Abstract
Good-quality sleep is defined by its ability to minimize disturbances, provide adequate duration, and maintain a balanced progression through sleep stages. Sleep disturbance is a common complaint in people living with HIV/AIDS. Despite the influence of sleep disturbance on treatment adherence, quality of life, work productivity, risk of chronic illness. Studies have reported sleep disturbances among HIV/AIDS patients in sub-Saharan African countries (SSA), yielding varied results at the country level. Therefore, conducting a systematic review and meta-analysis is essential. This systematic review and meta-analysis aimed to evaluate the prevalence of poor sleep quality and identify associated factors among HIV/AIDS patients in sub-Saharan African countries. We systematically searched across various databases, including PubMed, African Journals Online, Scopus, Cochrane Library, HINARI, and Science Direct. Additionally, we conducted searches using Google and Google Scholar search engines. Microsoft Excel was used for data extraction, and the data were analysed using STAT version 17.0. We assessed heterogeneity using Cochran’s Q test and I2 test and checked for small study effects using funnel plot symmetry and Egger’s test. Pooled prevalence and associated factors were estimated using a random-effects model at a 95% confidence interval (CI) and significance level of p < 0.05. To identify factors associated with poor sleep quality among individuals living with HIV/AIDS, odds ratios (ORs) and their corresponding 95% CI were calculated. This analysis combined data from 15 separate studies involving a total sample size of 5176 participants. The pooled prevalence of poor sleep quality among HIV/AIDS patients in SSA countries was 49.32% (95% CI 41.32–56.8%). Factors significantly associated with poor sleep quality included depression (OR 2.78; 95% CI 1.21–6.40) and CD4 count < 200 cells/mm3 (AOR 3.15; 95% CI 2.41–4.15). In this study the prevalence of poor sleep quality among HIV/AIDS patients in SSA was higher and differs across the countries, ranging from 21.7 to 73.7%. The findings underscore the urgent necessity for programs aimed at improving sleep quality, particularly in addressing factors such as participant income and depression that are linked to poor sleep quality in HIV/AIDS patients.
Systematic review registration: PROSPERO CRD42024517229.
Similar content being viewed by others
Introduction
Human immunodeficiency virus (HIV) infection and antiretroviral therapy (ART) can present with neurological complications. HIV persists in the central nervous system (CNS) because brain macrophages, microglial cells, and astrocytes serve as reservoirs1,2,3. As of 2022, there were 39.0 million HIV-positive individuals worldwide, with 76% of them residing in sub-Saharan Africa (SSA)4. According to the Ethiopia Public Health Institute’s 2023 report, the country recorded 603,537 people living with HIV5.
HIV/ADIS infected individuals are more vulnerable to sleep disturbances6. Poor sleep quality is a symptom that includes dysfunction related to the sleep and sleep stage, excessive somnolence, trouble falling and staying asleep, and difficulty starting and maintaining sleep7. According to various studies, between 50 and 70 million individuals in the United States experience sleep disturbances8. Sleep is one of the most important around the clock cycles in the human body system, which plays an important role in physiological and psychological functions9.
Sleep disturbance is frequently reported among individuals with chronic diseases, such as HIV infection10. The exact cause of sleep disturbance is not well known, but it may relate to HIV itself, antiretroviral drug side effects, and other HIV-related disorders11,12. Previous studies indicate that people living with HIV experience insomnia and other sleep difficulties at varying rates, ranging from 40 to 100%13. A meta-analysis reported a global pooled prevalence of sleep disturbances among adults living with HIV at 58%13. In Africa, adults living with HIV show significant rates of sleep disturbances, ranging from 39.4 to 94%14. Sleep disturbance rates vary significantly across different regions in Ethiopia. For instance, at Hawassa University Comprehensive Specialized Hospital, it was reported as 92.1%15; at Zewditu Memorial Hospital, the rate was 55.6%16; at Mettu Karl Referral Hospital, it was 57.1%17; and at Finote Selam General Hospital, the reported rate was 55.1%18.
Sleep disturbance is prevalent among individuals living with HIV/AIDS and significantly impacts treatment adherence, quality of life7,15,16,19,20,21. These issues may be overlooked due to sleep disturbances being seen as a typical consequence of the disease and its treatment or considered less significant compared to other HIV-related complications10,16. Additionally, cytokines such as TNF-alpha and interleukin-1 (IL-1), along with medications like Efavirenz used in treatment, are associated with sleep disturbances among HIV/AIDS patients22,23,24.
