Abstract
A core component of every blood program is the supply of safe blood and blood products. The elevated risk of transmission through these products is due to parvovirus B19 (B19V) resistance to the virus inactivation procedures. Our study aimed to screen asymptomatic blood donors for B19V at a tertiary care hospital in Chennai, Tamil Nadu, between September 2020 and June 2021. Sera from 106 healthy blood donors who tested negative for Human immunodeficiency virus (HIV), Hepatitis B surface antigen (HBsAg), Hepatitis C virus (HCV), syphilis, and malaria were tested for anti-B19V IgM and IgG using a qualitative indirect enzyme-linked immunosorbent assay (ELISA). In the study population, 23.5% (n = 25) of donors tested IgM positive, 38.6% (n = 41) tested IgG positive, and 7.5% (n = 8) tested positive for both IgM and IgG. A proportion of 61.3% (n = 65) of the blood donors tested IgG negative, suggesting they had no past B19V infection. B19V DNA was not detected in any of the subjects. The high seroprevalence of IgM indicates that blood donors may have been recently exposed to B19V, potentially posing a risk to immunocompromised individuals and those with hematological stress. Further longitudinal studies with a larger sample size are recommended to better understand the risk of B19V transfusion transmission.
Similar content being viewed by others
Introduction
A core component of every blood program is the supply of safe blood and blood products, as there is always a risk of transfusion-transmitted infections. In India, blood donors are routinely screened for HIV, Hepatitis B, Hepatitis C, syphilis, and malaria as per regulations set by the National Blood Transfusion Council (NBTC)1. Parvovirus B19 (B19V) is primarily transmitted by the respiratory route but can also be transmitted via blood transfusion, transplantation and vertically from mother to fetus2. B19V has a marked tropism for erythroid progenitor cells. Therefore, particular caution is necessary during blood transfusions to high-risk groups, such as immunodeficient individuals, those with hematological disorders, and pregnant women. In pregnant women, the infection can lead to hydrops fetalis, while among immunocompromised individuals and those with hematological disorders, it can cause chronic anemia, potentially resulting in transient aplastic crises. A suggestive precaution entails the provision of blood from seronegative donors and conducting nucleic acid detection tests (NAT) to identify B19V infections. The exclusion of donations with a high viral load ensures the safety of transfusions3. According to the United States Food and Drug Administration (FDA) and the European Pharmacopoeia Commission, a threshold of 104 IU/mL of B19V DNA should be maintained for blood and blood products4. B19V has demonstrated persistence in various tissues, including the brain, bone marrow, myocardium, synovium, and skin, for several months, existing at both low (< 103 IU/mL) and high titers (> 104 IU/mL)5. Unrecognized B19V infection in asymptomatic voluntary blood donors may lead to transmission of B19V infection.
The small size of the virus and the absence of a lipid envelope make B19V resistant to most virus inactivation procedures employed in the production of blood products, which increases the risk of transmission through blood and blood products6,7. Studies comparing subjects with and without blood transfusions show that those with transfusions exhibit significantly higher seropositivity8. Several nations, such as Japan, Germany, and the Netherlands, have adopted specific strategies to minimize the risk of B19V transfusion transmission, thereby enhancing the safety of blood transfusion practices9. In 1995, Japan experienced a minor epidemic of erythema infectiosum, leading to a higher rate of B19V viremia among blood donors, with an occurrence rate of 1 in 167 donors (0.6%). As a result, routine screening for B19V was implemented10. Recent studies in India have demonstrated an increase in the need for blood transfusions, primarily in high-risk groups. The findings of these studies have further highlighted the importance of introducing routine B19V screening of blood and blood products11. This study was conducted to assess the IgM and IgG seroprevalence and DNA detection of B19V among blood donors at a tertiary healthcare centre.
Materials and methods
A cross-sectional exploratory study was conducted between September 2020 and June 2021 at a tertiary care hospital in Chennai, Tamil Nadu. The study was approved by the Institutional Ethics Committee at Sri Ramachandra Institute of Higher Education and Research (SRIHER) (IEC-NI/19/APR/69/32) in accordance with the Declaration of Helsinki, 1964. Our study included donors who were eligible for blood donation according to Indian NBTC regulations.
