to assess the relationship between metals and TOFAT. The restricted cubic splines (RCS) were used to explore the dose–response relationship between metals and TOFAT. Multivariable linear regression was used to explore the associations of metals with SII. We also used g-computation analysis to explore the mixture effect of metals on TOFAT and SII. Finally, the criteria under which mediation analysis can be performed are: “statistically significant correlation between X and M” and “statistically significant correlation between M and Y”31. Once the conditions for mediation analysis were met, we used the R language package “bruceR” to analyze the mediating role of SII in the relationship between metals and TOFAT. The mediation analysis was carried out using the Bayesian Monte Carlo method to avoid the writing of fitting algorithms by specifying the observation and state equations for each model32. The number of simulations in this study was set to 1000 and the seed was set to 1, to repeat the analysis to obtain consistent results. The direct effect meant that the metal had a direct effect on TOFAT and the indirect effect meant that the metal affected TOFAT through the mediator33. Directed acyclic graphs were used to confirm the confounding variables adjusted in the analysis, and the results were shown in Fig. S1. A two-sided value of P of < 0.05 was regarded as statistically significant. All statistical analyses were performed with SPSS (version 24.0) or R (version 4.3.1).
Ethics statement
The study was approved by the National Center for Health Statistics Research Ethics Review Board. The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Results
Characteristics of participants and metals distribution
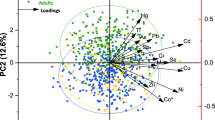
We included 3324 adults aged 18 years or older in our research. The demographic characteristics of the study subjects with low TOFAT or high TOFAT were listed in Table 1. Overall, gender, BMI, race, smoking, physical activity, energy intake, carbohydrate intake, and protein intake were statistically different between low TOFAT and high TOFAT participants. Participants with high TOFA were more likely to be female than those with low TOFAT. These subjects were also more likely to have an abnormal BMI and less time for physical activity. A difference has existed in SII between the low TOFAT group and the high TOFAT group (Table 1). As shown in Table S1, we also reported the demographic information of males and females. BMI, smoking, physical activity, and the intake of fat were different across TOFAT status in males and females. The geometric mean (GM) and quartiles of metal concentrations were shown in Table 2. The concentrations of Cd, Co, and Sn were higher in the high TOFAT group than in the low TOFAT group [GM (μg/g Cr): 0.166 vs 0.155, 0.388 vs 0.369, and 0.483 vs 0.400], while the opposite was observed for As and Pb [GM (μg/g Cr): 0.064 vs 0.079 and 0.288 vs 0.317]. The correlation analysis between the log-transform level of urinary metals was reported in Fig. S2.
Associations between metal concentrations and TOFAT
The associations between the level of metals and TOFAT were shown in Tables 3 and S2. As shown in model 3, after adjusting for confounders, 10 metals (As, Cd, Co, Cs, Hg, Mo, Mn, Pb, Sb, and Tl) had negative decreased trends of OR for TOFAT (all P for trend < 0.05). Compared with participants in the lowest quartile of As, Cd, Co, Cs, Hg, Mo, Mn, Pb, Sb, and Tl, participants in the highest quartile showed 59.2% (95%CI: 0.329, 0.505), 55.7% (95%CI: 0.342, 0.574), 31.8% (95%CI: 0.546, 0.852), 49.5% (95%CI: 0.407, 0.627), 50.9% (95%CI: 0.396, 0.608), 27.9% (95%CI: 0.586, 0.887), 46.0% (95%CI: 0.437, 0.667), 58.4% (95%CI: 0.330, 0.524) 19.1% (95%CI: 0.661, 0.989), and 27.3% (95%CI: 0.587, 0.900) decreased of TOFAT, respectively. In addition, RCS analyses showed nonlinear associations between Cd, Co, Cs, Tl, and TOFAT (with P-values for the nonlinearity of < 0.05). Non-linear associations were consistent between males and females at different metal concentrations (Fig. 2). The mixture effect of metals on TOFAT was shown in Fig. S3(a), and we found that Pb, Sn, As, Cd, and Co had a greater effect on TOFAT.
