Abstract
The objective of this study is to utilize optical coherence tomography angiography (OCTA) techniques for the purpose of identifying abnormalities in retinal and conjunctival vascular density among patients afflicted with renal hypertension. From October 2022 to October 2023, a cohort of sixteen patients diagnosed with renal hypertension (RH), comprising a total of 32 eyes, was selected from the Department of Nephrology at the First Affiliated Hospital of Nanchang University. Concurrently, a group of sixteen healthy individuals, carefully matched in characteristics, was recruited from volunteers at the Ophthalmology Research Center and designated as the healthy controls (HCs) group. Optical coherence tomography angiography was employed to assess and examine the superficial vascular plexus (SVP) and deep vascular plexus (DVP) of the macular retina in both eyes. Subsequently, a comparative analysis was conducted between the two groups, focusing on the superficial and deep retinal microvessels (MIR), macrovascular (MAR), and total microvascular (TMI). The present study employed the central annuli segmentation method (C1–C6), the hemispheric segmentation method (SL, IL, SR, IR), and the Early Treatment Diabetic Retinopathy Study (S, I, L, R) to evaluate deviations in retinal blood vessel density. The investigation aimed to examine the association between blood vessel density and TMI in conjunctival capillaries. A statistically significant difference (p < 0.05) in macular retinal vascular density was observed between the two groups based on the OCTA data. Specifically, in SVP, the density of TMI, MIR, and MAR in the RH group was significantly lower compared to the HCs group (p < 0.05). Additionally, the deep density of TMI and MIR in DVP of the RH group was significantly lower than that of the HCs group (p < 0.05). Furthermore, using the hemispheric segmentation method, both the superficial and deep retina showed a significant reduction in the density of SL, SR and IL regions (p < 0.05). In the ETDRS method, there was a significant decrease in superficial and deep retinal S, I, and L in the RH group (p < 0.05). When applying the central annuli segmentation methods, the RH group exhibited a significant decrease in the superficial retinal C1–3 region (p < 0.05) and a noticeable reduction in the deep retina in the C1–4 region (p < 0.05). Furthermore, a higher positive likelihood ratio was observed in the deep SL and superficial C2 region. There was a positive correlation between conjunctival capillary density and the region of TMI in depth. The results of the OCTA investigation revealed a significant disparity in the density of superficial and deep retinal blood vessels between RH group and the HCs group. Additionally, a notable correlation was observed between the depth of TMI and the density of conjunctival capillaries. These findings highlight the potential of retinal OCTA as a valuable tool for early detection and image-assisted diagnosis of retinopathy progression in patients with RH.
Similar content being viewed by others
Introduction
The etiology of high blood pressure in the majority of patients is uncertain, leading to their classification as having essential hypertension. However, 5–10% of adults with high blood pressure may have secondary hypertension, suggesting a potentially reversible cause1. In evaluating hypertension, both unilateral and bilateral renal dysfunction are considered as part of the overall clinical picture. Patients who are either younger than 30 years old or older than 50 years old, and who present with an abrupt onset of hypertension, unresponsiveness to antihypertensive medications2, visual impairment, and a 50% elevation in serum creatinine levels, should undergo evaluation for RH3. RH is more likely to progress to malignant hypertension than essential hypertension(especially renovascular hypertension) and is more likely to cause damage to target organs such as the fundus.
RH, a form of secondary hypertension, occurs when elevated blood pressure is caused by renal parenchymal disease or renal artery disease. Renal parenchymal hypertension, which encompasses several conditions including polycystic kidney disease, glomerulonephritis, diabetic nephropathy, chronic pyelonephritis, and hypertension after renal transplantation, is the predominant type of secondary hypertension4. Another condition that can occur is renal vascular hypertension, which is primarily caused by stenosis in the main or branch of the renal artery, either unilaterally or bilaterally5. This condition is commonly observed in cases of multiple arteritis, renal artery fibromuscular dysplasia, and atherosclerosis6.