The impact of HIV/AIDS in SSA countries includes increased mortality, morbidity, orphan hood, poor sleep quality, depression, anxiety, reduced productivity, as well as stigma and discrimination25,26. Individual study on poor sleep quality among HIV/AIDS patients in SSA have reported different levels of sleep disturbances, indicating the necessity for a systematic review and meta-analysis. This research aimed to determine the prevalence of poor sleep quality and identify associated factors among HIV/AIDS patients in the region.
Review questions
-
1.
What is the estimated pooled prevalence of poor sleep quality among patients with HIV/ADIS in sub-Saharan African countries?
-
2.
What factors are associated with poor sleep quality among HIV/ADIS patients in sub-Saharan African countries?
Methods
This protocol for this review has been officially registered in PROSPERO under protocol number CD42024517229, which can be access ay https://www.crd.york.ac.uk/PROSPERO# my PROSEPERO. This systematic review and meta-analysis strictly followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklists27.
The eligibility criteria
Inclusion criteria
Condition Poor sleep quality.
Context Both published and unpublished observational studies that reported the prevalence and/or associated factors of poor sleep quality in SSA countries were the context.
Population People living with HIV/ADIS were the population for this review.
Study design All observational studies that reported the prevalence and/or associated factors of poor sleep quality among people living with HIV/ADIS were included.
Language Studies written in the English language were included.
Publication year Studies published between 2014 and 2023.
Exclusion criteria
This study excluded case reports, case series, letters to the editor, studies without full-text availability, or studies that did not disclose the prevalence of poor sleep quality among patients with HIV/AIDS (Supplementary material 1).
Measurement of the outcome variable
The outcome variable in this study was poor sleep quality, defined using specific assessment tools. The PSQI consists of 19 questions across seven components: sleep quality, sleep onset latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. Each item is scored from 0 to 3, contributing to the global PSQI score, which ranges from 0 to 21. A global PSQI score above 5 indicates poor sleep quality28. Additionally, the Epworth Sleepiness Scale (ESS) was used, where individuals scoring > 7 out of 24 were classified as having abnormal sleepiness or sleep apnea29.
Search strategies
We systematically searched across various databases, including PubMed, African Journals Online, Scopus, Cochrane Library, HINARI, and Science Direct. Additionally, we conducted searches using Google and Google Scholar search engines, to identify primary studies relevant to our research objectives. Our search strategy was based on the CoCoPo principle (conditions, context, and population), ensuring that we defined all key components before commencing the review process30. Specifically, our aim was to identify studies that reported on the prevalence of poor sleep quality among individuals living with HIV/AIDS in sub-Saharan African (SSA) countries. Each database received a customized search strategy, utilizing Medical Subject Headings (MeSH) terms.
Study selection
All studies retrieved from various electronic databases were transferred to EndNote version 8. Following the removal of duplicate articles, the titles of all remaining articles were screened. Subsequently, both the abstracts and full texts were meticulously and independently reviewed by two authors (BA and LW). Any discrepancies between the reviewers were resolved through further discussion involving additional reviewers (MM and DE) when necessary.
Data extraction and management
Two authors (LL and MH) summarized the findings regarding the prevalence and associated factors of poor sleep quality among HIV/AIDS patients using a data extraction format developed with guidance from the Joanna Briggs Institute (JBI) data extraction tool for prevalence studies31. The extracted data were compared by two authors (EF and AD). Any discrepancies were resolved through consensus after discussion. The details of each study, including the first author’s name, publication year, study design, sample size, criteria for poor sleep quality, prevalence of poor sleep quality, and estimates of associated factors (such as sex, viral load, CD4 count) along with their standard errors, were extracted.
Risk of bias and quality assessment
We assessed the quality of the included studies using a validated modified version of a quality assessment tool specifically designed for prevalence studies32. Two authors, TD and AD, independently assessed the quality of the studies. Any disagreements in the quality appraisal between these two authors were resolved through consultation with a third reviewer, NW. The quality assessment tool consists of nine items that assess the risk of bias, with a maximum score of “9” and a minimum score of “0”. The risk of bias was classified as low (0–3), moderate (4–6), or high (7–9)33,34.
Data synthesis and analysis
The data extracted in Microsoft Excel were transferred to STATA version 17.0 software for further analysis35. The pooled estimate of the prevalence of poor sleep quality and its associated factors was determined using the random-effects model with Der Simonian Laird weights. Statistical heterogeneity was assessed using the Cochrane Q test and I2 statistics36. High heterogeneity among the included studies was observed in the random-effects model analysis. To address this, sensitivity, subgroup, and meta-regression analyses were conducted. Sensitivity analysis indicated that one study significantly influenced the overall estimates (Fig. 4).
