Abstract
The aim of the present research was to investigate the presence of heavy metals such as lead (Pb), copper (Cu), chromium (Cr), and cadmium (Cd) in blood samples from cows raised with irrigated wastewater, as well as in the wastewater itself, in the North-western region of Pakistan. A total of 60 blood samples were collected from five different locations in Kohat, namely Tappi Road (TR), Pindi Road (PR), Gul Malik Road (GMR), Markaz Road (MR), and a control group. The samples of both i.e. cow blood and wastewater were analyzed for the concentrations of heavy metals. The highest concentration of Cd was detected in the MR site with a mean value of 0.03 mg/L, and the highest concentration of Cu (0.04 mg/L) was recorded in the TR site, while the lowest level was found in the control group with a mean of 0.002 mg/L in blood samples. The highest Cr and Pb concentrations were found at the PR site, with mean values of 0.03 and 0.07 mg/L, respectively, whereas the control group had the lowest concentrations, with mean values of 0.002 and 0.01 mg/L. Similarly, heavy metal concentrations were analyzed in wastewater used for irrigation in the study area. Results indicated elevated concentrations of Cu and Cr in wastewater, although they remained below the World Health Organization (WHO) recommended values except for Cr (0.13 mg/L) in the GMR site, which exceeded permissible limits. Cd and Pb concentrations in wastewater were relatively low, but Cd concentration surpassed WHO limits, particularly with a mean concentration of 0.08 mg/L in the TR site. Comparison between heavy metal concentrations in blood and wastewater revealed higher values of Cd and Pb in blood samples than in wastewater, while Cu and Cr concentrations were higher in water compared to blood. Additionally, elevated levels of Super Oxide Dismutase (SOD), antioxidant enzyme Catalase (CAT), and oxidative stress marker malondialdehyde (MDA) were detected in blood samples. Cluster and principal component analyses were employed to assess heavy metal toxicity among the groups, indicating potential long-term adverse health effects on animals, transfer to humans, and toxicity in living organisms.
Similar content being viewed by others
Introduction
Heavy metal pollution poses a significant threat to the global environment, as these hazardous elements are widespread in the air, water, and food sources within the Earth's crust. The increasing concern over environmental pollution attributed to toxic heavy metals is evident1. These metals, known for their high density and harmful characteristics even in minute quantities, present a grave risk to ecosystems2. The abundance of toxic heavy metals results in adverse effects like destabilization of biological communities through their accumulation in various life forms. Consequently, this phenomenon has detrimental impacts on biota and may contribute to the decline of numerous living organisms3.
Various sources contribute to soil pollution, including wastewater, pesticides, mining activities, oil spills, and industrial effluents4,5. These factors introduce heavy metals into the environment, increasing the uptake of metals by crops. Wastewater, in particular, serves as a significant reservoir of harmful chemicals that negatively affect agricultural production and other organisms6. The combined influence of these sources underscores the urgency of addressing heavy metal pollution to protect both the environment and its inhabitants7.
Among the heavy metals, Copper (Cu), Cadmium (Cd), Mercury (Hg), and Lead (Pb) are highly toxic metals8. Cd, arsenic, silver, molybdenum, Pb, and selenium are heavy metals that cause carcinogenic, mutagenic, and teratogenic effects in the living body9. Cd produces oxidative stress. It stimulates the production of free radicals, produces harmful damage, and changes the balance between antioxidants in cells of living organisms10.
The heavy metals accumulation in plants is transferred to animals by consuming those exposed plants11. They cause toxicity in animal bodies as well as in humans12. Due to industrialization and urbanization, heavy metals enter the environment as gases, liquids, and solids. Heavy metals enter the food in different ways13. The industrial metallurgical process causes Cd, Pb, Cu, and Zn contamination. In animals, grazing around the industries, heavy metals are transferred through the food chain14.
The activities of enzymes can be inhibited due to some heavy metals’ attachment with substrate complexes, denaturalizing the protein of enzymes and interacting with the active sites of enzymes15. The role of oxidative stress is very important in the pathogenesis of various pathological conditions, include acute lung injury, mood disorders, ischemia–reperfusion injury (IRI), and neurodegenerative diseases16,17.
Sometimes oxidative stress is produced due to the lack of oxygen/or a large antioxidant amount of reactive oxygen/nitrogen species (ROS/RNS)18. The itemizations of superoxide anion O2- to water and hydrogen peroxide are catalyzed by Super Oxide Dismutase (SOD), and the Catalase (CAT) enzyme comes and detoxifies the remaining components19. The CAT and SOD enzymes of antioxidants compete with the toxicity of oxygen and work as a defensive system20,21. Malondialdehyde (MDA) is the ending product of lipid peroxidation, a charged molecule of minor molecular mass. Although it is an oxidative stress indicator, MDA can be measured in various biological samples. The MDA level is increased in animals and other organisms due to high concentrations of heavy metals22.
In the study area, industrial and urban wastewater is commonly used for irrigation, leading to the accumulation of various heavy metals accumulate in crops and grasses. Local cattle graze on areas irrigated with this wastewater, raising concerns about the impact and toxicity of heavy metals on plants, animals, humans, and the environment. The present study aimed to (a) assess the Pb, Cu, Cr, and Cd concentrations in blood samples of the cow (b) analyze heavy metals concentration in wastewater, (c) determine the level of SOD, CAT and lipid peroxidation products (MDA) in blood samples of the cow and to (d) compare heavy metals concentrations in blood of Cow feeding on wastewater irrigated areas with the control area.