Sample collection and processing
A separate blood draw from asymptomatic donors was performed before donation, and five mL of whole blood was collected in distinct vacutainers without anticoagulants for viral marker analysis. These samples were sourced from the hospital's blood bank for our study. At the time of sample collection, appropriate consent was obtained from the blood donors, and all methods were carried out as per relevant guidelines and regulations. The sample was centrifuged at 2500 rpm for ten minutes in the laboratory, and then the serum was stored in multiple aliquots at − 80 °C.
Determination of IgM and IgG antibodies by ELISA
B19V-specific IgM and IgG were determined using an indirect qualitative enzyme-linked immunosorbent assay (NovaLisa, ©Novatec, Germany). All steps were strictly followed as per the manufacturer's instructions. A volume of 100 μl of serum, cut-off control, and positive and negative controls were added to the antigen-precoated ELISA plate and incubated for one hour at 37 °C. The optical density was measured at 450 nm using an ELISA plate reader (ThermoScientific, Multiskan Ex). The assay results were recommended to be displayed as NovaTec units (NTU). The optical density of all samples was translated using the formula NTU = (Sample absorbance value/cut-off) X 10 and interpreted as follows: > 11 NTU was considered positive for B19V IgM and IgG antibodies, and < 9 NTU was considered negative. Clinical specimens previously confirmed to be B19V IgM-positive and IgG-positive in an external laboratory were utilized as internal positive controls. Furthermore, the assay was conducted using a commercially validated kit with positive control, demonstrating a diagnostic specificity of 99.28% and sensitivity of 97.42% for IgG and a specificity of 99.65% and sensitivity of 98.77% for IgM, respectively. Two investigators re-tested the samples to confirm the assay's reproducibility for IgM reactivity.
We evaluated the cross-reactivity of the B19V IgM ELISA kit by testing 35 IgM-positive samples of Dengue, Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Herpes Simplex virus (HSV), Rubella, and Varicella Zoster virus (VZV). To assess the potential for non-specific reactions, we also conducted B19V IgM tests on samples from 35 pregnant women. To confirm the presence of B19V-specific IgM, the immunoglobulin was purified using affinity chromatography at an external laboratory. Following purification, an in-house ELISA was conducted to verify the specificity.
Detection of B19V DNA by real-time quantitative polymerase chain reaction (qPCR)
DNA extraction was performed on all samples using the QIAamp blood DNA extraction kit (Qiagen, Germany), following the instructions provided by the manufacturer.
The final eluted volume was stored at − 20 °C for further use. Subsequently, the extracted DNA underwent qPCR, utilizing a commercial kit (Artus ParvoB19 RG PCR kit, Germany) to amplify a 76 base-pair region of the B19V genome. The standards ranged from 200 × 105 copies/mL to 2000 copies/mL, and the lower limit of detection was 50 copies/mL. The cycling conditions included denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s, and extension at 72 °C for 20 s.
The assay's performance was validated for each batch by including a positive control, which consisted of known plasmid DNA as the internal positive as well as positive control provided with the kit. Molecular-grade water was used as the negative control to check for cross-contamination.
The internal control provided in the kit was used to confirm the validity of the PCR results and to ensure that the PCR reaction proceeded without technical issues.
Statistical analysis
The chi-square test was performed to compare the IgM serostatus with the age, gender, and blood group of the donors. Statistical analysis was performed using SPSS version 29.0. A p-value < 0.05 was considered statistically significant. The odds ratio (OR) and 95% confidence interval (CI) were calculated.
Ethical approval
The current study was approved by the Institutional Ethics Committee.
Results
Demographics of blood donors
A total of 106 healthy blood donors were screened for antibodies against B19V. Of these, 98% (n = 104) were male, and approximately 2% (n = 2) were female. The blood donors were grouped into five age groups: < 20 years (n = 8), 21–30 years (n = 65), 31–40 years (n = 25), 41–50 years (n = 7), and 51–60 years (n = 1) (Fig. 1).
Seroprevalence of B19V IgG and IgM antibodies among blood donors
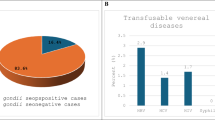
The overall IgG seroprevalence among the blood donors was 38.67% (n = 41). B19V IgG seroprevalence in the 18–30 year age group was 31.08% (n = 23) (Fig. 2). The overall B19V IgM seroprevalence among the blood donors was 23.5% (n = 25). The seroprevalence of B19V IgM in the 18–30 year age group was 25.6% (n = 19), and 7.5% (n = 8) of the study population were both IgM and IgG-positive (Fig. 3). A proportion of 61.3% (n = 65) of the blood donors was IgG-negative, indicative of no past B19V infection.