Publication bias
Publication bias, specifically the small study effect, was assessed through the use of funnel plots and statistically through Egger’s test37. To identify factors associated with poor sleep quality among individuals living with HIV/AIDS, odds ratios (ORs) and their corresponding 95% confidence intervals were calculated (Fig. 3).
Results
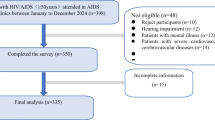
We conducted an electronic database search that yielded 5724 article records. After identifying and removing 689 duplicate entries, we reviewed the titles and abstracts of the remaining articles. Subsequently, 5709 articles were deemed unsuitable and excluded from the study. One article was excluded from the full-text review because of differences in the study population. During the full-text review, one article was excluded because of differences in the population under study. In addition, one article lacked full text, and another study lacked a validated assessment tool, resulting in their exclusion38,39. Ultimately, we included 15 full-text articles, comprising a total sample size of 5176, for further analysis (see Fig. 1). Of these 15 studies, two were from Cameroon32,40, eight were from Ethiopia14,22,31,41,42,43,44,45, and four were from Nigeria11,24,42,46,47 (Table 1). According to the Epworth Sleepiness Scale, poor sleep quality is defined by individual scores exceeding seven of 24 points32,40. According to the Pittsburgh Sleep Quality Index (PSQI), poor sleep quality is identified when an individual score above five out of 21 possible points14,31,41 (Fig. 1).
Prevalence of poor sleep quality among people living with HIV/AIDS in sub-Saharan Africa
The pooled prevalence of poor sleep quality among people living with HIV/AIDS in sub-Saharan Africa was 49.32% (95% CI 41.97–56.8%). The studies showed significant heterogeneity (I2 = 96.8%, p < 0.001). The prevalence ranged from 21.7% in Nigeria47, the lowest reported rate, to 73.7% in Ethiopia, the highest reported rate42 (Fig. 2).
Publication bias
Publication bias was assessed using both a Funnel Plot and Egger's regression test. The Funnel Plot analysis showed a symmetrical distribution, and Egger’s test produced non-significant results (estimated bias coefficient = 5.6 with a standard error of 8.5, p = 0.957), indicating that publication bias was not present (Fig. 3). Sensitivity analysis was conducted to detect influential studies on the pooled prevalence of poor sleep quality. As shown in Fig. 4, no study was found to significantly influence the pooled estimate. Omitting studies using leave-one-out meta-analysis did not affect the overall prevalence of poor sleep quality (Fig. 5).
We also performed and reported estimates from a subgroup analysis, considering other possible sources of variation, including assessment tools, study country, and sample size.
Sub group analysis of poor sleep quality based on assessment tools
The subgroup analysis of the prevalence of poor sleep quality, based on the assessment tool used, indicated variations between tools, with significant heterogeneity within studies (see Fig. 4). The results showed that the pooled prevalence of poor sleep quality was 50.33% (95% CI 39.34% 61.32%, I2 = 72.0%, p = 0.059) in studies with a moderate risk of bias.
Subgroup analysis of poor sleep quality based on study country
The subgroup analysis of the prevalence of poor sleep quality, based on study country, indicated variations in the prevalence across regions, with significant heterogeneity within studies (see Figs. 6, 7, and 8). The pooled prevalence of poor sleep quality in Cameroon among patients living with HIV/AIDS was 49.36% (95% CI 38.19–60.53%, I2 = 88.2%, p = 0.004). This was higher than the pooled prevalence in Nigeria among patients living with HIV/AIDS, which was reported at 41.61% (95% CI 25.09–54.12%, I2 = 95.7%, p < 0.001) (see Fig. 4).
Subgroup analysis was also performed on studies conducted in Ethiopia and Nigeria by omitting those from Cameroon to identify the influential studies on the pooled prevalence of poor sleep quality. However, omitting studies using leave-one-out meta-analysis did not influence the overall prevalence of poor sleep quality (Fig. 7). For instance, the estimated prevalence of poor sleep quality in Ethiopia was higher than in Nigeria, reported at 54% (95% CI 46–63%, I2 = 96.22, p value < 0.001).
Finally, we conducted another subgroup analysis focusing on country-specific studies. However, even after omitting certain studies, there was no influence on the overall prevalence of poor sleep quality (Fig. 8). Consequently, we observed a lower pooled prevalence of poor sleep quality in Nigeria, reported at 39% (95% CI 28–50%; I2 = 96.9%, p value < 0.001).