Associations between metal concentrations and SII
Figure 3 showed the relationship between metal concentrations and SII in the total, male, and female populations. In the total population, We found a positive connection between Cd, Co, Tu, and SII in the no covariate-adjusted models (model 1)[β = 54.48 (95%CI: 28.12, 80.84); β = 104.33 (95%CI: 67.59, 141.06); β = 35.90 (95%CI: 8.20, 63.60)] and in the fully adjusted model (model 3) [β = 29.70 (95%CI: − 2.46.65, 61.86); β = 79.37 (95%CI: 38.64, 120.11); β = 31.08 (95%CI: 3.36, 58.79)]. We also found that the reduction of SII was 35.03-fold (95%CI: − 58.81, − 11.25) and 58.12-fold (95%CI: − 81.41, − 34.83) for each unit increase in As and Hg concentrations. In males, the metal with which we found a statistically positive relationship with SII was only Cd [β = 68.01 (95%CI: 21.16, 114.86)], while in females, that was Co [β = 99.37 (95%CI: 46.47, 152.28)]. In males, the metals negatively associated with SII were As and Hg, while in females they were Hg and Pb. The mixture effect of metals on SII was shown in Fig. S3(b), we found that Hg, Cd, Co, Pb, and Tu contributed more to SII.
Associations between SII and TOFAT
Table S3 showed the associations between SII and TOFAT based on the multivariate logistic regression model. In the total, male, and female populations, SII always had a positive trend with TOFAT (P for trend < 0.001). In the total population, subjects in the highest quartile had an 82.1% increase in TOFAT compared to subjects in the lowest quartile of SII (Model 3), whereas TOFAT increased by 50.8% and 124.6% for males and females, respectively.
Mediation analyses
Based on the above results, the mediation analyses were performed. In the total population, after adjusting for confounders, SII had a mediated effect on the associations of Co with TOFAT, and the β of mediating effect was 0.9% (95%CI: 0.3%, 1.6%). The mediating effects of SII were not found on the associations of As, Cd, and Hg with TOFAT, with the β of mediating effect were − 0.4% (95%CI: − 1.0%, − 0.1%), 0.3% (95%CI: − 0.2%, 0.9%), and − 6.7% (95%CI: − 19.9%, 2.4%), respectively. Moreover, the direct effect coefficients of the associations between As, Co, Cd, Hg, and TOFAT were − 12.0% (95%CI: − 19.6%, − 5.6%), − 5.4% (95%CI: − 9.5%, − 1.4%), − 17.4% (95%CI: − 28.5%, − 6.2%), and − 44.3% (95%CI: − 48.4%, − 30.8%), respectively (Fig. 4). In males, SII was found to have a mediated role in the relationship between Cd, Hg, and TOFAT [βindirect = 1.3% (95%CI: 0.3%, 2.9%) and βindirect = − 13.9% (95%CI: − 20.1%, − 2.4%)] (Fig. S4). In females, SII was found to have a mediated effect on the associations of Co with TOFAT [βindirect = 1.3% (95%CI: 0.5%, 2.3%)] (Fig. S5).
Discussion
To the best of our knowledge, no current reports have explored the role of SII in the relationship between metals and TOFAT. Our study employed statistical methods, including linear and logistic regression, to investigate the relationship between metals, SII, and TOFAT, and to determine whether the conditions for mediation analysis were met. We then used mediation analysis with the quasi-Bayesian Monte Carlo method to examine the associations among metals, SII, and TOFAT in the total, male, and female populations. The results of our mediation analysis were satisfactory, providing valuable insights into the relationships.
Although the underlying mechanisms are not fully understood, it is plausible that metals play an important role in the metabolism of TOFAT. Interestingly, our study found that most metals had a negative trend with TOFAT, consistent with results from several other studies. For example, Vrijheid et al. found that Co was negatively associated with BMI in a study on environmental pollutants and childhood obesity34. Similarly, Su et al.35 observed that urinary As levels decreased with increasing BMI in a study comparing normal weight and obese students. Scinicariello et al.36 reported that adults in the highest quartile of Pb levels were less likely to be obese compared to those in the lowest quartile. Heavy metals such as Pb, Cd, and Hg are highly toxic and can disrupt normal cellular metabolic functions, leading to disturbances in energy metabolism and changes in TOFAT. Thayer et al.37 reported that heavy metals could disrupt endocrine function, interfere with insulin signaling, and inhibit normal glucose metabolism, leading to fat breakdown. Additionally, heavy metals can cause chronic inflammation, which damages fat cells and reduces fat storage, while the inflammatory response may accelerate fat breakdown and utilization38. Some heavy metals bind to proteins in the body, impairing the function of fat-metabolizing enzymes39. Exposure to heavy metals can also cause appetite loss and digestive disorders, affecting nutrient absorption and fat storage40,41. To minimize the health effects of heavy metals, effective measures should be taken to reduce exposure, such as improved environmental monitoring, safer management of food and water sources, and the promotion of healthy lifestyles.