Materials and methods
Study area
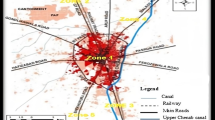
The research site i.e. District Kohat, is in the northwestern region of Pakistan, encompassing a total land area of 2,545 km2, with an altitude of approximately 508 m above sea level, at 71°26′32″ East longitude and 33°35′13″ North latitude23 (Fig. 1). Marked by a mountainous topography, the area witnesses an annual rainfall of around 638 mm and experiences a maximum temperature of 40 °C.
Map of the Study area and Digital Elevation Model (DEM). The software ArcGIS (version10.5; https://malagis.com/arcgis-enterprise-105-download.html) was use to draw the map of the country province, and detail study area.
The District comprises three tehsils: Kohat, Seni Gumbat, and Lachi, where the Tehsil Kohat distinguishes itself as a predominantly urbanized area in comparison to the others. This tehsil is characterized by a relatively high population and well-established agricultural infrastructure. The cow blood samples were collected from Tehsil Kohat, specifically from its five locations—Tappi Road (TR), Pindi Road (PR), Gul Malik Road (GMR), Markaz Road (MR), and a designated control group.
Sampling sites and blood sampling
A total of 66 blood samples, approximately 6 ml each, were collected from cows fed on various sites in the study area. in EDTA tubes with gentle handling to prevent hemolysis) The samples were collected in EDTA tubes with care to prevent hemolysis and refrigerated at 4 °C until various biochemical and heavy metals analyses were done.
Acid digestion of blood samples for heavy metal determination
Acid digestion was carried out to identify the selected heavy metals in blood samples. The 0.5 mL blood samples were put in 5 mL beakers with 3 mL of a nitric acid (15.7 M) and mixture of hydrogen peroxide (9.8 M) (Lab Alley and Bob's Best). Subsequently, 50 ml of water were added to each sample. For ten minutes, the solution was kept at equilibrium. Next, two milliliters of HNO3 and H2O2 were added to the samples, and they were digested at 60 °C. After the solution was heated until changed color, it was put into plastic bottles for storage24.
Elemental analysis of blood samples
The concentration of the selected heavy metals was determined using the atomic absorption spectrometer (PerkinElmer A Analyst 700, USA).
Erythrocytes from blood
The blood samples were prepared by following the method of25. Initially, 2 ml blood sample was mixed with 4 ml Sodium phosphate buffer and centrifuged twice at 4000 rpm for 10 min at 4 °C. The resultant supernatant was disposed of and the pellet was washed and stored with a phosphate buffer solution, from which the biochemical activities were conducted.
Determination of MDA contents in erythrocyte
The malondialdehyde content in both experimental and control blood samples were assessed by the method26. A mixture of 2 ml MDA solutionand0.5 ml of erythrocytes in phosphate buffer (pH0.1; 7.4 M) was heated on a boiling bath for 10 min. After cooling, the was centrifuged at 4000 rpm for 15 min, and the supernatant was collected. The absorbance readings were taken at 532 and 600 nm independently, and the intensity of lipid peroxidation was taken in absorbance units by using the given formula.
where A = Amount of enzyme solution, OD = Optical Density, V = Total volume of buffer solution, FW = Fresh weight of blood sample, e = Enzyme extract for research, andє = Ebsilon.
Analysis of SOD in erythrocytes
Superoxide dismutase (SOD) activity was analyzed by the method of27. A substrate composition consisting of 0.2 mg Riboflavin, 15.5 mg NBT, 485 mg methionine, and 100 mg Na (EDTA) was prepared in a reagent bottle, with addition of distilled water to achieve a volume of 250 ml. The reagents NBT and Riboflavin were combined separately with distilled water to create the reagent solution.
For the SOD activity, a 3mlsolutionwas prepared in a beaker, containing 0.025 ml water, 2.725 ml reaction substrate and 0.025 ml enzyme extract, and exposed to light for 20 min. Similarly, the control sample was subjected to the same conditions. Subsequently, SOD activity was determined by measuring absorbance at 560 nm.
The formula for SOD determination:
Where W is the sample's fresh weight, Vt is the amount of enzyme extract used in the reaction solution to test SOD, Ack is the OD value of the buffer solution used to extract the enzyme, and Ae is the OD value on the spectrophotometer.
Estimation of CAT in blood erythrocytes
The method of28 was used to find out the catalase activity. A 0.9 ml phosphate solution, 0.4 ml of H2O2and 0.1 ml of erythrocytes suspension, were added to a test tube. The reaction was halted after 60 s by adding 2 ml of acetic acid and potassium dichromate. The tubes were subsequently placed in a boiling water bath for 10 min, cooled, and the produced color measured at 620 nm. A 2.8 ml phosphate buffer (pH 7.4, 0.1 ml) and 0.1 ml of H2O2 were utilized. The activity was computed using the following formula:
W is the sample's fresh weight, and A is its activity or OD value. V is the total volume of the enzyme-extraction buffer solution. A is the amount of enzyme extract used in the reaction solution for testing, E is the activity constant or 39.4 mM/cm.
Wastewater sampling and treatment
Wastewater samples were collected in polyethylene bottles from different site of the study area mirroring the sites from where the blood samples were collected. The samples were cleaned with nitric acid and deionized water before use. Each bottle, containing one liter of wastewater, was preserved with 5 ml HNO3. The sampling was conducted by following the standard methods used by29. The samples were transported to the laboratory and stored until further analysis.
