Abstract
Extracellular vesicles (EVs) from mesenchymal stem cells (MSCs), specifically those preconditioned with deferoxamine (DFO) in canine adipose tissue-derived MSCs (cAT-MSCs), were explored for treating autoimmune diseases. This study assessed the effects of DFO-preconditioned EVs (EVDFO) in an experimental autoimmune encephalomyelitis (EAE) mouse model. cAT-MSCs were treated with DFO for 48 h, after which EVs were isolated. EAE mice received intranasal EV or EVDFO treatments and were euthanized following histopathologic analysis; RNA and protein expression levels were measured. Histologically, EV and EVDFO groups showed a significant reduction in inflammatory cell infiltration and demyelination. Immunofluorescence revealed increased CD206 and Foxp3 expression, indicating elevated M2 macrophages and regulatory T (Treg) cells, particularly in the EVDFO group. Treg cells also notably increased in the spleen of EVDFO -treated mice. STAT3 and pSTAT3 proteins were upregulated in the EAE groups compared to the naïve group. However, following EV treatment, STAT3 expression decreased compared to the EAE group, whereas pSTAT3 expression was similar in both the EV and EAE groups. In conclusion, EVDFO treatment resulted in reduced STAT3 expression, suggesting its role in T cell regulation and the potential of EVDFO in modulating the STAT3 pathway for reducing inflammation more effectively than non-preconditioned EVs.
Similar content being viewed by others
Introduction
Extracellular vesicles (EVs) in mesenchymal stem cells (MSCs) play an important role in transmitting molecules between cells1. They are released from cell membranes and contain proteins, metabolites, and nucleic acids, and several studies have shown that cells are modified by factors transmitted through EVs2. Many researchers have reported that MSC-derived EVs could be a therapeutic strategy for regulating immune cells using the transmitting function of EVs3. MSC-derived EVs showed immunomodulation of neutrophils, macrophages, and T cells into an anti-inflammatory phenotype, alleviating clinical signs in a mouse inflammation model4,5,6. MSC-derived EVs offer significant advantages over direct MSC cell therapies by eliminating risks associated with cell-based treatments, such as malignancy, undesired differentiation, and prolonged cell viability. Additionally, EVs can freely circulate through the body, penetrate physiological barriers, and are more cost-effective and easier to store and handle, making them a safer and more efficient therapeutic option7.
To enhance their therapeutic effect, research is being conducted to improve the function of EVs8. Hypoxic stimulation of MSCs is one strategy for improving the function of MSC-derived EVs9. As hypoxic conditions are evoked in MSCs, hypoxia-inducible factor-1 alpha (HIF-1α) accumulates in the nucleus of cells, and the cytokine levels under HIF-1α signaling are increased10. These cytokines are closely related to inflammation, proliferation, and angiogenesis11,12. To stably induce hypoxic conditions, the drug deferoxamine (DFO) was used in MSCs. DFO is a hypoxia-mimetic agent that inhibits the hydroxylation of HIF-1α by chelating the iron necessary for prolyl-4 hydroxylase13. In a previous study, we revealed that DFO-preconditioned canine adipose tissue-derived (cAT)-MSC-derived EVs (EVDFO) induce macrophages into an anti-inflammatory state, which was related to the STAT3 pathway in vitro14. Based on these results, we aimed to investigate their potential therapeutic application in autoimmune disease.
Multiple sclerosis (MS) is a severe autoimmune disease affecting the central nervous system (CNS)15. The experimental autoimmune encephalomyelitis (EAE) animal model is a representative model in MS research, and its clinical and pathological features are similar to those of patients with MS16. Although various treatments have been studied for MS and autoimmune diseases, current therapeutic strategies have limitations and research on new strategies is needed17. In the EAE model, activated T cells with the myelin oligodendrocyte glycoprotein35-55 (MOG35-55) peptide fragment target the CNS and trigger the infiltration of immune cells18. In the early stages of the inflammatory process, microglia/macrophages are transformed into the M1 type and release proinflammatory cytokines, which induce neuroinflammation and demyelination19. Thus, modulating macrophages and T cells into the anti-inflammatory (M2) phenotype is one of the treatment strategy in the EAE model20,21. One of the target factors for treatment in autoimmunity is the signal transducer and activator of transcription 3 (STAT3), and several studies about autoimmunity have reported that STAT3 is involved in regulating T cells22,23,24. In chronic inflammatory disease, it was revealed that STAT3 was a key factor in modulating the balance of T helper 17 and regulatory T (Treg) cells. As it promoted T cell proliferation, STAT3 contributed to chronic inflammation25,26.