Seroprevalence of IgM and IgG among blood donors in the study. Total IgG prevalence among blood donors was 38.67% (n = 41), whereas IgM prevalence was 23.5% (n = 25). A smaller group, 7.5% (n = 8), tested positive for both antibodies, indicating exposure to B19V. Notably, most (n = 65) lacked B19V IgG antibodies, implying no previous infection.
qPCR analysis for B19V DNA detection
All samples were tested by qPCR, and the internal control was amplified. However, B19V DNA was not detected in any of the samples.
Evaluation of cross-reactivity and non-specific reactions
Samples positive for IgM antibodies against Dengue, Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Herpes simplex virus (HSV), Rubella, and Varicella Zoster virus (VZV), as well as those from pregnant women, all tested negative for B19V IgM antibodies. IgM purification yielded a concentration of 800 µg/mL. Subsequently, an in-house ELISA confirmed the presence of B19V-specific IgM.
Statistical analysis
No correlation was observed between serostatus and gender or between serostatus and the blood group of the donors. However, a significant association was observed between IgG serostatus and age, specifically in donors > 30 years (OR: 3.5, 95% CI 1.5–8.2) (p-value = 0.003).
Discussion
B19V infection typically exhibits a biphasic clinical course. Initially, there is a phase of high viremia during the first few days, which gradually diminishes to undetectable levels by the eighth day as IgM production begins. The peak in antibody levels may coincide with a decline in viral load. Usually, by 10–12 days post-infection, seroconversion from IgM to IgG antibodies occurs. If B19V IgG is detected in two separate blood samples taken 6 months apart, it is considered 'B19V-safe' for patients with defined indications3. Blood donors who test positive for B19V IgM should be tested for B19V DNA by NAT; qPCR is the gold-standard method for most viral nucleic acid-based clinical diagnostics12.
The seroprevalence of B19V IgG antibodies in our study was 38.6% (Table 1), which is indicative of prior exposure to the infection. However, a large portion of the population has not yet been exposed to B19V and remains vulnerable. An earlier study from the same city in 2017 showed a B19V IgG seroprevalence of 44.3%13. Several studies conducted in India have reported a range of IgG seroprevalence from 27.96 to 63.7%, as shown in Table 1. These findings suggest that B19V is endemic within the Indian population14,15,16,17.
Blood donors represent a relatively healthy subset of the general population. The seroprevalence of B19V IgG among blood donors may provide insights into antibody prevalence within the healthy population.
An overview of the seroprevalence of B19V in various parts of the world reveals varying rates: 41.2% in Tehran and 46% in Yemen, while studies from Germany and Japan showed higher seroprevalences of 72.1% and 67.9%, respectively18,19,20. The heterogeneity in seroprevalence might be attributable to differences in the study period, the type of antigen used in ELISA, and the definition of the cut-off value used across various studies13. Our findings on seroprevalence are mainly consistent with other Asian studies. However, they show a significantly lower seroprevalence compared to Western studies, which can be attributed to the higher endemicity of B19V in colder climates14.
In our study, the total prevalence of IgM antibodies to B19V was 23.5% (Table 1). This is significantly higher when compared to other studies that have documented a B19V IgM seroprevalence of < 2%. However, a study by Kumar et al. has reported an IgM prevalence of 7.53% among Indian blood donors. Most B19V infections occur during late winter, early spring, or early summer. Since our study was conducted during this period, the high IgM seropositivity observed might be attributable to its alignment with the epidemic cycle21.
The possible reasons for the disparity in the male–female blood donor ratio in our study could be attributed to a higher prevalence of low hemoglobin percentage and low body weight among Indian women. In this study, most blood donors were between 18 and 30 years old (n = 65). We observed fewer donors in the age group of over 40 years. This could be due to the higher incidence of chronic conditions such as cardiovascular diseases, diabetes, and hypertension, which are more common in the older population in India and may exclude these individuals from qualifying as blood donors.
Our study participants were healthy donors who donated blood on a single occasion; consequently, there was no opportunity for subsequent testing or monitoring for seroconversion. Neutralizing IgG antibodies, which typically form about two weeks post-infection, effectively eradicate the virus from the bloodstream.