Laboratory analysis
The assessment of heavy metals was done by adding 5ml of concentrated HCland2ml of concentrated HNO3 to a 100 ml water sample. The solution was enclosed with a watch glass and heated at 95°C until its volume was reduced to 15ml, followed by cooling. Finally, the volume was attuned to 100 ml with distilled water. The concentrations of Cu, Cr, Cd, and Pb in water sample were determined using atomic absorption spectrophotometer.
Statistical analysis
The statistical analysis was performed using Graph Pad Prism 8.0 for one-way analysis of variance (ANOVA), while correlation analysis was done through SPSS. A significance level of p < 0.05 was considered significant. The Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) were done by Bio Vinci. Version: 3.0.0.
Ethical statement
The research involved a detailed study of cows, where blood samples were collected from the animals following all the research protocols and guidelines as reported by arrive (PLoS Bio 8(6), e1000412, 2010). All the experiments performed on cow were approved by the Ethical Research Committee, Department of Pharmacy, CUI, Abbottabad Campus (PHM.Eth/CS-M01-03-0323) and confirmed with the rulings of the Institute of Laboratory Animal, Resources, Commission (on Life Sciences,) National (Research Council (NRC, 2010).
Results and discussion
Analysis of heavy metals in cow’s blood samples
Heavy metals are the most dangerous and toxic substance that accumulates in animals, humans, and other organisms’ bodies. They enter the bodies through food, water, and other sources30. The concentrations of four toxic heavy metals in cow blood samples, Cr, Pb, Cu, and Cd, were analyzed through an atomic absorption spectrometer. The cow’s blood samples were collected from contaminated areas such as TR, PR, GMR, MR, and the unpolluted area as control group. The results showed that in contaminated areas, toxic heavy metals were found to be high with mean values, while low levels were found in the control group.
Concentration of Cd in blood samples
Cd is highly toxic to every animal and the human body. It accumulates and increases in the body with age31. The Cd concentration was found in contaminated and controlled areas with a mean value of 0.024, 0.026, 0.0172, 0.035, and 0.0018 mg/L in TR, PR, GMR, MR, and control group respectively. The highest mean value was found in MR and the lowest in the control group (Fig. 2). Analysis showed a significantly higher difference of Cd concentrations (P < 0.001) in cow blood samples of contaminated sites as compared to the control group, although below the permissible level (0.05 mg/L)32, (0.05 mg/L). Previous studies33,34,35 also reported a high level of Cd concentration in cow blood grazed in polluted areas. Heavy metals like Cd and Pb are passed quickly through the food chain and produces highly toxic impacts on humans and animals36,37 and should be monitored in cattle blood.
Concentration of Cu in blood samples
The Cu concentration was higher in contaminated areas than the control group in cows' blood samples. In the control area, Cu concentration was lower (0.0019 mg/L), whereas in contaminated areas, mean concentrations ranged from 0.04 to 0.031 mg/L (Fig. 3). Significant differences (P < 0.001)were found between control and contaminated sites, albeit it doesn’t exceeds the permissible limit of32 (Cu, 1.00 mg/L). The study’s findings align with previous research, which also reported elevated Cu concentrations in cow blood38,39.
Concentration of Cr in blood samples
Cr exists in various structures within food, posing risks to animals and humans upon exposure. A small amount of Cr is vital for humans and other organisms. However, when permissible limits are exceeded, it causes toxicity; 100 mg/L causes a threat to humans, while food levels of 5–7 mg/L become dangerous for human and animal consumption. Cu concentration was detected in TR, PR, GMR, MR, and control with a mean value of (0.024, 0.029, 0.019, 0.0024, and 0.0015 mg/L) respectively, as shown in Fig. 3. The highest value of Cr was detected in PR, while the lowest mean value of Cr was found in the control area, as shown in the Fig. 4 below. Statistically significant differences between control and contaminated sites (P < 0.001). The exposed area's blood samples were higher than the permissible level set by WHO/FAO, which is 1.00 mg/L. In contrast, the mean concentration of control was found below the32 limit40 reported a high level of Cr in cow blood that showed a resemblance to the present study33, which also studied Cr in cattle blood, which was higher than in the present study38, detected Cr concentration in cows' blood at lower levels than in the present study. Similarly33,41, also investigated Cr concentration in animal blood, and the concentration of Cr was lower than in the present study in cow blood samples.
Concentration of Pb in blood samples
The Pb accumulates in the body's vital organs like liver, bone, brain, and kidney42. The highest Pb mean value was observed in PR cow's blood samples, while the lowest was in the control area. Mean Pb concentration in contaminated areas (TR, PR, GMR, MR) and control group were 0.045, 0.069, 0.032, and 0.042 and 0.0061 mg/L, respectively (Fig. 5). Significant differences were noted between the contaminated and the control group (P < 0.001). WHO/FAO permissible level of Pb concentration is 0.50 mg/L, which was not exceeded in any group32,43. Researchers observed the high Pb concentrations in the cows grazed around the contaminated sites, which are aligned with our findings33,35,44.