Hypertension exerts deleterious effects on multiple organs within the body. In the early stages of the disease, retinal arterioles undergo spasms, which subsequently progress to arterial hardening as the disease advances7. Notably, ocular manifestations, particularly alterations in retinal microvessels, may serve as indicative markers of the prompt repercussions associated with elevated blood pressure8. Consequently, hypertensive retinopathy, characterized by aberrations in retinal circulation induced by high blood pressure, emerges as the most recent significant ocular consequence of this condition. Hypertensive retinopathy has been widely recognized as having a heightened propensity for systemic morbidity and mortality9. RH, in particular, exhibits a more malignant nature compared to primary hypertension, thereby posing a greater risk to the fundus target organs. The interplay between elevated blood pressure and renal illness can lead to a detrimental cycle, ultimately resulting in a poor prognosis. RH significantly impairs the functioning of the retina, choroid, and optic nerve10. Due to the different sensitivities of patients to diseases, some patients may exhibit more discomfort in the kidneys and ignore eye symptoms. Some patients may first experience eye symptoms such as decreased vision before increasing their attention to RH. Therefore, can we detect and treat early through changes in retinal microvasculature?
The utilization of optical coherence tomography angiography (OCTA), a non-invasive imaging technique, enables the expeditious acquisition of high-resolution flow data and angiographic images11. This technique facilitates the visualization of the retinal and choroidal perfusion vascular system in three dimensions, allowing for precise quantitative assessment of blood flow density and fundus microvascular system structure12. OCTA presents several advantages in comparison to fluorescence angiography. It offers a more comprehensive depiction of blood flow in the retinal and choroidal tissues, eliminating the requirement for dye injection13. Furthermore, by appropriately adjusting division boundaries, OCTA enables the examination of diverse capillary networks within the retina. Consequently, OCTA emerges as the most efficacious non-invasive technique for identifying microvascular changes induced by RH. The identification of ocular vascular anomalies has the potential to enhance the prognosis of renal hypertensive disease, as hypertension may have an earlier impact on ocular microvascular changes.
Insufficiently detailed studies assessing the outcomes of OCTA in patients with RH have been limited, with a scarcity of reports on identified ocular abnormalities. Consequently, the objective of this investigation was to examine the anomalies in conjunctival, deep, and superficial macular retinal microvascular density among RH patients undergoing OCTA.
Materials and methods
Subjects
From October 2022 to October 2023, the Department of Nephrology at the First Affiliated Hospital of Nanchang University conducted a study in which 16 patients (32 eyes) diagnosed with RH were chosen to constitute the RH group. Simultaneously, the eye center recruited 16 healthy individuals, matching them in terms of age and gender, to form the HCs group. Notably, these individuals exhibited no signs of any internal or ocular diseases. The potential influence of specific diseases on blood microcirculation in the eyes prompted the collection of general and professional examination data from patients. Subsequently, an ophthalmologist with expertise in the field conducted a blind assessment of each patient’s OCTA data.
Inclusion and exclusion criteria
The inclusion criteria for this study are as follows: (1) According to the criterion formulated by the Eighth National Joint Commission: Evidence-based Guidelines for the Management of Hypertension in Adults 2014. (2) In this study, renal hypertension is defined as hypertension (characterized by elevated diastolic blood pressure) caused by renal parenchymal lesions (e.g., primary diseases such as glomerulonephritis and pyelonephritis) and renal vascular lesions (e.g., renal vascular stenosis, usually secondary to atherosclerosis and fibromusculardysplasia). At present, the popular diagnostic criteria for hypertension in China: (1) Hypertension can be diagnosed when the systolic blood pressure ≥ 140 mmHg and the diastolic blood pressure ≥ 90 mmHg are measured separately or simultaneously on more than three occasions at rest on different days. (2) The study participants fall within the age range of 52–69. (3) The absence of ophthalmitis, choroiditis, retinal vasculitis, or any other eye disorders. (4) The absence of any other chronic medical conditions such as diabetes, uremia, chronic heart failure, and so on. (5) Initial detection of renal hypertension without a history of antihypertensive or hormonal medications.
The exclusion criteria encompass the following: (1) Presence of chronic medical conditions other than nephrogenic hypertension, such as diabetes, as well as other factors contributing to blood vessel stenosis. (2) Existence of retinopathy or choroidal illness, including glaucoma and arteriovenous disease. (3) History of ocular trauma or surgery. (4) Presence of other disorders that may impact fundus imaging. (5) Females who are currently pregnant or lactating.
Ethical of considerations
This study, conducted in accordance with the Helsinki Declaration, received approval from the Ethics Committee of Nanchang University’s First Affiliated Hospital. The participants’ right to informed consent was thoroughly respected, and a comprehensive explanation of the study was provided to them prior to their involvement. All participants willingly signed the informed consent statement.