Routine screening for B19V infection in blood donors is not universally practised. However, several countries, including Japan, Germany, and the Netherlands, have adopted specific measures to reduce the risk of B19V transmission and enhance the safety of blood transfusions. For instance, the Japanese Red Cross (JRC) implemented a receptor-mediated hemagglutination assay in September 1997 as a method for B19V screening. The JRC updated this method to a chemiluminescent enzyme immunoassay to increase detection sensitivity in 2008. In Germany, routine screening for B19V DNA began in 2000 with the minipool NAT technique. Similarly, the Netherlands Health Council has recommended a targeted approach for high-risk populations, advocating using B19V-safe blood in cellular blood products where B19V IgG antibodies are detected in two separate blood samples collected 6 months apart9. Despite these efforts, there is no global consensus on the optimal test for preventing transfusion-transmitted B19V infection due to the high costs associated with molecular testing and the typically low levels of viremia.
Reports on the persistence or disappearance of B19V-specific IgM antibodies vary. This variability may be attributed to the initial formation of IgM antibodies against both the conformational epitopes of the VP1 and VP2 capsid proteins and the linear epitopes of VP1. Notably, IgM antibodies targeting the conformational epitopes of the VP2 capsid protein tend to diminish relatively earlier than those targeting the linear epitopes of the VP1 capsid protein22.
The persistence of IgM might account for 7.5% of healthy blood donors who have both IgM and IgG antibodies. It has also been documented that B19V-specific IgM antibodies can persist in the body for more than 5 months23. It is known that B19V can persist in the solid tissues of infected individuals for life. However, the mechanism of persistence remains ambiguous5.
Typically, the co-existence of IgM and IgG suggests an acute resolving infection. At the same time, the presence of IgG alone indicates a chronic phase and possibly a persistent B19V infection. Future longitudinal studies should be undertaken to better understand this phenomenon among people who are both IgM and IgG-positive.
In this study, all samples tested negative for B19V by qPCR. The absence of viral DNA in the 25 IgM-positive donors might be due to the timing of sample collection, which could coincide with post-clearance periods or phases of low viral replication. Additionally, variations in individual immune responses could lead to the persistence of IgM antibodies even after the virus has been cleared3,23.
Approximately 39% of donors who tested negative for B19V DNA had anti-B19V IgG antibodies, suggesting some donors had prior exposure to the virus and subsequently recovered. While these results are encouraging, it is essential to note that they cannot be generalized because they represent testing conducted on small batches. Continuous monitoring is recommended to ensure ongoing vigilance and accuracy in the detection of viral DNA.
The global detection rate of B19V DNA appears to be more variable, with documented frequencies in several countries as follows: Argentina (0.95%), Brazil (1.1%), Ghana (1.3%), the United Kingdom (0.9%), the United States (0.9%), South Africa (0.9%) and Germany 0.013%24,25. Differences in prevalence may be attributed to variations in the primers used, nucleic acid-based detection methods, and genetic diversity, all of which can influence the results. Contributing factors to these variations may include the type of study, the sampling technique, the period of study, and natural variations in viral circulation. B19V incidence is influenced by spring outbreaks and significant epidemics every few years as it is an epidemic-endemic infection.
Given that our study was conducted during the COVID-19 pandemic, there is a possibility that several donors might have been exposed to COVID-19. However, we did not test donors for SARS-CoV-2 infection. The potential interactions between B19V and SARS-CoV-2 and their combined impact on the immune response still need to be examined. It is worth noting that factors such as immunosuppression and other viral co-infections are established risk factors for the reactivation of B19V26. This is the first study from India to document the presence of B19V during the COVID-19 pandemic.
Our study has certain limitations. For example, it only involved a single donation per donor, precluding the possibility of follow-up tests or monitoring for seroconversion, which may affect our understanding of the persistence or resolution of B19V infection. Our study had a demographic skew towards younger donors (18–30 years), as older adults are often ineligible to donate due to health concerns. Consequently, the findings might not accurately reflect the seroprevalence across all age groups. Based on small sample sizes, the results may not be widely generalizable. As the study was conducted during the COVID-19 pandemic, not testing for SARS-CoV-2 may overlook possible interactions between these two viruses and their impact on the immune response. We have not established a link between donor and recipient infectivity using pre- and post-transfusion samples in cases where the donors tested positive for IgM. This limitation precludes a comprehensive understanding of the risk of transfusion-transmitted infection and its impact on recipient health.