Oxidative stress, a state where there is an imbalance between the production and removal of free radicals, causes cellular damage and is linked to many metals, including Cd, Co, and Hg. These metals can lead to elevated oxidative stress levels, triggering inflammation42,43. For instance, Cd can replace metal ions in certain metalloenzymes, leading to enzyme inactivity and increased oxidative stress44. It can also deplete intracellular glutathione, an antioxidant, rendering cells unable to effectively scavenge reactive oxygen species45. Co has been reported to stabilize hypoxia-inducible factors, mimicking a hypoxic environment and promoting reactive oxygen generation46,47. Both Cd and Hg can disrupt mitochondrial function, leading to the overproduction of reactive oxygen species48,49. Inhaled metal particles can cause local irritation and systemic inflammation50. Our study found that the levels of metals were consistent with those reported in other studies, reflecting widespread metal exposure in the general U.S. population51,52. In the total population, we found that Cd and Co were positively related to inflammation, consistent with the finding of Zhang et al.53. Liu et al.54 also found that markers of inflammation and oxidative stress in breath were associated with occupational exposure to Co. We also found that As level was negatively correlated with SII, likely because the total As concentration was included55. As is an indispensable element for the human body, with evidence obtained which indicates that As is of physiological importance, especially when methionine metabolism is stressed56. Interestingly, we found gender differences in the metals associated with SII: As and Cd were significant in males, while Co was significant in females. Additionally, Hg was negatively correlated with SII in all populations, and more experiments are needed to elucidate the exact mechanism.
Our mediation analysis revealed that SII played a mediating role in the association between Co and TOFAT. Although Co had a direct negative effect on TOFAT, its indirect effect through SII was positive, suggesting that Co exposure increased the level of TOFAT through the mediating effect of SII. Co-induced inflammation typically involves immune cell activation, platelet aggregation, and changes in lymphocyte counts57. In inflammatory states, the process of lipolysis may be inhibited, leading to fat accumulation58. Localized adipose tissue inflammation can alter the metabolic properties of adipocytes, leading to increased fat synthesis and decreased lipolysis59. Our results also reflected that the mediating effect of SII between Co and TOFAT was more pronounced in females. In addition, the mediating effect of SII was not statistically different on the associations of As, Cd, and Hg with TOFAT. Further investigations are needed to elucidate the role of metals, SII, and TOFAT. The effects of metals on immune cells, adipose precursor cell differentiation, and adipogenesis can be observed in cell culture systems. Metal exposure can be simulated in experimental animals to measure SII, pro-inflammatory cytokines, insulin sensitivity, and total fat levels. Changes in SII and total fat levels and their correlation can be evaluated in high metal exposure populations. The findings of the present study suggest that inflammatory markers may play a role in the effect of metals on TOFAT levels, while the current study only explored the role of SII, a novel inflammatory marker, and therefore more inflammatory markers need to be investigated. This study had the following advantages: Firstly, the study had a large sample (3,324 participants) and a long investigation time (NHANES 2011-2018). Secondly, we first analyzed the role of SII in the relationship between metals and TOFAT. Thirdly, we included all covariates associated with TOFAT to ensure that the results of this study were stable and reliable. In addition, our study has some limitations. Firstly, some of the covariates contain reporting information bias. Secondly, this study was cross-sectional and weak in determining causal functions.
Conclusions
In conclusion, our results indicated that most metals had a negative trend with TOFAT. In addition, Co had a positive association with TOFAT which was completely mediated by SII. Our findings have important research implications at a time when obesity health problems are becoming serious and harmful metal exposure is widespread. More investigations are needed to explore the potential biological mechanisms underlying the relationship between SII, metals, and TOFAT.
Data availability
The datasets generated and/or analysed during the current study are available in the NHANES repository, https://www.cdc.gov/nchs/nhanes/index.htm.
References
Raviv, S., Dixon, A. E., Kalhan, R., Shade, D. & Smith, L. J. Effect of obesity on asthma phenotype is dependent upon asthma severity. J. Asthma 48, 98–104. https://doi.org/10.3109/02770903.2010.534220 (2011).
Rizvi, M. K. et al. Astounding health benefits of jamun (Syzygium cumini) toward metabolic syndrome. Molecules 27, 21. https://doi.org/10.3390/molecules27217184 (2022).