Clinical examinations
All participants underwent a comprehensive set of clinical assessments, encompassing blood pressure measurement, intraocular pressure (IOP) measurement, visual acuity (VA) measurement, tear rupture time of sodium fluorescein application (tBUT), corneal fluorescence staining, conjunctival aminosalicylic acid green for eye staining score (OSS), Schirmer test (SIT), infrared light lacrimal river height (TMH) measurement, and the Hospital Anxiety Rating Scale (HADS).
OCTA
There are two distinct physiological layers within the retinal capillary bed, which vary depending on their ___location. The superficial vascular plexus (SVP), found in the retinal nerve fiber and ganglion cell layer, is made up of arterioles, venules, and capillaries, while the deep vascular plexus (DVP), found in the inner and outer plexus, is primarily made up of capillary-sized blood vessels14. To obtain a 3 × 3 mm2 retinal area centered on the fovea, the Zeiss AngioplexTM OCTA (Carl Zeiss Meditec, Dublin, CA)15 was employed. Each participant’s eye was captured with a 3 mm by 3 mm OCTA frontal angiography image. Sub-regions of the anterior image depicting the SVP and DVP were subsequently isolated, and subjected to specialized software for processing. The image size of 245 × 245 pixels was adjusted to 1024 × 1024 pixels to facilitate blood vessel segmentation16. The total blood vessels (TMI) refer to the blood vessels prior to segmentation. Segmentation is performed on images using various image processing techniques such as inversion, equalization, and the removal of background noise and non-vascular objects. This process aims to distinguish the primary blood arteries from the microvascular network and extract the microvessels, resulting in a binary image17. A blood vessel is categorized as a macrovascular (MAR) when its diameter exceeds 25 μm. It is differentiated from the remaining blood vessels, commonly known as microvascular (MIR). Additionally, the application is used to remove the big blood vessel shadow graphic projection artifact in SVP that is projected onto the DVP.
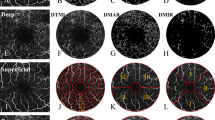
The macular area was evaluated by segmenting the image using three methods as follows. Firstly, the Hemispherical segmentation method was employed, wherein the picture was divided into four sections (superior right, superior left, inferior left, and inferior right) by the horizontal and vertical diagonals (SR, SL, IL, IR) (Fig. 1). Additionally, the Early Treatment Diabetic Retinopathy Study (EDTRS) was utilized, dividing the macular area into four quadrants based on the diagonal of the two quadrants. The segmentation method employed in this study, as depicted in Fig. 1, involves the removal of a non-vascular middle area measuring 0.6 mm in diameter from a circular area ranging from 0.6 to 2.5 mm in diameter. The remaining area is then divided into six thin rings, each with a bandwidth of 0.16 mm, denoted as C1–6 in an inside-out fashion. It is important to note that all participants in this study utilized their right eye. In order to acquire the mirror image and associated data, the left eye data was subjected to a horizontal flip. These resultant figures were then merged with the data from the right eye and subjected to averaging. Subsequently, fractal analysis was conducted within each subpartition utilizing the box counting method, as facilitated by the fractal analysis Toolbox (TruSoft Benoit Pro 2.0, TruSoft International, Inc, St. Petersburg, FL). The resulting fractal dimension (Dbox), serving as an indicator of the blood vessel density within each respective zone, was subsequently obtained.
Statistical analysis
The mean standard deviation (SD) of the data was presented after performing data analysis using IBM’s SPSS 22.0 (Armonk, NY, USA). The mean ± standard deviation was displayed to represent the results. The two-sample t test was employed to examine the results for the RH and HCs groups, while ANOVA methods and Bonferroni correction were used for comparing data between groups. Receiver operating characteristic (ROC) curves were utilized to determine the density of conjunctival vessels and superficial and deep retinal microvessels. The present study employs GraphPad Prism (Version 9.0; GraphPad Software) to investigate the correlation between macular retinal blood vessel density and conjunctival blood vessel density through Pearson correlation analysis. Additionally, a linear correlation analysis is conducted to examine the relationship between the conjunctival capillary plexus and the density of STMI and DTMI. To account for multiple comparisons, P-values are adjusted using the error finding rate (FDR), with a significance level set at 0.05.