Therefore, in this study, we assessed whether EVDFO could alleviate neurological signs of the EAE model more effectively than non-preconditioned EV treatment. We also evaluated the immunomodulation effect of EVDFO and analyzed molecular changes in splenocytes and peripheral blood mononuclear cells (PBMC).
Results
Characterization of cAT-MSC-derived EVs and elevation of protein levels in DFO-preconditioned MSCs
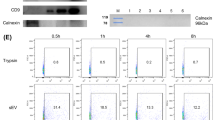
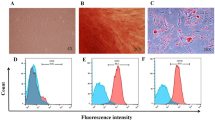
The cAT-MSCs were characterized for surface markers using flow cytometry and differentiation (Fig. 1A,B). EVs were extracted from cAT-MSC-culture media. CD63 and CD9 were detected as positive EV markers and β-actin was used as a negative marker in western blotting (Fig. 1C). The EVs had a round shape with diameters ranging from 100 to 200 nm, per the electron microscope analysis (Fig. 1D). Using particle-size analysis, the diameters of EVs were confirmed to be similar in size by electron microscopic analysis (Fig. 1E).
Characterization of MSCs and EVs. (A) Analysis of surface markers of cAT-MSCs using flow cytometry; cAT-MSC was negative for CD34 and CD45 and positive for CD29, CD44, and CD96. (B) cAT-MSCs differentiated as adipocytes, osteocytes, and chondrocytes. (C) Analysis of EV markers using western blotting. EVs derived from cAT-MSCs were positive for CD63 and CD9 but negative for β-actin. (D) EVs were round in shape, with diameters ranging from 100 to 200 nm in electron microscopic analysis. (E) The diameters of EVs were measured at 100 to 200 nm using particle-size analysis.
Considering previous studies, we decided to use 100 μM DFO in the cAT-MSCs14,27. To confirm hypoxia preconditioning with DFO, HIF-1α was detected in DFO-preconditioned cAT-MSCs using western blotting (Fig. 2A). The protein expression of STAT3 was decreased in MSCDFO; however, pSTAT3 expression was increased in MSCDFO compared to that in non-preconditioned MSCs. The expression of COX-2 also increased in MSCDFO compared to that in non-preconditioned MSCs (Fig. 2B). COX-2 protein expression was also measured in 25 μg of EVs and was found to be higher in EVDFO than in non-preconditioned EVs (Fig. 2C).
Protein expression of non-/preconditioned MSCs and EVs. (A) In DFO-preconditioned cAT-MSCs, the expression of HIF-1α was increased. (B) In DFO-preconditioned cAT-MSCs, the expression of STAT3 decreased, while pSTAT3 (Tyr705) and COX-2 expression increased. (C) The expression of COX-2 in the EVDFO group as well as in the cytosols of non-/preconditioned cAT-MSCs increased compared to the EV group.
cAT-MSC-derived EVs and EVDFO alleviated clinical signs and histological changes in the EAE mouse model
Clinical signs were evaluated daily, and the onset of neurological signs occurred on day 7 and peaked on day 21 (Fig. 3A). The day of onset was similar to that of the EAE group as well as the EAE + EV and EAE + EVDFO groups. In the EV and EVDFO groups, clinical scores were significantly reduced compared to those in the EAE group, and clinical signs were milder in the EVDFO group. No adverse effects related to the intranasal injections were observed (Fig. 3B).
EAE mouse model and clinical signs. (A) A schematic diagram of the in vivo experiment. The mice were immunized with 200 μg of MOG35-55 emulsified with 400 μg complete Freund’s adjuvant on day 0. Subsequently, 200 ng of pertussis toxin (Sigma, USA) was injected intraperitoneally on day 0 and day 2. From day 9, 10 μg aliquot of EVs were injected intranasally every 24 h for 14 days. (B) Clinical scores of EAE mice. The onset of neurological signs occurred on day 7 and peaked on day 21. The day of onset in EAE mice was similar to the EAE group and the EAE + EV and EVDFO treatment groups. In mice treated with EVs and EVDFO, clinical scores were significantly reduced, and the EVDFO-treated group showed lower scores compared to the EV group. Additionally, compared to the EAE group, the clinical scores of the EAE + EV group were significantly different on day 20 compared to day 23, and the clinical scores of the EAE + EVDFO group were significantly different on day 15 compared to day 23. Results are shown as means ± standard deviation. **P < 0.01, ***P < 0.001.