Park, T. J. et al. Myonectin inhibits adipogenesis in 3T3-L1 preadipocytes by regulating p38 MAPK pathway. BMB Rep. 54, 124–129. https://doi.org/10.5483/BMBRep.2021.54.2.262 (2021).
Kopp, B., Zalko, D. & Audebert, M. Genotoxicity of 11 heavy metals detected as food contaminants in two human cell lines. Environ. Mol. Mutagen 59, 202–210. https://doi.org/10.1002/em.22157 (2018).
Eom, S. Y. et al. Allele frequencies of the single nucleotide polymorphisms related to the body burden of heavy metals in the Korean population and their ethnic differences. Toxicol. Res. 32, 195–205. https://doi.org/10.5487/tr.2016.32.3.195 (2016).
Mwove, J., Imathiu, S., Orina, I. & Karanja, P. Environmental exposure assessment of lead and cadmium in street vended foods sold in selected locations in Kenya. Food Sci. Nutr. 11, 2610–2619. https://doi.org/10.1002/fsn3.3344 (2023).
Luo, K. H. et al. Use of generalized weighted quantile sum regressions of tumor necrosis factor alpha and kidney function to explore joint effects of multiple metals in blood. Int. J. Environ. Res. Public Health 19, 12. https://doi.org/10.3390/ijerph19127399 (2022).
González-Domínguez, Á., Millán-Martínez, M., Domínguez-Riscart, J., Lechuga-Sancho, A. M. & González-Domínguez, R. Metal homeostasis and exposure in distinct phenotypic subtypes of insulin resistance among children with obesity. Nutrients 15, 2347. https://doi.org/10.3390/nu15102347 (2023).
Kupsco, A. et al. Prenatal metal concentrations and childhood cardiometabolic risk using Bayesian Kernel machine regression to assess mixture and interaction effects. Epidemiology 30, 263–273. https://doi.org/10.1097/ede.0000000000000962 (2019).
Maret, W. The metals in the biological periodic system of the elements: Concepts and conjectures. Int. J. Mol. Sci. 17, 66. https://doi.org/10.3390/ijms17010066 (2016).
Bordoni, A. et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 57, 2497–2525. https://doi.org/10.1080/10408398.2014.967385 (2017).
Mahemuti, N. et al. Association between Systemic Immunity-Inflammation Index and Hyperlipidemia: A population-based study from the NHANES (2015–2020). Nutrients 15, 1177. https://doi.org/10.3390/nu15051177 (2023).
Holub, K. et al. Analysis of systemic inflammatory factors and survival outcomes in endometrial cancer patients staged I-III FIGO and treated with postoperative external radiotherapy. J. Clin. Med. 9, 5. https://doi.org/10.3390/jcm9051441 (2020).
Xu, X. et al. Association between systemic immune inflammation level and poor prognosis across different glucose metabolism status in coronary artery disease patients. J. Inflamm. Res. 16, 4031–4042. https://doi.org/10.2147/jir.s425189 (2023).
Chen, Y., Xie, K., Han, Y., Xu, Q. & Zhao, X. An easy-to-use nomogram based on SII and SIRI to predict in-hospital mortality risk in elderly patients with acute myocardial infarction. J. Inflamm. Res. 16, 4061–4071. https://doi.org/10.2147/jir.s427149 (2023).
Vega-Millán, C. B. et al. Inflammation biomarkers associated with arsenic exposure by drinking water and respiratory outcomes in indigenous children from three Yaqui villages in southern Sonora, Mexico. Environ. Sci. Pollut. Res. Int. 28, 34355–34366. https://doi.org/10.1007/s11356-021-13070-x (2021).
Baek, K. & Chung, I. Cadmium exposure is associated with monocyte count and monocyte to HDL ratio, a marker of inflammation and future cardiovascular disease in the male population. J. Korean Med. Sci. 32, 1415–1422. https://doi.org/10.3346/jkms.2017.32.9.1415 (2017).
Zhong, Q. et al. Independent and combined associations of blood manganese, cadmium and lead exposures with the systemic immune-inflammation index in adults. Toxics 11, 659. https://doi.org/10.3390/toxics11080659 (2023).
Osborn, O. & Olefsky, J. M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374. https://doi.org/10.1038/nm.2627 (2012).
Stolarczyk, E. et al. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell Metab. 17, 520–533. https://doi.org/10.1016/j.cmet.2013.02.019 (2013).