Ethical approval and consent to participate
The study methods and protocols were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (Nanchang, China) and followed the principles of the Declaration of Helsinki. All subjects were notified of the objectives and content of the study and latent risks, and then provided written informed consent to participate.
Results
Subjects
The basic data and clinical examination results of the RH group and the HCs group were analyzed using an independent sample rank sum test. There were no significant differences observed in age, gender, or intraocular pressure (IOP) (P > 0.05). However, significant differences (P < 0.05) were found in visual acuity (VA), tear break-up time (tBUT), ocular surface staining (OSS), Schirmer’s test (SIT), and tear meniscus height (TMH) between the two groups. Additionally, the systolic blood pressure (SBP) and diastolic blood pressure (DBP) also exhibited statistically significant differences (P < 0.05) between the two groups. (Table 1).
Superficial retinal microvessel density
Table 2 presents a comparison of retinal density in the superficial macular region among various locations. Notably, the RH patients exhibited a significant decrease in superficial TMI, MIR, and MAR compared to the HCs group (P < 0.05, Fig. 2A,B,C). Furthermore, employing the hemispheric segmentation approach, it was observed that the capillary density of SL, SR, and IL in the superficial retinal capillaries was significantly lower in RH patients compared to the HCs (P < 0.05, Figs. 2A,B,D). The S and I areas exhibited a significant decrease when ETDRS segmentation was implemented, in comparison to HCs (P < 0.05, Fig. 2A,B,E). The central annuli segmentation method resulted in a substantial reduction in the superficial portions of C1–3 (P < 0.05, Fig. 2A,B,F).
Analysis of the superficial retinal layer’s macular retinal microvessel density in the RH and HCs. (A) The superficial retinal vascular density as seen in OCTA pictures. (B) A comparative study between three different grouping methods for retinal vascular density. Results of an examination of the superficial retinal vascular density in every subregion across groups are exhibited in (C–F).
Table 3 presents the findings of the comparative analysis conducted on retinal density in the deep macular region across different locations. The deep TMI and MIR of RH patients exhibited a significant decrease (P < 0.05, Fig. 3A) compared to the HCs group. Our hemispherical segmentation method revealed a notable decrease in capillary density in the SR, SL, and IL regions of deep retinal capillaries compared to the HCs group (P < 0.05, Fig. 3B,D). Additionally, the utilization of the ETDRS segmentation approach demonstrated a significant reduction in MIR within the S, I, and L regions when compared to the HCs group (P < 0.05, Fig. 3B,E). A notable reduction in vascular density (P < 0.05, Fig. 3B,F) was observed in the deep regions of C1–4 through the utilization of the Central annuli segmentation approach.
Analysis of the deep retinal layer’s macular retinal microvessel density in the RH and HCs. (A) The deep retinal vascular density as seen in OCTA pictures. (B) A comparative study between three different grouping methods for retinal vascular density. Results of an examination of the superficial retinal vascular density in every subregion across groups are exhibited in (C–F).
ROC evaluation of retinal microvessel density in the superficial and deep layers
The study successfully demonstrated the ability of OCTA-measured macular retinal microvascular density to differentiate between individuals with RH and HCs. Statistical analysis revealed significant differences in the superficial retina subregions of MIR, TMI, MAR, SL, SR, IL, S, I, L, and C1–C3 between the two groups. Notably, the C2 zone of retinal vascular density exhibited an area under the curve (AUC) of 0.9878 (95% CI = 0.9680–1) when compared to the HCs group, indicating a higher diagnostic sensitivity for detecting retinal microangiopathy caused by RH (Fig. 4A). The RH group demonstrated significant modifications in various areas of the deep retinal layer, including MIR, TMI, SR, SL, IL, S, I, L, and C1–4. The model with the highest positive likelihood ratio, SL, had an AUC of 0.978 (CI = 0.9493–1). It is probable that RH diagnostics will exhibit greater sensitivity in the SL area, as depicted in Fig. 4B.
Correlation between the density of superficial and deep retinal microvessels and the density of conjunctival capillaries
In our investigation of the relationship between retinal vascular density and conjunctival capillary density, we observed a significant positive correlation between the deep area of TMI and conjunctival capillary density (correlation coefficient = 0.4229, P = 0.0198). However, no such correlation was found with the superficial area of TMI. (Fig. 5).