On day 25 post-EAE induction, mice were euthanized, and the spinal cord was isolated with DPBS. To evaluate inflammatory cell infiltration, paraffinized spinal cord sections were stained with H&E. In the EAE group, cells with nuclei, which were suspected to be inflammatory cells, were significantly infiltrated, and in the EAE + EVDFO groups, the number of infiltrated cells was lower than that in the EAE group (Fig. 4). To evaluate demyelination of the spinal cord, paraffinized sections were stained with LFB. In the EAE group, the demyelination area increased significantly; in the EAE + EV and EAE + EVDFO groups, we observed attenuated demyelination compared to the EAE group (Fig. 4).
Histology of the cord in the EAE mouse model. (A) Histology of EAE mice. Infiltration of inflammatory cells was confirmed by H&E staining. Nucleated cells were significantly infiltrated in the spinal cord of the EAE mouse model (yellow arrows). In the EAE + EV and EAE + EVDFO groups, the number of infiltrated cells was reduced compared to the EAE group (upper line). LFB staining confirmed demyelination. In the EAE group, the demyelination area was significantly increased compared to the naïve group (yellow arrows). The EAE + EV and EAE + EVDFO groups showed attenuated demyelination compared to the EAE group (middle line). Infiltrated cells were analyzed using immunofluorescent staining. In the EAE group, the expression of PE-CD11b, which is a macrophage marker, increased, while FITC-CD206 expression, which is a M2 polarized macrophage marker, was not observed. However, in the EAE + EV and EAE + EVDFO groups, the expression of FITC-CD206 increased. Additionally, in the EAE + EVDFO groups, the expression of FITC-Foxp3 increased significantly compared to the EAE and EAE + EV groups (lower line).
We evaluated the phenotype of macrophages infiltrated in the spinal cord using immunofluorescence. In the EAE group, the expression of PE-CD11b increased; however, in the EAE + EV and EAE + EVDFO groups, the expression of PE-CD11b decreased, and the expression of FITC-CD206 increased (Fig. 4). To evaluate the population of Treg cells infiltrating the spinal cord, Foxp3 was detected using immunohistochemical analysis. In the EVDFO group, the expression of FITC-Foxp3 increased significantly (Fig. 4).
mRNA expression of cytokine and protein level changes in the spinal cord of EV-treated mice
We analyzed the mRNA expression of cytokines in the spinal cord of all mice. The mRNA expression of CD206, which is related to the M2-type macrophages, was significantly elevated in the EAE + EVDFO group; in the EAE + EV group, the mRNA expression of CD206 did not increase. The mRNA expression of iNOS, which is related to the M1-type macrophages, was significantly elevated in the EAE group but significantly lower in the EAE + EV and EAE + EVDFO groups. No significant difference was observed between the EV and EVDFO groups. The mRNA expression of Foxp3, which is related to Treg cells, was significantly elevated in the EAE + EV and EAE + EVDFO groups compared to that in the naïve group; however, only the expression in the EAE + EVDFO group was significantly elevated compared to that in the EAE group. The mRNA expression levels of TNF-α, IFN-γ, and IL-1β, cytokines related to inflammation, were significantly elevated in the EAE group; however, the mRNA expression level of the inflammatory cytokine IL-6 did not increase. In the EAE + EV and EAE + EVDFO groups, the mRNA expression of these cytokines was significantly lower than that in the EAE group; in particular, the group treated with EVDFO showed a significant reduction in TNF-α and IL-6 levels compared to the EV-treated group (Fig. 5A).