Heavy metals analysis in wastewater
Four heavy metals (Cd, Cr, Pb, and Cu) were analyzed in irrigation waste water (Table 1). The concentrations of Cd range from 0.06to 0.02 mg/L (mean value: 0.08 mg/L) in site TR, 0.017–0.014mg/L (mean value: 0.02 mg/L) in site PR 0.012–0.045 (mean value: 0.052 mg/L) in site MR, and 0.025–0.001 mg/L (mean value: 0.035 mg/L) in site GMR. The high mean Cd value was in site TR (0.08 mg/L), while the lowest was recorded in site PR (0.017 to 0.041). Notably, the Cd mean value was above the permissible limit (0.01 mg/L)32.
The Cr concentration varied across the study sites, ranging from 0.034 to 0.025 mg/L (mean value: 0.023mg/L),0.012 to 0.07 mg/L (mean: 0.021mg/L), and 0.024 to 0.002 mg/L (mean value: 0.03mg/L) in TR, PR, and MR sites of the study area, while it ranged from 0.13 to 0.041 mg/L (mean value: 0.07 mg/L) in wastewater samples of the study area. The highest mean value (0.13–0.014) was in site GMR while the lowest (0.021 mg/L) was recorded mg/Lin the site PR. All the values were within the permissible limit32 except the site GMR. The Cu concentrations ranged from 0.073 to 0.056 mg/L (mean value: 0.087 mg/L), 0.03 to 0.07 mg/L (mean value: 0.043 mg/L), 0.62 to 0.07 mg/L (mean value: 0.21 mg/L), and 0.07 to 0.05 mg/L (mean value: 0.03 mg/L) in the TR, PR, MR and GMR sites of the study area. The highest (0.21 mg/L) and the lowest (0.043 mg/L) mean value was found in MR and PR sites, respectively. All the values of Cu were within the permissible limit (1.00 mg/L)32. The Pb concentrations ranged from 0.043 to 0.031 mg/L (mean value: 0.040 mg/L), 0.084 to 0.060 mg/L (mean: 0.091 mg/L), 0.12 to 0.06 mg/L (mean value: 0.010 mg/L) and 0.013 to 0.003 mg/L (mean value: 0.016 mg/L) in wastewater samples of the sites TR, PR, MR, and GMR. The highest mean value was found in site MR whiles the lowest in the GMR site. All Pb concentrations were within the permissible limits (0.50 mg/L)32.
Heavy metals comparison in blood and wastewater
Comparison of heavy metals in blood and wastewater reveals that the Cd and Pb were higher in blood samples of animals as compared to wastewater while the Cu and Cr were higher in wastewater as compared to blood samples (Fig. 6). This suggests that contamination of cow’s blood is not only from waste water but may include other factors like fertilizer, insecticide and pollution from vehicle combustion. Burning of Pb and its discharge into the environment can be accountable for elevated concentration of Pb in some flora, roadside areas, soil, air, water and plants. Additionally, the contamination may also occur from industrial waste and from fertilizer uses45,46.
Principal component analysis and biplots
The PCA assists in providing knowledge on the most important variables, which indicate the entire data set, understanding and data minimization with the least damage to the original data47. Results of PCA for heavy metals in the blood of Cows are mentioned in Table 2. PCA of HMs in blood samples indicated three components. The first component, F1, with an Eigen value of (1.65) contributed 89.01% of the cumulative variability of Cd, Cu, Cr, and Cr with positive loading values (0.36, 0.69, 0.58 and 0.90, respectively), as shown in Table 2. This component demonstrated that anthropogenic pollution of HMs was prevalent, with fertilizers, pesticides, and contamination in irrigation water contributing to most of these metals. All of the positive loading values on F1 pointed to the same/common source of contamination of selected metals in blood samples, namely anthropogenic sources such as domestic waste/sludge, and commercial and industrial48,48,49. F2 had a cumulative variability of 88.04 with negative loading values of Cd and Cu (− 0.35 and − 0.18), whereas Cr and Pb have positive values of 0.21 and 71. PC3 had a 99.01% cumulative variance, with maximum negative loading values (− 0.02) and Pb (0.34), respectively, while Cu and Cr have negative loading values (0.43 and 0.02). The factors that influence mainly originated from natural phenomena (sediment, rock, soil, dust, etc.) and human-related activities (fish breeding, mining, residential activities, agricultural activities, etc.)50,51.
The biplot is a useful tool for determining the magnitude and sign of each variable influencing the first two or more factor loadings, as well as for seeing how each variable visualizes in terms of loadings47. It might have a complex structure based on the projected loadings from the unrotated factor investigation. The main objective of factor rotation is to find a parameterization where each variable has a limited number of large loadings. The biplot (Fig. 7a) shows the distribution of sample stations and variables. A correlation biplot approach based on parameters and sample sizes was used to create the diagram. According to the biplot, the first two components accounted for 88.04% of the overall variance, with the initial components loaded at 63.28%. The biplot showed that the majority of the variables, including Pb, Cu, and Cd were located on the lower right side of biplot F1. Figure 7b displays the variable distribution in the biplot versus F1 to F2. The diagram also revealed that the first two components accounted for 88.04% of the variance, with the majority of the variables located on the lower right side of the diagram (F1), while only one variable was located at the upper right side F1. The results demonstrated that the initial components had the highest loading to the other components and that the major parameters in F1 were significantly related.