Discussion
This study investigates the microvascular density in the superficial and deep retinal layers using the OCTA technique. The findings suggest that retinal microvessel damage may occur due to RH, as evidenced by significantly lower blood vessel density in the superficial and deep retina of individuals in the RH group compared to HCs group. Additionally, the higher positive probability ratios observed in the deep SL region and superficial C2 region suggest a potentially greater diagnostic significance for detecting retinal microvascular alterations induced by RH. Furthermore, it was observed that the deep TMI region exhibited a robust positive association with conjunctival capillary density, whereas no significant correlation was found between the superficial TMI area and patients with RH. Therefore, the alteration of retinal microvascular OCTA is expected to be one of the criteria for adjuvant diagnosis of renal hypertension.
It is estimated that 10% of high blood pressure cases are classified as secondary hypertension, with kidney disease being responsible for 50% of these instances18. The two most common types of nephropathy that predispose to hypertension are diabetic nephropathy and renal vascular disease, which tend to have a higher mortality rate19. In the majority of kidney disorders, there is a clear correlation between the degree of renal impairment and the onset of hypertension. Specifically, when the glomerular filtration rate (GFR) falls below 50 ml/min, there is an inverse relationship with the prevalence of hypertension. The prevalence of high blood pressure among individuals with end-stage renal disease (ESRD) ranges from eighty to ninety percent. This can be attributed to various factors, including the activation of the RAAS, reduced renal perfusion, and structural damage leading to renin release. Consequently, the synthesis of angiotensin II20is stimulated, which directly causes vasoconstriction in peripheral blood arteries. Additionally, angiotensin II promotes the absorption of sodium in the proximal and distal tubules through the release and action of aldosterone21. However, as glomerular filtration rate (GFR) decreases to stages 4 and 5 of chronic kidney disease (CKD), the significance of salt and water retention increases. The decline in GFR due to renal disease leads to a reduction in sodium excretion, thereby contributing to the development of hypertension22. Conversely, excessive release of angiotensin II results in vasospasm and heightened vasomotor tension, leading to systemic vascular stenosis7. The aforementioned outcome is attributed to the myogenic and metabolic mechanisms responsible for regulating local blood flow, with the aim of managing blood volume within the vascular bed23. Arterial stiffness can be induced by elevated blood pressure, leading to retinal ischemia due to heightened resistance to blood flow and reduced perfusion24.
Hypertension-mediated organ damage (HMOD), a condition where high blood pressure damages organs, can occur. One type of HMOD caused by several vascular alterations in the retinal microcirculation is hypertensive retinopathy25. Six to fifteen percent of non-diabetic adults 40 years of age and older have hypertensive retinopathy7, however this figure is probably much lower than the actual number26. The detrimental effects of hypertension extend to the retinal nerve fiber layer (RNFL) and the retinal microcirculation27. This phenomenon has the potential to result in a progressive and painless loss of vision among certain groups of patients. The microvascular structure of the retina, which is unique to the human body, can be observed directly, allowing for close monitoring of any alterations that may transpire28. In addition to hypertensive retinopathy, high blood pressure can also aggravate diabetic retinopathy (DR), elevate IOP, and induce thromboembolic disorders, thereby affecting ocular health29.
The measurement of retinal microcirculation using OCTA allows for rapid and non-invasive assessment. Parameters such as vascular density (VD), foveal avascular zone (FAZ), and radial peripapillary capillary (RPC) network are utilized to evaluate microvascular changes in the retina and choroid30. This technique possesses the capability to detect subtle deviations in capillary structure, serving as an early indicator of ischemia and hypoxia before observable changes occur in arterioles and venules31. Moreover, a significant association exists between the decline in retinal and choroidal visual impairment and the presence of coronary artery and branch stenosis. Consequently, OCTA emerges as a non-invasive method for early detection and assessment of coronary heart disease (CHD), potentially mitigating the risk of myocardial infarction (MI)32. It is worth noting that while OCTA lacks the capability to measure retinal blood vessel diameter, it can effectively evaluate both blood vessel density and hemodynamics of retinal blood flow, distinguishing it from spectral ___domain OCT (SD-OCT)33,34. In order to ascertain the effect of hypertension on peripheral circulation and, consequently, on the evolution of the disease, it may be helpful to monitor changes in retinal blood vessel density in patients who are at risk for hypertension by increasing the frequency of OCTA follow-up.