RNA/protein expression in the cord of the EAE mouse model. (A) RNA expression in the spinal cord of the EAE mouse model. The mRNA expression of CD206 increased significantly in the EAE + EVDFO group compared to the EAE and EAE + EV groups. The mRNA expression of iNOS increased significantly in the EAE group compared to the naïve group, while in the EAE + EV and EAE + EVDFO groups, the mRNA expression of iNOS decreased significantly. The mRNA expression of Foxp3 increased significantly in the EAE + EV and EAE + EVDFO groups compared to the naïve group. The mRNA expression of inflammatory cytokines, TNF-α, IFN-γ, and IL-1β, increased significantly in the EAE group compared to the naïve group. In the EAE + EV and EAE + EVDFO groups, the mRNA expression of TNF-α, IFN-γ, IL-1β, and IL-6 decreased significantly compared to the EAE group; the EVDFO-treated group showed an especially significant reduction in TNF-α and IL-6 mRNA expression compared to the EV-treated group. (B) The protein expression of STAT3 decreased in the EAE + EV and EAE + EVDFO groups compared to the EAE group. pSTAT3 (Tyr705) expression increased significantly in the EAE group compared to the naïve group but decreased in the EAE + EVDFO group compared to the EAE group. Results are shown as means ± standard deviation. *P < 0.1, **P < 0.01, ***P < 0.001; ns, not significant.
To evaluate the relationship between STAT3 and EV treatment, we assessed STAT3 expression in mouse brain tissue. The expression of STAT3 was lower in the EAE + EV and EAE + EVDFO groups than that in the EAE group. pSTAT3 expression was significantly lower in the EAE group than in the naïve group, while its expression was higher in the EAE + EVDFO group than in the EAE group (Fig. 5B).
EVs altered the Treg cell population in the EAE mouse model
On day 25 post-EAE induction, the spleens were dissected and isolated as splenocytes after RBC lysis. To evaluate the Treg cell population, splenocytes were bound to CD4, CD25, and Foxp3 antibodies, and CD4 + CD25 + Foxp3 + cells were evaluated as Treg cells. The percentage of Agroups in the Treg population increased significantly, and the EVDFO group showed a significant increase in the Treg population compared with the EV-treated group (Fig. 6A).
Analysis of Treg cells, RNA and protein expression, and secretory cytokines in the splenocytes of the EAE mouse model. (A) Analysis of CD4+CD25+Foxp3+Treg cells in splenocytes. The numbers of CD4+ (upper line) and CD4+CD25+Foxp3+ (lower line) cells were significantly reduced in the EAE group compared to the naïve group. In the EV and EVDFO groups, the Treg cell population increased significantly compared to the EAE group. (B) RNA expression in splenocytes. The mRNA expression of CD206 increased significantly in the EAE + EV and EAE + EVDFO groups compared to the EAE group. The mRNA expression levels of iNOS and TNF-α, however, were unchanged in the EAE and EAE + EV groups but decreased significantly in the EAE + EVDFO group. The mRNA expression of IFN-γ increased significantly in the EAE group compared to the naïve group, while in the EAE + EVDFO group, its expression decreased significantly compared to the EAE and EAE + EV groups. (C) Cytokine levels in the culture media of splenocytes. The levels of IL-1β and IL-6 increased significantly in the EAE group. In the EAE + EV and EAE + EVDFO groups, the levels of these cytokines were decreased compared to the EAE group; the IL-6 level was especially lower in the EAE + EVDFO group than that in the EAE + EV group. (D) Protein expression in splenocytes. The protein expression of STAT3 and pSTAT3 (Tyr 705) increased in the EAE group compared to the naïve group; their expression levels were also significantly decreased in the EAE + EVDFO groups compared to the EAE and EAE + EV groups. Results are shown as means ± standard deviation. *P < 0.1, **P < 0.01, ***P < 0.001; ns, not significant.
Cytokine and protein level changes in the spleen in EV-treated mice
The expression levels of inflammatory cytokines were also evaluated. The expression of CD206 increased significantly in the EAE + EV and EAE + EVDFO groups. The expression of TNF-α did not increase in the EAE and EAE + EV groups; however, in the EAE + EVDFO group, the expression was significantly lower than that in the EAE group. IFN-γ expression increased significantly in the EAE group, and there was no significant difference between the EAE and EAE + EV groups. However, in the EAE + EVDFO group, the expression of IFN-γ was significantly lower than that in the EAE and EAE + EV groups (Fig. 6B).
The culture media from MOG35-55-activated splenocytes were obtained, and the inflammatory cytokines were evaluated. IL-1β and IL-6 levels were significantly increased in the EAE group. However, in the EAE + EV and EAE + EVDFO groups, levels of these cytokines were reduced compared to those in the EAE group (Fig. 6C).
To evaluate the relationship between STAT3 and EV treatment, we assessed STAT3 levels in mouse splenocytes. The expression levels of STAT3 and pSTAT3 were higher in the EAE group than in the naïve group. In contrast, STAT3 and pSTAT3 expression decreased in the EAE + EV and EAE + EVDFO groups compared to that in the EAE group, showing a greater decrease in the EVDFO group than in the EV group (Fig. 6D).