Hierarchical cluster analysis (HCA) of heavy metals
The HCA of heavy metals in the blood samples of cows from five different locations is shown in a heat map (Fig. 8). The color scheme represents the concentrations of four heavy metals studied. Similar elements refer to the same source of origin and entrance into the environment. The analysis showed similarities and dissimilarities among the metals. The metals were classified into one main cluster consisting of two sub-groups i.e., Cu and Cd and Pb and Cr. These sub-groups had a close relationship indicating a common source for each pair. The results showed agreement with the finding of52, metals' concentration in blood was framed into the three clusters of Cr, Cu, Pb, and Cd. The heavy metal level in this type of sample is considerable, and it also has the same or a relevant source, as well as affecting factors38.
Malondialdehyde (MDA)
The mean MDA level was variable in contaminated sites, and the control area (Fig. 9). The highest mean level of MDA was found in TR (7.75 µmol/L), while the lowest was in the control group (1.51 µmol/L). In stressful conditions, MDA contents increase in cells which may damage the living cells. Malondialdehyde was significantly high (P < 0.001) in all blood samples was taken from wastewater-irrigated areas as compared to the control area. The present results align with the previous studies showing the same pattern22,53. In oxidative stress, MDA is a familiar lipid peroxidation indicator whenever exposure occurs to Cd in the bodies of living organisms54,55. Disturbance of heavy metals can disruptantioxidant protective systems and metabolism processes, exacerbating susceptibility to various diseases56. When Cd and Pb combined accumulate in the body at the same concentration, they can adversely affect the activity of antioxidant enzymes57.
Superoxide dismutase (SOD)
SOD activity in cow blood samples from contaminated areas (TR, PR, GMR, MR) ranged from 1.17 to 1.73 U/mL (Fig. 10). The highest activity was in the control 2.32 group (2.48 U/mL), and the lowest in PR (1.17 U/mL). Whenever the value of heavy metals such as Cd, Pb, and Cr increases in the living body can disturb the SOD activity. There was significant difference (P < 0.001) between the blood samples of contaminated and control areas. Literature supports the present findings58. Zinc and Cu play a vital role in changing SOD activity. Cu ions contribute to getting alternate oxidation, while ions of Zn might work as stabilizers59,60. Studies show that Cd combined with the imidazole groups of superoxide dismutase is the most important for the cessation of H2O2 in cells60, leading to decreased SOD activity.
Catalase (CAT)
The CAT activity varied across the contaminated and controlled area samples and the difference (P < 0.001) was significant (Fig. 11). The highest CAT activity was rin the control area blood samples (12.37 U/mL), while lower activity was in PR site (3.71 U/mL) among the polluted areas. Similar results has been reported by the literature61,62. The CAT and SOD are primary antioxidants that exist in mammalian cells. The SOD catalyzes the ROS and produces O2 in the cell, then converts it to water by CAT63.
Heavy metals with antioxidants and MDA by using Pearson correlation analysis
Pearson correlation was done to recognize oxidants and antioxidants' response to toxic heavy metals in blood samples (Table 3). The results showed a significantly high (P < 0.01) positive correlation between Cd and Cu. While Cd and Cr did not show a positive correlation, Pb and declared a significant (P < 0.05) positive correlation. Similarly, Cd and Pb have a significantly weak positive correlation64, reported a positive correlation linking Pb and Cd in blood, which agrees with the present study's results. The correlation between Cu and Cr was not significant but positive, while Cr and Pb have a highly significant correlation (P < 0.01). The Pb and Cr were not significantly positively correlated22 also find out a significant positive correlation between these toxic metals. Cd and Pb showed a strong positive correlation with MDA, while Cu and Cr have the significantly highest positive correlation with MDA (P < 0.01). In a previous study, there were significant positive correlations between MDA and Cr, which resembled the present study with those reported by65. The results of the current research show similarity with previous study results; there was also a significantly high positive correlation among MDA with Cd and Pb in blood samples collected from polluted areas. The increases in MDA depend on the amount of Pb and Cd in cells; if the amount of the metal is high, ROS will be produced by decreasing glutathione and SH group of the protein-bound; due to this process, ROS generates a high amount48.
SOD with Cd, Cu, Cr, and Pb showed a significant (P < 0.01) negative correlation66 Suggested that Cd concentration negatively correlated with SOD activity, like the present study results. The negative correlation is explained here, which showed high Cd concentration in cells causes oxidative stress, which may create ROS, and replace Fe with Cu in some number of membrane proteins and cytoplasmic, which might cause loss and intensification occurring in the level of unbound Cu and Fe ions67. Similarly, previous literature showed a significant negative correlation between Pb and SOD in the blood68.
CAT with Cr and Pb also significantly negatively correlated. At the same time, Cd and Cu with CAT have a non-significant negative correlation; Cu, Cr, and Pb have a significantly high negative correlation. Similarly, CAT with Cr and Pb also significantly negatively correlated, and Cd and Cu with CAT showed a significant correlation. The present study showed similarities with previous results; there was a significantly weak negative correlation between Cd and Pb with CAT in blood samples collected from contaminated areas. Whenever increases occur in the concentration of Cd and Pb in the cells, it increases the generation of radicals and damages the protection of antioxidant functions to control the problem69.
While MDA with CAT and SOD are also negatively non-significantly correlated. Catalase and superoxide showed a significant positive correlation. Hydrogen peroxide with SOD and CAT has a significantly higher negative correlation, while MDA with CAT and SOD also has a negative non-significant correlation. SOD and CAT show a negative non-significant correlation with MDA, resembling the present study results70.