Conclusion
Individuals with blood disorders, pregnant women, and immunocompromised people are at a higher risk for B19V infection and may face severe health consequences. The comprehensive range of conditions associated with B19V infection may not be widely recognized, which poses a risk of underdiagnosis. Therefore, stringent screening criteria for B19V should be applied to the transfusion of blood and blood products for such high-risk groups. Additionally, it is essential to raise awareness among clinicians about the potential for transfusion-transmitted B19V infection.
The enigma of B19V infection by transmission transfusion is not yet fully understood. The advent of routine screening for Hepatitis B, Hepatitis C, and HIV has dramatically diminished the transmission risk of these viruses via transfused blood. Nevertheless, universal B19V screening in blood products is not economically possible. A more financially sustainable option for blood centers might involve focusing on high-risk populations. In developing countries like India, where NAT is not yet a standard practice in donor screening, it may be prudent to adopt a policy of testing blood donors for B19V IgM.
Further studies with larger sample sizes are recommended to assess the feasibility of implementing IgM antibody screening for B19V in healthy blood donors (Supplementary Information S1).
Data availability
De-identifiers were used as per International Council of Medical Journal Editors (ICMJE) guidelines to protect donor privacy. The de-identified data from blood donors in this study is accessible in the supplementary information file, sourced from the Sri Ramachandra Hospital blood bank database. All data is available with the corresponding author in case of any clarification.
References
Guidelines for Blood Donor Selection and Blood Donor Referrals. National Blood Transfusion Council. https://naco.gov.in/national-blood-transfusion-council-nbtc-0 (2017).
Landry, M. L. Parvovirus B19. Microbiol Spectr. 4, 1. https://doi.org/10.1128/microbiolspec.DMIH2-0008-2015 (2016).
Juhl, D. Parvovirus B19: What is the relevance in transfusion medicine?. Front. Med. 5, 4. https://doi.org/10.3389/fmed.2018.00004 (2018).
US Food and Drug Administration. Nucleic acid testing (NAT) to reduce the possible risk of parvovirus B19 transmission by plasma-derived products. Rockville (MD): FDA Center for Biologics Evaluation and Research. FDA draft guidance for industry (2008).
Corcioli, F. et al. Tissue persistence of parvovirus B19 genotypes in asymptomatic persons. J. Med. Virol. 80, 2005–2011. https://doi.org/10.1002/jmv.21289 (2008).
Lefrère, J.-J., Mariotti, M. & Thauvin, M. B19 parvovirus DNA in solvent/detergent-treated anti-haemophilia concentrates. The Lancet. 343, 211–212. https://doi.org/10.1016/s0140-6736(94)90993-8 (1994).
Schmidt, I., Blümel, J., Seitz, H., Willkommen, H. & Löwer, J. Parvovirus B19 DNA in plasma pools and plasma derivatives. Vox Sang. 81, 228–235. https://doi.org/10.1046/j.1423-0410.2001.00120.x (2001).
Lin, K. H. et al. Seroepidemiology of human parvovirus B19 in Taiwan. J. Med. Virol. 57, 169–173. https://doi.org/10.1002/(SICI)1096-9071(199902)57:2%3c169::AID-JMV14%3e3.0.CO;2-1 (1999).
Jia, J., Zhang, M., Ma, Y. & Zhang, J. Human parvovirus B19 research concerning the safety of blood and plasma derivatives in China. Ann. Blood. 4(2), 1. https://doi.org/10.21037/aob.2019.01.01 (2019).
Yoto, Y. et al. Large-scale screening for human parvovirus B19 DNA in clinical specimens by dot blot hybridization and polymerase chain reaction. J. Med. Virol. 47, 438–441. https://doi.org/10.1002/jmv.1890470424 (1995).
Sinha, S., Seth, T., Colah, R. B. & Bittles, A. H. Haemoglobinopathies in India: Estimates of blood requirements and treatment costs for the decade 2017–2026. J. Community Genet. 11, 39–45. https://doi.org/10.1007/s12687-019-00410-1 (2020).
Chen, W. et al. Novel nucleic acid detection for human parvovirus B19 based on pyrococcus furiosus argonaute protein. Viruses. 15, 595. https://doi.org/10.3390/v15030595 (2023).
Shoganraj, S. & Arumugam, P. Seroprevalence of human parvovirus B19 among voluntary blood donors in Chennai—a cross sectional study. IJSR Int. J. Sci. Res. (IJSR) 8, 24–26 (2019).