Sub group analysis based on sample size
To address heterogeneity, we conducted a subgroup analysis of the prevalence of poor sleep quality based on sample size, categorizing studies with mean sample sizes of ≥ 300 and < 300 (Fig. 9). The analysis revealed a higher prevalence of poor sleep quality among studies with a sample size of ≥ 300, reported at 52% (95% CI 44–59%; I2 = 96.45; p value < 0.001).
Sub group analysis of poor sleep quality based on study year
We conducted another subgroup analysis to address heterogeneity, and as depicted in Fig. 10, no individual study was found to significantly influence the overall pooled prevalence of sleep quality. In this analysis, a higher prevalence of poor sleep quality was observed in studies conducted from 2010 to 2023, reported at 48% (95% CI 39–57%; I2 = 97.51%; p value < 0.001).
Factors associated with pooled quality among people living with HIV/AIDS
To explore factors associated with the pooled prevalence of poor sleep quality among people living with HIV/AIDS in sub-Saharan African (SSA) countries, we conducted separate random-effects pooled estimate analyses on extracted factors. The results indicated that variables such as depression and CD4 count < 200 were statistically significant predictors of poor sleep quality. According to the pooled meta-analysis (Fig. 11), individuals with HIV/AIDS in SSA who experienced depression were 2.78 times more likely to have poor sleep quality compared to those without depression (OR 2.78, 95% CI 1.21–6.40). Similarly, in Fig. 12, the analysis showed that HIV/AIDS patients in SSA with a CD4 count less than 200 had a 3.15 times higher risk of poor sleep quality than those with a CD4 count greater than 200 (OR 3.15, 95% CI 2.40–4.05).
Discussion
Poor sleep quality is a major health concern among people with HIV in sub-Saharan Africa. Many studies across the region show varied findings. Hence, this systematic review and meta-analysis estimated the pooled prevalence and associate factors poor sleep quality among people living with HIV/ADIS in sub-Saharan Africa.
According to the study, the pooled prevalence of poor sleep quality among people living with HIV/AIDS in sub-Saharan African countries was 49.32% (95% CI 41.97–56.8%). This finding is consistent with the 49.54% reported in studies conducted in Iran12.
However, it was lower than a result reported by Jie Wu et al. at prevalence 58%13, and studies in the USA48, and in Mexico49, which reported 75% and 58.6%, respectively. These differences might be attributed to variations in assessment tool cut-off points, sociodemographic factors, viral load, CD4 count, the presence of opportunistic infections, and levels of depression and anxiety. Overall, these factors contribute to the variation in poor sleep quality among HIV patients15,29,50.
A significant heterogeneity was observed between the included primary studies. To address this, we conducted a subgroup analysis considering factors such as the country, assessment tool, study year and sample size. Despite this effort, heterogeneity persisted.
Our analysis indicated that studies using the Pittsburgh Sleep Quality Index (PSQI) reported a higher prevalence of poor sleep quality compared to those using the Epworth Sleepiness Scale and the Insomnia Severity Index (ISI). This study is in line with a study conducted by Márcio Flávio Moura de Araujo et al.51. This discrepancy might be due to the use of different questionnaires or scales to measure sleep quality, each with its own criteria and cut-off points. These variations can lead to differences in what is classified as “poor sleep quality” across studies52,53,54,55.
Result from sub group analysis based on the country indicated that Ethiopia had the highest prevalence of poor sleep quality, estimated at 54.29%. In contrast, Nigeria exhibited the lowest prevalence among the countries studied. The prevalence of poor sleep quality in Ethiopia is consistent with earlier research, such as Kathryn et al., who reported a prevalence of 56%56. However, it was lower than result from Taiwan, which reported, 60%57, and higher than those in China, 15%58, Bangladesh, 38.8%59, and Vietnam, 41.2%60. Differences in HIV/AIDS care across countries, influenced by healthcare infrastructure, could explain varying levels of sleep quality. Better access to antiretroviral therapy, specialized clinics, and mental health support improves sleep outcomes. Conversely, limited access to treatment and support services in certain nations may exacerbate stress, anxiety, and depression among patients, worsening sleep quality. Socioeconomic factors like income and housing stability also contribute to sleep disturbances. Cultural attitudes toward HIV/AIDS, including stigma, often deter patients from seeking care and adhering to treatment, leading to increased psychological distress and poorer sleep quality48,61,62.