Conclusions
The study finds that the concentration of heavy metals in blood samples surpasses the permissible limits in the contaminated areas as compared to a control area. Additionally, increased levels of heavy metals have been noted in wastewater.
The antioxidant activities like SOD and CAT, vary between contaminated and control area, where elevated levels of MDA were detected in contaminated areas. Grazing in wastewater-irrigated areas may have contributed to heavy metal accumulation in cow blood.
It is crucial to emphasize that heavy metals pose a threat to animal health and can be transferred to the human body through the food chain. The results suggest that cows in the study areas are unsuitable for human consumption. The absence of proper wastewater management in these areas underscores the need for regular monitoring of heavy metal levels for public health protection.
To address this issue, it is recommended to implement preliminary treatment plants for purifying wastewater used in irrigation. This proactive measure is essential to mitigate potential health risks associated with consuming products derived from animals exposed to contaminated environments.
Data availability
The authors confirm that the data and materials supporting the findings of this study are available within the article.
References
Hosseini, N. S., Sobhanardakani, S., Cheraghi, M., Lorestani, B. & Merrikhpour, H. Heavy metal concentrations in roadside plants (Achillea wilhelmsii and Cardaria draba) and soils along some highways in Hamedan, west of Iran. Environ. Sci. Pollut. Res. 27, 13301–13314 (2020).
Mukherjee, A. G. et al. Heavy metal and metalloid contamination in food and emerging technologies for its detection. Sustain. 15, 1195 (2023).
Mitra, S. et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 34, 101865 (2022).
Akhtar, N., Syakir Ishak, M. I., Bhawani, S. A. & Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water (Switzerland) 13, 2660 (2021).
Subhanullah, M. et al. The detrimental effects of heavy metals on tributaries exert pressure on water quality, Crossocheilus aplocheilus, and the well-being of human health. Sci. Rep. 14, 1–17 (2024).
Jayakumar, M., Surendran, U., Raja, P., Kumar, A. & Senapathi, V. A review of heavy metals accumulation pathways, sources and management in soils. Arab. J. Geosci. 14, 2156 (2021).
Martínez-Oviedo, A., Monterrubio-Martínez, E. & Tuxpan-Vargas, J. Assessing the water contaminants in San Luis Potosi and its effects on its inhabitants: An interdisciplinary study on environmental contamination and public health. J. Hazard. Mater. 464, 132828 (2024).
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R. & Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12, 1–19 (2021).
Briffa, J., Sinagra, E. & Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6, e04691 (2020).
Branca, J. J. V. et al. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 9, 1–21 (2020).
Rehman, A. U. et al. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 321, 114455 (2021).
Sun, K. et al. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 773, 145403 (2021).
Bakhshalizadeh, S. et al. Nickel and cadmium tissue bioaccumulation and blood parameters in Chelon auratus and Mugil cephalus from Anzali free zone in the south Caspian Sea (Iran) and Faro Lake (Italy): A comparative analysis. J. Trace Elem. Med. Biol. 72, 126999 (2022).
Castro-Bedriñana, J., Chirinos-Peinado, D., Ríos-Ríos, E., Machuca-Campuzano, M. & Gómez-Ventura, E. Dietary risk of milk contaminated with lead and cadmium in areas near mining-metallurgical industries in the Central Andes of Peru. Ecotoxicol. Environ. Saf. 220, 112382 (2021).
Zhang, S. W., Zhang, M., Wang, F. & Zeng, H. Direct electrochemistry and enzyme-involved photo-electrocatalysis of oxygen reduction for the electrode on the basis of titanium dioxide-graphene oxide nano-complex with laccase accommodation. Chem. Eng. J. 430, 132619 (2022).
Rahman, M. H. et al. Redox effects of molecular hydrogen and its therapeutic efficacy in the treatment of neurodegenerative diseases. Processes 9, 1–17 (2021).
Howard, M. D., Greineder, C. F., Hood, E. D. & Muzykantov, V. R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control. Release 177, 34–41 (2014).
Brzóska, M. M. & Moniuszko-Jakoniuk, J. Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: Studies on a rat model of human environmental exposure. Toxicol. Sci. 82, 468–477 (2004).
Wang, K. et al. Engineering ultrasmall ferroptosis-targeting and reactive oxygen/nitrogen species-scavenging nanozyme for alleviating acute kidney injury. Adv. Funct. Mater. 32, 1–13 (2022).
Makris, K. C., Heibati, B. & Narui, S. Z. Chrono-modulated effects of external stressors on oxidative stress and damage in humans: A scoping review on night shift work. Environ. Int. 178, 108048 (2023).
Khan, K. et al. Assessment of heavy metals and associated oxidative stress in occupationally exposed workers from Bannu and Karak Districts in Pakistan. Environ. Geochem. Health 45, 5915–5925 (2023).
Raeeszadeh, M., Khoei, A. J., Parhizkar, S., Rad, F. T. & Salimi, B. Assessment of some heavy metals and their relationship with oxidative stress and immunological parameters in aquatic animal species. Biol. Trace Elem. Res. 201, 4547–4557 (2023).
Faheem, H. et al. Groundwater potential zone mapping using geographic information systems and multi-influencing factors: A case study of the Kohat District Khyber Pakhtunkhwa. Front. Earth Sci. 11, 1–18 (2023).
Kazi, T. G. et al. Evaluation of zinc status in whole blood and scalp hair of female cancer patients. Clin. Chim. Acta 379, 66–70 (2007).