The central retinal artery (CRA), the first intraorbital branch of the eye artery, is the main source of the retinal vascular system. After leaving the optic nerve and entering the eye at the optic disc’s center, CRA splits into two main branches. These branches subsequently subdivide the arterioles responsible for nourishing the peripheral retina. Within each quadrant of the retina, every arteriole provides arterial blood supply to the inner nerve layer. The larger venous blood vessels within the retina are situated in close proximity to the inner border membrane. Two layers of capillaries are formed by the arteries: the DVP, which is situated deeper in the inner core layer, and the SVP, which is found in the layers of ganglion cells and nerve fibers35. Our study observed a significant decrease (p < 0.05) in the density of the SL, SR, IL, S, I, L, and C1–C3 regions compared to the HCs group. The ___location of these subregions suggests that vascular occlusion may occur in the superficial temporal retinal arteriole during RH, potentially impacting the trophic nerve fibers. These findings may be attributed to microvascular obstruction in patients with RH. The blood flow from the superficial capillary plexus enters the deep venules through the DVP, which is the terminal part of the retinal capillary unit36. In RH patients, the significant decrease in deep macular retina thickness and macular ischemic retinal density may be caused by small microvascular obstructions. Conversely, the density of SL, SR, IL, S, I, L, and C1–C4 regions lessened (p < 0.05), suggesting that temporal DRL perfusion may be preferentially affected by slowed retinal blood flow.
The utilization of OCTA has facilitated a more comprehensive comprehension of the intricate characteristics of the retinal vascular system37,38. The positioning of these subregions presents the potential for occlusion of the deep temporal retinal arteriole in cases of RH. The predictive value of progressive retinopathy’s distinctive lesions for HMOD has been established39. The status of retinal vessels as terminal vessels and their increased susceptibility to blood pressure fluctuations may serve as an indication of the overall vascular system in cases of RH13. As hypertensive retinopathy advances, vision gradually and painlessly deteriorates, ultimately leading to blindness.
In order to assess the influence of the illness on retinal involvement, we conducted an analysis of the area under the receiver operating characteristic (AUC) curve for various macular retinal partitioning approaches in patients with RH. Our findings indicate that OCTA exhibits a favorable diagnostic capacity for retinal microangiopathy, with a positive probability ratio of up to 0.9878 for C2 density in the superficial layer of the retina and up to 0.978 for SL density in the deep layer. A previous investigation on the microcirculation in the bulbar conjunctiva yielded comparable findings, indicating a significant alteration in red blood cell velocity among individuals with hypertension in comparison to their healthy counterparts40. Furthermore, hypertensive individuals exhibited a reduced normalization of red blood cell velocity following a period of hypoxia. In our study, a significant positive correlation was observed between conjunctival capillary density and the deep area of TMI in patients with RH. This finding supports the hypothesis that conjunctival capillary damage in RH is associated with deep retinal TMI.
One research revealed that hypertensive retinopathy was prevalent in more than 70% of the 224 non-diabetic patients with hypertension and CKD41. Moreover, subsequent investigations42 confirmed that the severity of CKD was independently associated with the progression of retinopathy. Recent studies43 have indicated that patients with both CKD and hypertension exhibit lower density of SVP and DVP. The presence of low retinal vascular density in OCTA has been noted as a potential predictor for acute kidney damage (AKI) and its onset44. Our findings align with this observation, suggesting that alterations in retinal microvascular density could serve as an early indicator of disease progression prior to the development of severe RH. Consequently, the utilization of OCTA to identify retinal microvascular circulation offers a practical and effective approach to improve disease prognosis.
However, it is important to acknowledge the limitations of this study. Firstly, the sample size of the study participants is relatively small, rendering them susceptible to external factors such as drug influences. Secondly, RH is primarily induced by kidney disease, while essential hypertension can also contribute to the development of kidney disease. Given the potential interplay between these two conditions, distinguishing them clinically can pose a significant challenge. Consequently, it is worth noting that essential hypertension can potentially lead to retinal alterations. Moreover, the present investigation lacked adequate data to substantiate the dynamic monitoring of alterations in macular microvessel density during the progression of RH. To overcome the constraints of this study and enhance the practical applicability of its findings, additional extensive prospective longitudinal studies are imperative.