The subgroup analysis based on sample size revealed that studies with ≥ 300 participants consistently showed a higher prevalence of poor sleep quality compared to those with < 300 participants. This suggests that larger sample sizes may increase the likelihood of detecting poor sleep quality. Larger samples provide greater statistical power for more precise prevalence estimates and may capture a broader range of individuals affected by poor sleep quality, enhancing the study's comprehensiveness. The subgroup analysis based on study year found no individual study significantly influencing the overall pooled prevalence of sleep quality. However, it revealed a higher prevalence of poor sleep quality from 2010 to 2023 compared to the period from 2013 to 2018. This trend might be attributed to several factors such as changes in healthcare practices, evolving treatment protocols, or shifts in the population demographics over time.
According to this review, poor sleep quality showed a significant correlation with CD4 count and depression. Specifically, participants with depression were more likely to experience poor sleep quality compared to HIV/AIDS patients without depression, which aligns with findings from a study conducted in Iran19,63,64,65. People living with HIV/AIDS and comorbid depression are particularly vulnerable to poor sleep quality. Depression often disrupts sleep cycles, leading to difficulties in falling asleep, staying asleep, or achieving restorative sleep. This may result in hypersomnia, insomnia, or other sleep-related issues. Additionally, psychological distress such as rumination, worry, and negative thoughts associated with depression can significantly interfere with sleep onset and maintenance64,66,67,68. In addition, the complex relationship between depression and HIV/AIDS exacerbates insomnia. The regulation of sleep, emotional processing, alterations in the hypothalamic–pituitary–adrenal axis, psychopathological symptoms, and disturbances in the sleep–wake cycle are all interconnected in this complex situation. Additionally, both circumstances are linked to a decline in69,70.
According to the study, people living with HIV/AIDS who had a CD4 count of less than 200 cells/mm3 were more likely to have poor sleep quality than those who had a count of more than 200 cells/mm3. This study is supported by a study conducted in Mexico49 and the USA71. This could be because a CD4 count of less than 200 cells/mm3 indicates severe immune suppression, which increases a person's susceptibility to opportunistic infections and comorbidities, some of which have a direct or indirect impact on sleep quality72.
Limitations of the study
Despite conducting sensitivity, subgroup, and meta-regression analyses to mitigate the impact of heterogeneity, the level of heterogeneity among the included studies remained high. This could be attributed to variations in the study area, methodology, study period, assessment tools for diagnosing poor sleep quality, and other unexplained factors. Therefore, it is crucial for clinicians and policymakers to consider these factors when interpreting the results. Another limitation of our study was the restriction of literature searches to specific languages, predominantly English, which might have led to the exclusion of relevant studies published in other languages. Additionally, limited access to databases or journals could have resulted in incomplete retrieval of important studies, particularly those published in local or regional journals.
Conclusions
This review revealed that HIV/AIDS patients in SSA had a significantly greater prevalence of poor sleep quality than did the general population, highlighting the urgent need for mental health services and treatments to improve participant health. This astounding frequency highlights how urgently programs to enhance sleep quality are needed. The results from the HIV/AIDS study revealed relationships between participant income, depression, and poor sleep quality.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Klatt, E. C. Pathology of HIV/AIDS 6–50 (The Mercer University of Medicine Savannah, 2017).
Johnston, L. et al. HIV/AIDS: Risk and Protective Behaviors Among Adults Ages 21 to 40 in the US, 2004–2016 (Institute for Social Research, The University of Michigan, 2017).
Kandula, U. R. & Wake, A. D. Promising stem cell therapy in the management of HIV and AIDS: A narrative review. Biol. Targets Ther. 16, 89–105 (2022).
HIV JUNPo. World AIDS Day Report 2022: Dangerous Inequalities (UN, 2022).
Institute, E. P. H. HIV Related Estimates and Projections in Ethiopia for the Year 2021–2022 (The Ethiopian Public Health Institute Addis Ababa, 2022).
Phillips, K. D. & Skelton, W. D. Effects of individualized acupuncture on sleep quality in HIV disease. J. Assoc. Nurses AIDS Care 12(1), 27–39 (2001).
Nelson, K. L., Davis, J. E. & Corbett, C. F. Sleep quality: An evolutionary concept analysis. Nurs. Forum 57, 144–151 (2022).
Taibi, D. M. Sleep disturbances in persons living with HIV. J. Assoc. Nurses AIDS Care 24(1), S72–S85 (2013).
Sankri-Tarbichi, A. G. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna J. Med. 2(01), 3–8 (2012).