Raturi, G., Kaur, P. & Kaur, G. Seroprevalence of human parvovirus B19 amongst North Indian blood donors-do current donor testing guidelines need a relook?. Transfusion Apheresis Sci. 57, 646–650. https://doi.org/10.1016/j.transci.2018.07.017 (2018).
Kumar, S. et al. Seroprevalence of human parvovirus B19 in healthy blood donors. Med. J. Armed Forces India. 69, 268–272. https://doi.org/10.1016/j.mjafi.2012.11.009 (2013).
Kishore, J., Srivastava, M. & Choudhury, N. Serological study on parvovirus B19 infection in multitransfused thalassemia major patients and its transmission through donor units. Asian J. Transfus Sci. 5, 140–143. https://doi.org/10.4103/0973-6247.83239 (2011).
Jain, P. et al. Prevalence and genotypic characterization of human parvovirus B19 in Children with hemato-oncological disorders in North India. J. Med. Virol. 87, 303–309. https://doi.org/10.1002/jmv.24028 (2015).
Abdelrahman, D. et al. Prevalence and phylogenetic analysis of parvovirus (B19V) among blood donors with different nationalities residing in Qatar. Viruses. 13, 540. https://doi.org/10.3390/v13040540 (2021).
Röhrer, C. et al. Seroprevalence of parvovirus B19 in the German population. Epidemiol. Infect. 136(11), 1564–1575. https://doi.org/10.1017/S0950268807009958 (2008).
Ihara, T. et al. A population-based epidemiological survey of human parvovirus B19 infection: A project of the Kyushu and Okinawa Population Study (KOPS). Arch Virol. 158, 2465–2472. https://doi.org/10.1007/s00705-013-1746-z (2013).
Vuković, V. et al. Seroepidemiology of Human Parvovirus B19 Infection among the Population of Vojvodina, Serbia, over a 16-Year Period (2008–2023). Viruses. 16, 180. https://doi.org/10.3390/v16020180 (2024).
Heegaard, E. D. & Brown, K. E. Human Parvovirus B19. Clin. Microbiol. Rev. 15, 485–505. https://doi.org/10.1128/CMR.15.3.485-505.2002 (2002).
Marano, G. et al. Human Parvovirus B19 and blood product safety: A tale of twenty years of improvements. Blood Transfus. 13, 184–196. https://doi.org/10.2450/2014.0174.14 (2015).
Adamo, M. P. et al. Human parvovirus B19 frequency among blood donors after an epidemic outbreak: Relevance of the epidemiological scenario for transfusion medicine. Heliyon. 6, e03869. https://doi.org/10.1016/j.heliyon.2020.e03869 (2020).
Healy, K. et al. Prevalence of parvovirus B19 viremia among german blood donations and the relationship to ABO and rhesus blood group antigens. J. Infect. Dis. 227, 1214–1218. https://doi.org/10.1093/infdis/jiac456 (2023).
Lai, P. Y., Vu, A., Sarva, S. T., Jayaraman, G. & Kesavan, R. Parvovirus reactivation in COVID-19. Cureus. 13, e17796. https://doi.org/10.7759/cureus.17796 (2021).
Acknowledgements
We would like to acknowledge Dr. Amita Jain, Professor, Department of Microbiology, KGMU, Lucknow, and express thanks for the provision of the B19V positive control used in this study.
Funding
This research was partially funded by our institute (GATE research fund) and Scheme for the Promotion of Academic and Research Collaboration (SPARC), MHRD, Government of India.
Author information
Authors and Affiliations
Contributions
S.K.—contributed to the collection and processing of clinical samples, performed ELISA and PCR assays, interpreted data, and drafted the manuscript. R.K.T.—Contributed to laboratory work, data interpretation, and drafted the manuscript. S.R.—contributed to the collection of clinical samples, laboratory work, and patient details. B.R.—contributed to the laboratory work, data interpretation, and drafted the manuscript. S.S.—contributed to the collection of clinical samples and laboratory work. R.K.M.—contributed to the collection of samples and patient details. P.S.—contributed to the conception and design of the study, interpretation of data, critical revision of intellectual content, and final approval of the version to be published. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kumari, S., Kuruvilla Thomas, R., Sruthi, S. et al. Increased parvovirus B19 seropositivity in healthy blood donors in India. Sci Rep 14, 20497 (2024). https://doi.org/10.1038/s41598-024-68095-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68095-2