Adedara, I. A., Vaithinathan, S., Jubendradass, R., Mathur, P. P. & Farombi, E. O. Kolaviron prevents carbendazim-induced steroidogenic dysfunction and apoptosis in testes of rats. Environ. Toxicol. Pharmacol. 35, 444–453 (2013).
Stocks, J. & Dormandy, T. L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br. J. Haematol. 20, 95–111 (1971).
Fridovich, I. & Freeman, B. Antioxidant defenses in the lung. Annu. Rev. Physiol. 48, 693–702 (1986).
Sinha, A. K. Colorimetric assay of catalase. Anal. Biochem. 47, 389–394 (1972).
Rawan, B. et al. Assessments of Roof-Harvested rainwater in Disctrict Dir lower. Khyber Pakhtunkhwa Pakistan. Water 14, 3270 (2022).
Zaynab, M. et al. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 34, 101653 (2022).
Kamaly, H. F. & Sharkawy, A. A. Health risk assessment of metals in chicken meat and liver in Egypt. Environ. Monit. Assess. 195, 802 (2023).
Permissible level set by FAO/ WHO, 2004—Google Search. https://www.google.com/search?q=permissible+level+set+by+FAO%2F+WHO%2C+2004+&ei=8szvY8rSHsHXkwWq35DQDQ&ved=0ahUKEwjKk_2HnZ39AhXB66QKHaovBNoQ4dUDCA8&uact=5&oq=permissible+level+set+by+FAO%2F+WHO%2C+2004+&gs_lcp=Cgxnd3Mtd2l6LXNlcnAQA0oECEEYAFAAWABg2gpoAHAAeACAAQCIAQCSAQCYAQCgAQKgAQHAAQE&sclient=gws-wiz-serp.
Pompilio, C.-G. N., Francisco, C.-S., Tulio, F. d. M.-T. M., Samuel, S.-M. S., & Elisa, G.-J. F. Heavy metals in blood, milk and cow's urine reared in irrigated areas with wastewater. Heliyon, 7(4), 10–24 (2021).
Monteverde, V. et al. Heavy metal levels in milk and serum of dairy cows from different farms located near an industrial area. Animals 12, 2574 (2022).
Chirinos-Peinado, D.M. & Castro-Bedriñana, J.I. Lead and cadmium blood levels and transfer to milk in cattle reared in a mining area. Heliyon 6, 0–4 (2020).
Tahir, I. & Alkheraije, K. A. A review of important heavy metals toxicity with special emphasis on nephrotoxicity and its management in cattle. Front. Vet. Sci. 10, 1149720 (2023).
Khatun, J., Intekhab, A. & Dhak, D. Effect of uncontrolled fertilization and heavy metal toxicity associated with arsenic (As), lead (Pb) and cadmium (Cd), and possible remediation. Toxicology 477, 153274 (2022).
Aluc, Y. & Ekici, H. Investigation of heavy metal levels in blood samples of three cattle breeds in Turkey. Bull. Environ. Contam. Toxicol. 103, 739–744 (2019).
Kanici-Tarhane, A., Aluc, Y., Kiziltepe, S. & Ekici, H. An investigation of heavy metal concentrations in the sera of cattle grazed in different locations in the Kars Province of Türkiye. Bull. Environ. Contam. Toxicol. 112, 1–10 (2024).
Malik, M. I. et al. Effects of dietary chromium supplementation on blood biochemical parameters in dairy cows: A multilevel meta-analytical approach. J. Dairy Sci. 107, 301–316 (2024).
Felix, O. C., John, N. A. & Ekene, E. V. Assessment of lead (Pb) residues in organs and muscles of slaughtered pigs at Nsukka and Environs in Enugu state Nigeria. J. Adv. Vet. Anim. Res. 3, 392–398 (2016).
Collin, M. S. et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 7, 100094 (2022).
Boudebbouz, A. et al. Heavy metals levels in raw cow milk and health risk assessment across the globe: A systematic review. Sci. Total Environ. 751, 141830 (2021).
Scott, S. R. et al. The effects of a known exposure source on Pb isotopes in bones from Pb-dosed cows. Appl. Geochemistry 121, 104699 (2020).
Hanfi, M. Y., Mostafa, M. Y. A. & Zhukovsky, M. V. Heavy metal contamination in urban surface sediments: Sources, distribution, contamination control, and remediation. Environ. Monit. Assess. 192, 1–21 (2020).
Enb, A., Donia, M. A. A., Abd-Rabou, N. S., Abou-Arab, A. A. K. & El-Senaity, M. H. Chemical composition of raw milk and heavy metals behavior during processing of milk products. Glob. Vet. 3, 268–275 (2009).
Lanjwani, M. F. et al. Spatial distribution of hydrochemistry and characterization of groundwater of taluka Bakrani, Larkana, Sindh Pakistan. Arab. J. Geosci. 15, 380 (2022).
Bux, R. K. et al. Assessment of heavy metal contamination and its sources in urban soils of district Hyderabad, Pakistan using GIS and multivariate analysis. Int. J. Environ. Sci. Technol. 19, 7901–7913 (2022).
Anaman, R. et al. Identifying sources and transport routes of heavy metals in soil with different land uses around a smelting site by GIS based PCA and PMF. Sci. Total Environ. 823, 153759 (2022).