McGrath, L. & Reid, S. Sleep and quality of life in HIV and AIDS. In Sleep and Quality of Life in Clinical Medicine (eds Verster, J. C. et al.) 505–514 (Humana Press, 2008).
GebreEyesus, F. A., Degu, F. S., Yohanes, Y. B. & Azagew, A. W. Sleep quality and associated factors among adult people living with HIV on follow-up at Dessie Town Governmental Health Facilities Antiretroviral Therapy Clinics, Northeast, Ethiopia, 2020, A multicenter cross-sectional study. BMC Psychiatry 23(1), 132 (2023).
Mohammadnejhad, S. et al. Sleep traits and associated factors among people living with HIV/AIDS in Iran: A two-step clustering analysis. Sci. Rep. 14(1), 5076 (2024).
Wu, J., Wu, H., Lu, C., Guo, L. & Li, P. Self-reported sleep disturbances in HIV-infected people: A meta-analysis of prevalence and moderators. Sleep Med. 16(8), 901–907 (2015).
Peltzer, K. & Phaswana-Mafuya, N. The symptom experience of people living with HIV and AIDS in the Eastern Cape, South Africa. BMC Health Serv. Res. 8, 1–8 (2008).
Bedaso, A., Abraham, Y., Temesgen, A. & Mekonnen, N. Quality of sleep and associated factors among people living with HIV/AIDS attending ART clinic at Hawassa University comprehensive specialized Hospital, Hawassa, SNNPR, Ethiopia. PLoS ONE 15(6), e0233849 (2020).
Mengistu, N. et al. Quality of sleep and associated factors among people living with HIV/AIDS on follow up at Ethiopian Zewditu Memorial Hospital, 2018. Sleep Sci. Pract. 5, 1–8 (2021).
Abdu, Z. & Dule, A. Poor quality of sleep among HIV-positive persons in Ethiopia. HIV/AIDS-Res. Palliat. Care 12, 621–628 (2020).
Adane, M., Amha, H., Tafere, Y. & Alem, G. Poor sleep quality and associated factors among people attending anti-retroviral treatment clinic at Finote selam general hospital, Amhara, Ethiopia. Sleep Med. X 4, 100054 (2022).
Mousavi, M. E. et al. Association between psychological discomforts and sleep quality among people living with HIV/AIDS. AIDS Res. Ther. 20(1), 78 (2023).
Leng, M. et al. Sleep quality and health-related quality of life in older people with subjective cognitive decline, mild cognitive impairment, and Alzheimer disease. J. Nerv. Ment. Dis. 208(5), 387–396 (2020).
Joo, H. J., Joo, J. H., Kwon, J., Jang, B. N. & Park, E.-C. Association between quality and duration of sleep and subjective cognitive decline: A cross-sectional study in South Korea. Sci. Rep. 11(1), 16989 (2021).
Reid, S. & Dwyer, J. Insomnia in HIV infection: A systematic review of prevalence, correlates, and management. Psychosom. Med. 67(2), 260–269 (2005).
Rosekind, M. R. The epidemiology and occurrence of insomnia. J. Clin. Psychiatry 53, 4–6 (1992).
Gray, J. & Young, B. Acute onset insomnia associated with the initiation of raltegravir: A report of two cases and literature review. AIDS Patient Care STDs 23(9), 689–690 (2009).
Amuche, N. J., Emmanuel, E. I. & Innocent, N. E. HIV/AIDS in sub-Saharan Africa: Current status, challenges and prospects. Asian Pac. J. Trop. Dis. 7(4), 239–256. https://doi.org/10.12980/apjtd.7.2017D6-366 (2017).
Andrews, G., Skinner, D. & Zuma, K. Epidemiology of health and vulnerability among children orphaned and made vulnerable by HIV/AIDS in sub-Saharan Africa. AIDS Care 18(3), 269–276 (2006).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Interm. Med. 151(4), 65–94 (2009).
Legas, G., Beyene, G. M., Asnakew, S., Belete, A. & Desie, T. Poor sleep quality and associated factors among HIV-positive pregnant women in Northwest, Ethiopia: A facility-based, cross-sectional study. BMC Psychiatry 22(1), 559 (2022).
Njoh, A. A. et al. Likelihood of obstructive sleep apnea in people living with HIV in Cameroon–preliminary findings. Sleep Sci. Pract. 1, 1–9 (2017).
Munn, Z., Stern, C., Aromataris, E., Lockwood, C. & Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 18, 1–9 (2018).