Arpón, A. et al. Methylome-wide association study in peripheral white blood cells focusing on central obesity and inflammation. Genes Basel 10, 444. https://doi.org/10.3390/genes10060444 (2019).
Chen, C., Ye, Y., Zhang, Y., Pan, X. F. & Pan, A. Weight change across adulthood in relation to all cause and cause specific mortality: Prospective cohort study. Bmj 367, l5584. https://doi.org/10.1136/bmj.l5584 (2019).
Kelly, T. L., Wilson, K. E. & Heymsfield, S. B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One 4, e7038. https://doi.org/10.1371/journal.pone.0007038 (2009).
Huang, Z. et al. Relationship of blood heavy metals and osteoporosis among the middle-aged and elderly adults: A secondary analysis from NHANES 2013 to 2014 and 2017 to 2018. Front. Public Health 11, 1045020. https://doi.org/10.3389/fpubh.2023.1045020 (2023).
Zhong, Q. et al. Exposure to multiple metals and the risk of hypertension in adults: A prospective cohort study in a local area on the Yangtze River, China. Environ. Int. 153, 106538. https://doi.org/10.1016/j.envint.2021.106538 (2021).
Lin, Z. et al. Correlation between sedentary activity, physical activity and bone mineral density and fat in America: National Health and Nutrition Examination Survey, 2011–2018. Sci. Rep. 13, 10054. https://doi.org/10.1038/s41598-023-35742-z (2023).
Liu, Y. et al. Association of serum 25(OH)D, cadmium, CRP with all-cause, cause-specific mortality: A prospective cohort study. Front. Nutr. 9, 803985. https://doi.org/10.3389/fnut.2022.803985 (2022).
Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462. https://doi.org/10.1136/bjsports-2020-102955 (2020).
National Research Council Subcommittee on the Tenth Edition of the Recommended Dietary, A. In Recommended Dietary Allowances: 10th Edition (National Academies Press, 1989).
Wang, Z. et al. The relationship between triglyceride-glucose index and albuminuria in United States adults. Front. Endocrinol. Lausanne 14, 1215055. https://doi.org/10.3389/fendo.2023.1215055 (2023).
Wu, S. et al. Joint effect of multiple metals on hyperuricemia and their interaction with obesity: A community-based cross-sectional study in China. Nutrients 15, 552. https://doi.org/10.3390/nu15030552 (2023).
Hajizadeh, N., Pourhoseingholi, M. A., Baghestani, A. R., Abadi, A. & Zali, M. R. Bayesian adjustment for over-estimation and under-estimation of gastric cancer incidence across Iranian provinces. World J. Gastrointest. Oncol. 9, 87–93. https://doi.org/10.4251/wjgo.v9.i2.87 (2017).
Chen, L. et al. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. 20, 207. https://doi.org/10.1186/s12916-022-02403-3 (2022).
Vrijheid, M. et al. Early-life environmental exposures and childhood obesity: An exposome-wide approach. Environ. Health Perspect. 128, 67009. https://doi.org/10.1289/ehp5975 (2020).
Su, C. T. et al. The relationship between obesity, insulin and arsenic methylation capability in Taiwan adolescents. Sci. Total Environ. 414, 152–158. https://doi.org/10.1016/j.scitotenv.2011.10.023 (2012).
Scinicariello, F., Buser, M. C., Mevissen, M. & Portier, C. J. Blood lead level association with lower body weight in NHANES 1999–2006. Toxicol. Appl. Pharmacol. 273, 516–523. https://doi.org/10.1016/j.taap.2013.09.022 (2013).
Thayer, K. A., Heindel, J. J., Bucher, J. R. & Gallo, M. A. Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environ. Health Perspect. 120, 779–789. https://doi.org/10.1289/ehp.1104597 (2012).
Moon, S. S. Association of lead, mercury and cadmium with diabetes in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabet. Med. 30, e143-148. https://doi.org/10.1111/dme.12103 (2013).
Aquino, N. B., Sevigny, M. B., Sabangan, J. & Louie, M. C. The role of cadmium and nickel in estrogen receptor signaling and breast cancer: Metalloestrogens or not?. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 30, 189–224. https://doi.org/10.1080/10590501.2012.705159 (2012).
Vázquez, M. et al. Toxic trace elements at gastrointestinal level. Food Chem. Toxicol. 86, 163–175. https://doi.org/10.1016/j.fct.2015.10.006 (2015).