Rashid, A. et al. Mapping human health risk from exposure to potential toxic metal contamination in groundwater of Lower Dir, Pakistan: Application of multivariate and geographical information system. Chemosphere 225, 785–795 (2019).
Akbar, T. A. et al. Principal Component Analysis (PCA)–geographic information system (GIS) modeling for groundwater and associated health risks in Abbottabad, Pakistan. Sustainability 14, 14572 (2022).
Shakeri, M. T., Nezami, H., Nakhaee, S., Aaseth, J. & Mehrpour, O. Assessing heavy metal burden among cigarette smokers and non-smoking individuals in Iran: Cluster analysis and principal component analysis. Biol. Trace Elem. Res. 199, 4036–4044 (2021).
Dhaliwal, R. S. & Chhabra, S. Effect of heavy metals on oxidative stress parameters of cattle inhabiting Buddha Nallah area of Ludhiana District in Punjab. J. Vet. Sci. Technol. 7, 10000352 (2016).
Shen, X., Min, X., Zhang, S., Song, C. & Xiong, K. Effect of heavy metal contamination in the environment on antioxidant function in wumeng semi-fine wool sheep in Southwest China. Biol. Trace Elem. Res. 198, 505–514 (2020).
Shaikh, Z. A., Vu, T. T. & Zaman, K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 154, 256–263 (1999).
Ajarem, J. S. et al. Heavy metal accumulation, tissue injury, oxidative stress, and inflammation in dromedary camels living near petroleum industry sites in Saudi Arabia. Animals 12, 707 (2022).
Pandya, C. D., Pillai, P. P. & Gupta, S. S. Lead and cadmium co-exposure mediated toxic insults on hepatic steroid metabolism and antioxidant system of adult male rats. Biol. Trace Elem. Res. 134, 307–317 (2010).
Wrzecińska, M., Kowalczyk, A., Cwynar, P. & Czerniawska-Piątkowska, E. Disorders of the reproductive health of cattle as a response to exposure to toxic metals. Biology (Basel). 10, 1–16 (2021).
Forman, H. J. & Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 20, 689–709 (2021).
Li, Y. et al. Jasmonic acid alleviates cadmium toxicity through regulating the antioxidant response and enhancing the chelation of cadmium in rice (Oryza sativa L.). Environ. Pollut. 304, 119178 (2022).
Grassi, G., Simonetti, A., Gambacorta, E. & Perna, A. Decrease of activity of antioxidant enzymes, lysozyme content, and protein degradation in milk contaminated with heavy metals (cadmium and lead). JDS Commun. 3, 312–316 (2022).
Alburaidi, B. S. et al. Comparative evaluation of cadmium-induced oxidative stress in camel and bovine erythrocytes. J. King Saud Univ. Sci. 34, 101772 (2022).
Koner, D. et al. Molecular characterization of superoxide dismutase and catalase genes, and the induction of antioxidant genes under the zinc oxide nanoparticle-induced oxidative stress in air-breathing magur catfish (Clarias magur). Fish Physiol. Biochem. 47, 1909–1932 (2021).
Li, K. et al. Associations between blood heavy metal(loid)s and serum heme oxygenase-1 in pregnant women: Do their distribution patterns matter?. Environ. Pollut. 286, 117249 (2021).
Lu, M. et al. Manganese overexposure induces Parkinson-like symptoms, altered lipid signature and oxidative stress in C57BL/6 J mouse. Ecotoxicol. Environ. Saf. 263, 115238 (2023).
Turan, F., Eken, M., Ozyilmaz, G., Karan, S. & Uluca, H. Heavy metal bioaccumulation, oxidative stress and genotoxicity in African catfish Clarias gariepinus from Orontes river. Ecotoxicology 29, 1522–1537 (2020).
Nguyen, H. D. Prognostic biomarker prediction for glioma induced by heavy metals and their mixtures: An in-silico study. Toxicol. Appl. Pharmacol. 459, 116356 (2023).
Al-fartosy, A. J. M., Shanan, S. K. & Awad, N. A. Biochemical study of the effects of some heavy metals on oxidant/antioxidant status in gasoline station workers. Int. J. Sci. Res. Publ. 7, 83–94 (2017).
Goncharuk, E. A. & Zagoskina, N. V. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium: Review. Molecules 28, 3921 (2023).
Shi, L. et al. Effects of heat stress on serum immune and antioxidative indexes and relative expression of related genes in sheep. Chinese J. Anim. Nutr. 32, 5275–5284 (2020).
Acknowledgements
The authors extend their appreciation to University Higher Education Fund for funding this research work under Research Support Program for Central labs at King Khalid University through the project number CL/CO/E/6.
Author information
Authors and Affiliations
Contributions
The manuscript's language and conducted additional numerical simulations. The ultimate W.U., K.A., G.R., K.U. and I.K. bolstered the manuscript by conducting analyses and numerical results. A.M., M.S. and B.R. conceived the primary concept, analyzed the data, and aided in restructuring the manuscript. S.H. and K.U. meticulously validated the findings, revised the manuscript, and secured funding. Furthermore, I.K. and K.U. improved manuscript version, prepared for submission, reflects a consensus achieved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ullah, W., Ahmad, K., Rehman, G. et al. Heavy metal impacts on antioxidants in cow blood from wastewater-irrigated areas. Sci Rep 14, 16918 (2024). https://doi.org/10.1038/s41598-024-67792-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67792-2