Conclusion
The utilization of OCTA has facilitated a deeper understanding of the macular retinal microcirculation in individuals with RH. Upon comparing the RH group to the HCs group, a significant reduction in the density of both superficial and deep retinal blood vessels was observed. This alteration could potentially be attributed to the activation of the RAAS, leading to peripheral microvascular spasm. Additionally, the superficial C2 region and deep SL exhibit a higher positive likelihood ratio, suggesting a greater diagnostic significance for retinal microangiopathopathy in RH patients. In patients with RH, a significant positive correlation was observed between the density of conjunctival capillaries and the deep TMI region. Consequently, the utilization of retinal OCTA holds promise in facilitating the diagnosis of RH progression by enabling early detection of retinopathy.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Unger, T. et al. International society of hypertension global hypertension practice guidelines. Hypertension 75(6), 1334–1357 (2020).
Ma, Y. J., Dong, Y. X. & He, Y. Diagnosis and treatment of renal hypertension in clinic. Med. Innov. China 21(01), 179–184 (2024).
Whelton, P. K. et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension 71(6), 1269–1324 (2018).
Zhai, R. N., Zheng, L. Y., Xue, R., Gui, D. K. & Wang, N. S. Research progress on renal hypertension pathogenesis and its diagnosis and treatment. World Clin. Drug 38(5), 305–310 (2017).
Weinberger, M. H. Which renal vascular hypertension was induced. J. Am. Soc. Hypertens. 7(5), 327 (2013).
Wang, Y. et al. The predictive potential of altered spontaneous brain activity patterns in diabetic retinopathy and nephropathy. Epma J. 10(3), 249–259 (2019).
Bhargava, M., Ikram, M. K. & Wong, T. Y. How does hypertension affect your eyes?. J. Hum. Hypertens. 26(2), 71–83 (2012).
Newman, A., Andrew, N. & Casson, R. Review of the association between retinal microvascular characteristics and eye disease. Clin. Exp. Ophthalmol. 46(5), 531–552 (2018).
Dziedziak, J. et al. Impact of arterial hypertension on the eye: A review of the pathogenesis, diagnostic methods, and treatment of hypertensive retinopathy. Med. Sci. Monit. 28, e935135 (2022).
Ren, Y. F. et al. Observation of the relationship between microcirculatory changes in the fundus and visual impairment in patients with renal hypertension using OCTA*. Rare Uncommon Dis. 30(9), 31–3235 (2023).
Campbell, J. P. et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci. Rep. 7, 42201 (2017).
Ye, L. et al. Retinal microvasculature alteration in active thyroid-associated ophthalmopathy. Endocr. Pract. 24(7), 658–667 (2018).
Ikram, M. K. et al. Retinal vascular caliber measurements: Clinical significance, current knowledge and future perspectives. Ophthalmologica 229(3), 125–136 (2013).
Kur, J., Newman, E. A. & Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin Eye Res. 31(5), 377–406 (2012).
Rosenfeld, P. J. et al. ZEISS angioplex™ spectral ___domain optical coherence tomography angiography: Technical aspects. Dev. Ophthalmol. 56, 18–29 (2016).
Jiang, H. et al. Automated segmentation and fractal analysis of high-resolution non-invasive capillary perfusion maps of the human retina. Microvasc. Res. 89, 172–175 (2013).
Mwanza, J. C. et al. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-___domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52(11), 7872–7879 (2011).
Lu, X., Wen, P. & Xing, Q. Hypertension secondary to renal hypoplasia in a child. Eur. Heart. J. 44(39), 4200 (2023).
Kalra, P. A. Renal specific secondary hypertension. J. Ren. Care 33(1), 4–10 (2007).
Vaajanen, A. et al. Does the renin-angiotensin system also regulate intra-ocular pressure?. Ann. Med. 40(6), 418–427 (2008).
Choudhary, R. et al. Therapeutic targets of renin-angiotensin system in ocular disorders. J. Curr. Ophthalmol. 29(1), 7–16 (2017).
Johnson, R. J. & Schreiner, G. F. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int. 52(5), 1169–1179 (1997).
Pournaras, C. J. et al. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 27(3), 284–330 (2008).
Katsi, V. et al. Impact of arterial hypertension on the eye. Curr. Hypertens. Rep. 14(6), 581–590 (2012).