Charkiewicz, A. E., Omeljaniuk, W. J., Nowak, K., Garley, M. & Nikliński, J. Cadmium toxicity and health effects— brief summary. Molecules 28, 6620. https://doi.org/10.3390/molecules28186620 (2023).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453-462. https://doi.org/10.1016/j.cub.2014.03.034 (2014).
Yu, S., Wang, X., Zhang, R., Chen, R. & Ma, L. A review on the potential risks and mechanisms of heavy metal exposure to Chronic Obstructive Pulmonary Disease. Biochem. Biophys. Res. Commun. 684, 149124. https://doi.org/10.1016/j.bbrc.2023.149124 (2023).
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A. & Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 17, 3782. https://doi.org/10.3390/ijerph17113782 (2020).
Glusic, M., Ropret, P., Vogel-Mikus, K. & Grdadolnik, J. The binding sites of cadmium to a reduced form of glutathione. Acta Chim. Slov. 60, 61–69 (2013).
Wang, G. L. & Semenza, G. L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. U. S. A. 90, 4304–4308. https://doi.org/10.1073/pnas.90.9.4304 (1993).
Kaelin, W. G. Jr. & Ratcliffe, P. J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402. https://doi.org/10.1016/j.molcel.2008.04.009 (2008).
Wu, X. et al. A brief exposure to cadmium impairs Leydig cell regeneration in the adult rat testis. Sci. Rep. 7, 6337. https://doi.org/10.1038/s41598-017-06870-0 (2017).
Jia, G., Aroor, A. R., Martinez-Lemus, L. A. & Sowers, J. R. Mitochondrial functional impairment in response to environmental toxins in the cardiorenal metabolic syndrome. Arch. Toxicol. 89, 147–153. https://doi.org/10.1007/s00204-014-1431-3 (2015).
Gawda, A. et al. Air particulate matter SRM 1648a primes macrophages to hyperinflammatory response after LPS stimulation. Inflamm. Res. 67, 765–776. https://doi.org/10.1007/s00011-018-1165-4 (2018).
Nwanaji-Enwerem, J. C. et al. Individual species and cumulative mixture relationships of 24-hour urine metal concentrations with DNA methylation age variables in older men. Environ. Res. 186, 109573. https://doi.org/10.1016/j.envres.2020.109573 (2020).
Sobel, M. et al. Environmental-level exposure to metals and metal-mixtures associated with spirometry-defined lung disease in American Indian adults: Evidence from the Strong Heart Study. Environ. Res. 207, 112194. https://doi.org/10.1016/j.envres.2021.112194 (2022).
Zhang, H., Yan, J., Niu, J., Wang, H. & Li, X. Association between lead and cadmium co-exposure and systemic immune inflammation in residents living near a mining and smelting area in NW China. Chemosphere 287, 132190. https://doi.org/10.1016/j.chemosphere.2021.132190 (2022).
Liu, L. et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ. Health Perspect. 117, 668–674. https://doi.org/10.1289/ehp11813 (2009).
Kesici, G. G. Arsenic ototoxicity. J. Otol. 11, 13–17. https://doi.org/10.1016/j.joto.2016.03.001 (2016).
Uthus, E. O. Evidence for arsenic essentiality. Environ. Geochem. Health 14, 55–58. https://doi.org/10.1007/bf01783629 (1992).
Assinger, A., Schrottmaier, W. C., Salzmann, M. & Rayes, J. Platelets in sepsis: An update on experimental models and clinical data. Front. Immunol. 10, 1687. https://doi.org/10.3389/fimmu.2019.01687 (2019).
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465. https://doi.org/10.1038/s42255-021-00493-6 (2021).
Kawai, T., Autieri, M. V. & Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 320, C375-c391. https://doi.org/10.1152/ajpcell.00379.2020 (2021).
Acknowledgements
The authors thank the National Center for Health Statistics of the Centers for Disease Control and Prevention for sharing the data.
Funding
No external funding source was required for the current study.
Author information
Authors and Affiliations
Contributions
Weipeng Zhang: Conceptualization, Writing – original draft. Cong Zhang: Supervision, Writing – review & editing, Validation. Dengqiu Lu and Junfeng Nie: Software, Data curation, Writing – review & editing. Zhumin Hu: Methodology, Writing – review & editing. Cuiyao Xian and Minxing He: Visualization, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, W., Zhang, C., Lu, D. et al. The mediation effect of Systemic Immunity Inflammation Index between urinary metals and TOFAT among adults in the NHANES dataset. Sci Rep 14, 14940 (2024). https://doi.org/10.1038/s41598-024-65925-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65925-1