Evaluation of the effect of EVs in canine PBMCs through RNA and protein expression analyses
After stimulation of canine PBMCs with Con A, the cells were treated with EVs or EVDFO for 24 h. The expression of CD4 was not significantly different between the groups. The expression of CD25 increased significantly in the Con A-stimulated groups; however, even with EV treatment, its expression did not change significantly compared to that in the Con A-stimulated group. The expression of Foxp3 increased significantly in the EVDFO group compared to that in the Con A-stimulated and EV treatment groups. The expression of IL-6, TNF-α, and IFN-γ increased significantly in the Con A-stimulated group compared to the naïve group; however, EVDFO treatment significantly decreased their expression compared to the Con A-stimulated group. In the EV group, the expression of IL-6 and IFN-γ also decreased significantly compared to that in the Con A-stimulated group; however, the expression of TNF-α did not decrease compared to the Con A-stimulated group (Fig. 7A).
RNA and protein expression of Con A-stimulated canine PBMCs. (A) RNA expression in PBMCs. The expression of CD4 was unchanged after stimulation with Con A and treatment with EVs. The expression of CD25 increased significantly in the Con A-stimulated groups. EV treatment did not affect the expression of CD25 compared to the Con A-stimulated group. The expression of Foxp3 increased significantly in the EVDFO group compared to the Con A-stimulated and EV-treatment groups. The expression of IL-6, TNF-α, and IFN-γ increased significantly in the Con A-stimulated group compared to the naïve group. Treatment with EVDFO significantly decreased the expression of IL-6, TNF-α, and IFN-γ compared to the Con A-stimulated group. (B) Protein expression in PBMCs. The protein expression of pSTAT3 (Tyr 705) increased in all Con A-stimulated groups. However, STAT3 expression decreased in the EV and EVDFO groups; the decrease in STAT3 expression in the EVDFO group was greater than that in the EV group. Results are shown as means ± standard deviation. *P < 0.1, **P < 0.01, ***P < 0.001; ns, not significant.
The protein expression of pSTAT3 increased in all Con A-stimulated groups, whereas the expression of STAT3 decreased in the EV and EVDFO groups. The expression of STAT3 in the EVDFO group, in particular, decreased more than that in the EV group (Fig. 7B).
Discussion
Several studies have been conducted on the immunomodulatory function of MSCs, and their therapeutic efficacy has been actively studied in chronic inflammation and autoimmune disease28,29,30. Furthermore, in previous studies, cell conditions were reflected in the contents of EVs31,32; based on these results, EVs derived from MSCs have been investigated as a treatment option for inflammation33,34. Pretreatment, or hypoxia treatment, is often employed to increase the therapeutic effectiveness of EVs derived from MSCs35. The immunomodulatory effects of MSC-derived EVs are also influenced by preconditioning with hypoxia, which alters the composition of the EV cargo6,36. In this study, we used DFO to induce hypoxic conditions in cAT-MSCs and identified the changes in cAT-MSC-derived EVs. Moreover, we intranasally administered EVDFO in an EAE mouse model—an autoimmune disease model used in MS research—and confirmed their therapeutic efficacy.
In our EAE mouse model, the intensity of neurological signs was significantly reduced in the EV and EVDFO groups. Upon histological examination, inflammatory cell infiltration significantly decreased, and demyelination was alleviated. Considering the size of EVs, which is under 1 μm, EVs have the possibility of crossing the blood–brain-barrier (BBB) through nasal injection37. Through clinical improvement in the EAE mouse model by EV nasal injection, it can be assumed that the EV passed the BBB and showed enhancing the efficiency of therapeutic agents that could target neurological conditions. Also, intranasal injection is less invasive and associated with reduced pain and discomfort compared to other injection methods.
Immunofluorescence staining revealed that the expression of CD206, a marker of the M2 macrophages, and Foxp3, a marker of Treg cells, increased significantly in the EAE + EVDFO group compared to the EAE and EAE + EV groups. In addition, the expression of inflammatory factors, TNF-α, IFN-γ, IL-1β, and IL-6, was significantly lower in the EAE + EVDFO group than that in the EAE and EAE + EV groups. Therefore, we assumed that EVDFO modulated macrophages into M2 type macrophages and Treg cells in the spinal cord. Considering the previous EAE model, demyelination was associated with inflammatory cell infiltration38; modulating the immune system is important to alleviate demyelination. Therefore, as the immune system was modulated to an anti-inflammatory state through EVDFO, demyelination also decreased in histologic analysis.