Munn, Z., Tufanaru, C. & Aromataris, E. JBI’s systematic reviews: Data extraction and synthesis. AJN Am. J. Nurs. 114(7), 49–54 (2014).
Hoy, D. et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 65(9), 934–939 (2012).
Dagnew, B., Andualem, Z., Angaw, D. A., Alemu Gelaye, K. & Dagne, H. Duration of exposure and educational level as predictors of occupational respiratory symptoms among adults in Ethiopia: A systematic review and meta-analysis. SAGE Open Med. 9, 20503121211018120 (2021).
De Cassai, A. et al. Enhancing study quality assessment: An in-depth review of risk of bias tools for meta-analysis—A comprehensive guide for anesthesiologists. J. Anesth. Analg. Crit. Care 3(1), 44 (2023).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Controll. Clin. Trials 7(3), 177–188 (1986).
Higgins, J. P. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions (The Cochrane Collaboration, 2011).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Njamnshi, A. et al. Sleep disorders in HIV–/INS; AIDS patients in Cameroon, sub-Saharan Africa. J. Neurol. Sci. 333, e710 (2013).
Yunusa, M. A. & Obembe, A. Prevalence of self reported sleep problems among patients with HIV infection in Sokoto, Nigeria. Br. J. Med. Med. Res. 15(5), 1–9 (2016).
Massongo, M. et al. Sleep apnea syndrome: Prevalence and comorbidity with other non-communicable diseases and HIV infection, among hospitalized patients in Yaounde, Cameroon. Sleep Disord. 2022, 1–8 (2022).
Mekonnen, W., Hailesilassie, H., Gizaw, A. T. & Tesfaye, E. Sleep quality status and its associated factorsamong people having follow up treatment in Jimma University Medical Center ART Clinic, Ethiopia. East Afr. J. Health Sci. 2, 231–249 (2020).
Tadesse, A., Badasso, K. & Edmealem, A. Poor sleep quality and associated factors among people living with HIV/AIDS attending ART clinic at Tirunesh Beijing Hospital, Addis Ababa, Ethiopia. AIDS Res. Treat. 2023(1), 6381885 (2023).
Oshinaike, O. et al. Quality of sleep in an HIV population on antiretroviral therapy at an urban tertiary centre in Lagos, Nigeria. Neurol. Res. Int. 2014, 1–6 (2014).
Bisong, E. Predictors of sleep disorders among HIV out-patients in a tertiary hospital. Recent Adv. Biol. Med. 2017(3), 2747 (2017).
Ogunbajo, A. et al. Poor sleep health is associated with increased mental health problems, substance use, and HIV sexual risk behavior in a large, multistate sample of gay, bisexual and other men who have sex with men (GBMSM) in Nigeria, Africa. Sleep Health 6(5), 662–670 (2020).
Adeoti, A., Dada, M., Ogundipe, K. & Akolawole, M. Burden of insomnia and associated risk factors in people living with HIV/AIDS in a tertiary hospital in sub-Saharan Africa. J. AIDS Clin. Res. Sex Transm. Dis. 5, 19 (2018).
Osiyemi, A. O. et al. Sleep disturbance and associated factors among Nigerian adults living with HIV in the dolutegravir era. Front. Sleep 1, 963529 (2022).
Gutierrez, J. et al. Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea. SAGE Open Med. 7, 2050312119842268 (2019).
Rodríguez Estrada, E., Iglesias Chiesa, M. C., Fresán Orellana, A. & Reyes-Terán, G. Factors associated with poor sleep quality among HIV-positive individuals in Mexico City. Salud Ment. 41(3), 123–129 (2018).
Patil, S. P., Schneider, H., Schwartz, A. R. & Smith, P. L. Adult obstructive sleep apnea: Pathophysiology and diagnosis. Chest 132(1), 325–337 (2007).
Uchôa, L. R. A. et al. Poor sleep quality in persons living with HIV: A systematic review and meta-analysis. J. Nurs. Health Sci. 4(2), 8 (2018).
Buysse, D. J., Reynolds, C. F. III., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28(2), 193–213 (1989).
Nokes, K. M. & Kendrew, J. Correlates of sleep quality in persons with HIV disease. J. Assoc. Nurses AIDS Care 12(1), 17–22 (2001).
Lee, K. A. et al. Types of sleep problems in adults living with HIV/AIDS. J. Clin. Sleep Med. 8(1), 67–75 (2012).