Muiesan, M. L. & Grassi, G. Assessment of retinal vascular changes in hypertension: New perspectives. J. Hypertens. 24(5), 813–814 (2006).
Ong, Y. T. et al. Hypertensive retinopathy and risk of stroke. Hypertension 62(4), 706–711 (2013).
Lee, H. M. et al. Changes in thickness of central macula and retinal nerve fibre layer in severe hypertensive retinopathy: A 1-year longitudinal study. Acta Ophthalmol. 96(3), e386–e392 (2018).
Vaajanen, A. et al. Angiotensin (1–7) reduces intraocular pressure in the normotensive rabbit eye. Invest. Ophthalmol. Vis. Sci. 49(6), 2557–2562 (2008).
Holappa, M., Valjakka, J. & Vaajanen, A. Angiotensin(1–7) and ACE2, “The Hot Spots” of renin-angiotensin system, detected in the human aqueous humor. Open Ophthalmol. J. 9, 28–32 (2015).
Kashani, A. H. et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 60, 66–100 (2017).
Chua, J. et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hypertens. 37(3), 572–580 (2019).
Wang, J. et al. Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study. Biomed. Opt. Express 10(4), 1532–1544 (2019).
Konstantinidis, L. & Guex-Crosier, Y. Hypertension and the eye. Curr. Opin. Ophthalmol. 27(6), 514–521 (2016).
Hillard, J. G. et al. Retinal arterioles in hypo-, normo-, and hypertensive subjects measured using adaptive optics. Transl. Vis. Sci. Technol. 5(4), 16 (2016).
Huang, X. et al. Abnormal regional spontaneous neural activity in visual pathway in retinal detachment patients: a resting-state functional MRI study. Neuropsychiatr. Dis. Treat. 13, 2849–2854 (2017).
Garrity, S. T. et al. Considerations in the understanding of venous outflow in the retinal capillary plexus. Retina 37(10), 1809–1812 (2017).
Cheung, C. Y. et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J. Hypertens. 29(7), 1380–1391 (2011).
Zhang, M. et al. Projection-resolved optical coherence tomographic angiography. Biomed. Opt. Express 7(3), 816–828 (2016).
Porta, M., Grosso, A. & Veglio, F. Hypertensive retinopathy: There’s more than meets the eye. J. Hypertens. 23(4), 683–696 (2005).
Sasongko, M. B., Wong, T. Y. & Wang, J. J. Retinal arteriolar changes: Intermediate pathways linking early life exposures to cardiovascular disease?. Microcirculation 17(1), 21–31 (2010).
Chillo, P. et al. Hypertensive retinopathy and associated factors among nondiabetic chronic kidney disease patients seen at a tertiary hospital in Tanzania: A cross-sectional study. Int. J. Nephrol. Renovasc. Dis. 12, 79–86 (2019).
Farrah, T. E. et al. The eye, the kidney, and cardiovascular disease: Old concepts, better tools, and new horizons. Kidney Int. 98(2), 323–342 (2020).
Vadalà, M. et al. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefes. Arch. Clin. Exp. Ophthalmol. 257(8), 1687–98 (2019).
Alan, G. et al. Retinal vascular density as a novel biomarker of acute renal injury after acute coronary syndrome. Sci. Rep. 9(1), 8060 (2019).
Funding
Supported by National Natural Science Foundation of China (No.82160195); Jiangxi Double-Thousand Plan High-Level Talent Project of Science and Technology Innovation (No.jxsq2023201036); Key R & D Program of Jiangxi Province (20223BBH80014); Science and Technology Project of Jiangxi Province Health Commission of Traditional Chinese Medicine (No. 2022B258); Science and Technology Project of Jiangxi Health Commission (No. 202210017).
Author information
Authors and Affiliations
Contributions
L.W. and JY.W. edited and revised the article, C.C. revised the article, M.K. made Fig. 1, SH.X. made Fig. 3, H.W. made Fig. 5, L.Q. made table 1, LQ.H. made table 2, J.Z. made table 3, X.C. made Fig. 4, P.Y. made Fig. 2, H.H. collected the data, Y.S. provided funding and research ideas, All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Wang, JY., Chen, C. et al. OCTA evaluates changes in retinal microvasculature in renal hypertension patients. Sci Rep 14, 28910 (2024). https://doi.org/10.1038/s41598-024-68690-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68690-3