We further assessed the experimental changes in the spleen using the EAE model. The results showed a reduction in the number of CD4+CD25+Foxp3+ Treg cells, which was related to the loss of immune homeostasis39. Conversely, treatment with either EVs or EVDFO resulted in an increased ratio of Treg cells, with a notable rise in CD206 expression in the EV-treated groups, suggesting that EVs promote the shift towards anti-inflammatory M2 macrophages. Moreover, EVDFO administration was more effective than EV treatment in modulating T cells and M2 macrophage. These findings imply that intranasally administered EVs impact the central nervous system and the overall immune system, stimulating Treg cell differentiation and the induction of anti-inflammatory M2 macrophages. Consequently, through the modulation of T cells and macrophages, EVDFO holds the potential to restore immune homeostasis40,41,42.
Considering that STAT3 is critical in immune cell regulation in the EAE mouse model22,43 and the close association between MSCs and STAT3 pathway phosphorylation44,45, we investigated STAT3 expression changes in the spleen and spinal cord following treatment. In the spleen, STAT3 and pSTAT3 protein levels were elevated in the EAE groups compared to those in the naïve group. However, when treated with EVs, STAT3 expression decreased compared to the EAE group. We hypothesized that EVs changes STAT3 status as decreasing STAT3 and increasing proportion of pSTAT3 in the spleen and spinal cord, contributing to the activation of Treg cells and the modulation of macrophages46,47. Additionally, the greater decrease in STAT3 expression observed in the EAE + EVDFO group than in the EAE + EV group suggests that EVDFO treatment is more effectively changing STAT3 status and regulating the immune system. Although the spinal cord is characterized by lower STAT3 expression compared to other organs48, and thus shows relatively subtle changes, there was still a reduction in STAT3 expression in spinal cord of EAE + EVDFO group. Additionally, in the EAE group treated with EVDFO, there was a noticeable decrease in inflammatory cell infiltration in the spinal cord, and the state of the inflammatory cells were changed. Considering these factors, it is reasonable to conclude that the spinal cord was affected similarly to the spleen.
To confirm the direct effect of EVs and EVDFO on T cells, PBMCs were treated with EVs and analyzed for expression of inflammatory factors and markers of T cells. After treatment with EVs or EVDFO, the levels of inflammatory factors were reduced compared to those in Con A-stimulated PBMCs. Notably, when analyzing the expression of CD4/CD25/Foxp3 in PBMC, there was no change in CD4 expression, but CD25 expression increased in all groups stimulated by Con A. CD25 is not a specific marker of Treg cells, and its expression may have increased due to external stimulation49. As the expression of Foxp3 increased significantly in the EVDFO group, we assumed that the Treg cell population increased with EVDFO treatment50. In PBMCs, we also confirmed the changes in STAT3 expression with EV and EVDFO treatment. When stimulated by Con A, expression of both STAT3 and pSTAT3 increased; however, the expression of STAT3 decreased with EV and EVDFO treatment, more so with EVDFO treatment. Therefore, we suggest that EVDFO effectively regulates T cells, and the regulation mechanism is associated with changing STAT3 status.
Previous studies have suggested a relationship between COX-2 and PGE2 with the upregulation of Foxp3 + Treg cells51. For instance, COX-2 activators can increase the population of Treg cells and enhance their immunosuppressive function, thereby preventing autoimmune reactions52. On the other hand, the use of COX-2 inhibitors may decrease the number and efficacy of Tregs53. Also, in the present study, the EVDFO group contained higher levels of COX-2 than the non-preconditioned EV group (Fig. 2C). Additionally, overexpression of COX-2 is known to be related with the phosphorylation of STAT354. We estimated that when EVDFO, which had enriched COX-2 molecules, were administered, the status of STAT and pSTAT3 expression in T cells were changed and this changes lead to activation of Treg cells and alleviated clinical signs in EAE mouse model. Another possibility is that EVDFO might synchronized T cells to the same state as DFO-preconditioned cAT-MSCs55. In cAT-MSCs, preconditioning with DFO decreased the expression of STAT3; conversely, the expression of pSTAT3 increased (Fig. 2B), indicating that DFO-induced hypoxia increased STAT3 phosphorylation rather than increasing its expression56. In T cells treated with EVDFO, the expression of STAT3 and pSTAT3 was similar to that in cells treated with MSCDFO; therefore, EVs may perform a synchronizing function by transmitting molecules such as miRNAs.