Junqueira, P., Bellucci, S., Rossini, S. & Reimão, R. Women living with HIV/AIDS: Sleep impairment, anxiety and depression symptoms. Arq. Neuro-psiquiatr. 66, 817–820 (2008).
Yang, Y. et al. Prevalence of poor sleep quality in perinatal and postnatal women: A comprehensive meta-analysis of observational studies. Front. Psychiatry 11, 161 (2020).
Teong, A. C. A., Diong, A. X., Omar, S. Z. & Tan, P. C. The impact of self-reported sleep on caesarean delivery in women undergoing induction of labour: A prospective study. Sci. Rep. 7(1), 12339 (2017).
Xu, X., Liu, D., Zhang, Z., Sharma, M. & Zhao, Y. Sleep duration and quality in pregnant women: A cross-sectional survey in China. Int. J. Environ. Res. Public Health 14(7), 817 (2017).
Liu, R.-Q. et al. Association between depressive symptoms and poor sleep quality among Han and Manchu ethnicities in a large, rural, Chinese population. PLoS ONE 14(12), e0226562 (2019).
Huong, N. T. T. & Thuy, N. T. H. Quality of sleep among pregnant women. Int. J. Clin. Med. 10(1), 16–25 (2018).
Gómez-Olivé, F. X., Rohr, J. K., Roden, L. C., Rae, D. E. & von Schantz, M. Associations between sleep parameters, non-communicable diseases, HIV status and medications in older, rural South Africans. Sci. Rep. 8(1), 17321 (2018).
Wibbeler, T., Reichelt, D., Husstedt, I.-W. & Evers, S. Sleepiness and sleep quality in patients with HIV infection. J. Psychosom. Res. 72(6), 439–442 (2012).
Womack, J. A. et al. Sleep disturbance among HIV-infected and uninfected veterans. JAIDS J. Acquir. Immune Defic. Syndr. 74(4), e117–e120 (2017).
Huang, X. et al. Burden of sleep disturbances and associated risk factors: A cross-sectional survey among HIV-infected persons on antiretroviral therapy across China. Sci. Rep. 7(1), 3657 (2017).
Huang XiaoJie, H. X. et al. Burden of sleep disturbances and associated risk factors: A cross-sectional survey among HIV-infected persons on antiretroviral therapy across China. Sci. Rep. https://doi.org/10.1038/s41598-017-03968-3 (2017).
Fang, H., Tu, S., Sheng, J. & Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 23(4), 2324–2332 (2019).
Xu, W.-Q. et al. The role of depression and anxiety in the relationship between poor sleep quality and subjective cognitive decline in Chinese elderly: Exploring parallel, serial, and moderated mediation. J. Affect. Disord. 294, 464–471 (2021).
Jiang, Y., Jiang, T., Xu, L.-T. & Ding, L. Relationship of depression and sleep quality, diseases and general characteristics. World J. Psychiatry 12(5), 722 (2022).
Li, G. et al. Sleep dysfunction mediates the relationship between hypothalamic-insula connectivity and anxiety-depression symptom severity bidirectionally in young adults. NeuroImage 279, 120340 (2023).
Raniti, M. B. et al. Sleep duration and sleep quality: Associations with depressive symptoms across adolescence. Behav. Sleep Med. 15(3), 198–215 (2017).
Seay, J. S. et al. Self-reported sleep disturbance is associated with lower CD4 count and 24-h urinary dopamine levels in ethnic minority women living with HIV. Psychoneuroendocrinology 38(11), 2647–2653 (2013).
Shittu, R. et al. Short sleep duration and correlates among sero-positive HIV patients in Nigeria, West Africa. Br. J. Med. Med. Res. 10(7), 1–10 (2015).
Acknowledgements
We would like to thank the database owners as well as the primary study researchers.
Author information
Authors and Affiliations
Contributions
Data curation: E.T.F., M.H., and A.A.; Methodology: E.T.F., M.H., and A.A.; Project administration: A.A., M.G.M., and M.M.; Software: T.E. and H.W.A., B.A.M.; Supervision: M.M., M.G.M., and T.E.; Validation: A.A. and M.G.M., B.A.M.; Visualization: T.E., and H.W.A.; Writing–review and editing: M.M., A.M.D., D.E., L.W.L., and N.K.W., B.A.M., H.W.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Melese, M., Mengistie, B.A., Delie, A.M. et al. Poor sleep quality and its associated factors among HIV/ADIS patients living in sub-Saharan African countries: a systematic review and meta-analysis. Sci Rep 14, 16955 (2024). https://doi.org/10.1038/s41598-024-68074-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68074-7