One limitation of this study is that it was unclear whether other substances, such as miRNA in EVs, regulate the STAT3 pathway. Therefore, additional research would be required to analyze the molecules in EV and the changes in EVDFO. However, this report suggests that preconditioning with DFO in MSC is effective in enhancing the clinical efficacy of MSC-derived EV. Moreover, EVDFO is a good candidate for cell-free therapy, which has the potential to alleviate CNS inflammation and modulate auto-immunity.
To our knowledge, this is the first report to confirm the effectiveness of DFO-preconditioned EVs in an EAE mouse model. We found that EVDFO treatment had a relatively higher efficacy in reducing inflammation than non-preconditioned EVs. Moreover, our findings indicate that STAT3 regulation plays a major role in regulating EAE and that EVs control chronic inflammation by regulating STAT3.
Methods
Cell isolation and culture
The Institutional Animal Care and Use Committee of Seoul National University (protocol no. SNU-180621-27, SNU-220818-2) approved the protocol for obtaining canine adipose tissue. Canine adipose tissue was obtained from a healthy dog during routine spaying surgery. The tissues were cut into small pieces and digested with collagenase type IA for 1 h at 37 °C (1 mg/mL; Sigma-Aldrich, St. Louis, MO, USA). After digestion, the cell pellet was obtained by centrifugation at 1200×g for 5 min to remove debris and filtered through a 70 µm Falcon cell strainer (Fisher Scientific, Waltham, MA, USA). The cells were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM; Welgene, Korea) with 10% fetal bovine serum (FBS; PAN Biotech, Aidenbach, Germany) at 37 °C in a humidified atmosphere containing 5% CO2.
The cAT-MSCs were characterized by detecting stem cell markers, namely, FITC-conjugated CD29, PE-conjugated CD34, FITC-conjugated CD44, FITC-conjugated CD45, and PE-conjugated CD9, using flow cytometry on a FACS Aria II installed at the National Center for Inter-University Research Facilities at Seoul National University. The cells were differentiated into adipocytes, osteocytes, and chondrocytes to confirm their multilineage features. After characterization, cAT-MSCs at passages 3–4 were used in subsequent experiments. When cAT-MSCs were approximately 70% confluent, 100 μM DFO was added, followed by incubation for 48 h.
Isolation and characterization of EVs from cAT-MSCs
The cAT-MSCs were cultured for 48 h in DMEM (Welgene) supplemented with 10% exosome-depleted FBS (Thermo Fischer Scientific). The culture media were collected and centrifuged at 3000×g for 20 min to remove cells and debris. Each supernatant was transferred to a fresh tube, and ExoQuick-CG (System Biosciences, USA) was added according to the manufacturer’s instructions.
Isolated EVs were characterized using protein markers against CD63 (Novus Biologicals, CO, USA) and CD9 (GeneTex, Irvine, CA, USA). The morphology of the EVs was identified using transmission electron microscopy. Briefly, the EV suspension (10 μL) was placed on a 300-mesh formvar/carbon-coated electron microscopy grid and stained with uranyl acetate. EVs were observed under a transmission electron microscope (LIBRA 120; Carl Zeiss, Germany) at 120 kV. The size of the EV particles was measured using a zeta potential and particle size analyzer (ELSZ-1000ZS, Otsuka Electronics, Osaka, Japan).
EAE induction and therapy
Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University, and all protocols were approved by the relevant guidelines (protocol no. SNU-220328-11). The EAE mouse model was induced in six-week-old female C57BL/6 mice. Eight mice were used in each of the three groups, and six mice were used in the naïve group, totaling 30 mice. Every group was divided randomly into two cages, with four mice in each cage. Each mouse had an identifiable mark on its tail, and the cage was also numbered. Mice were immunized with 200 μg of MOG35-55 (Prospecbio, Israel) emulsified with 400 μg of complete Freund’s adjuvant (CFA; Sigma-Aldrich). The emulsification was injected into both flanks of each mouse. Subsequently, 200 ng of pertussis toxin (Sigma-Aldrich) was injected intraperitoneally on days 0 and 2.
From day 9, we intranasally injected 10 μg of EVs every 24 h for 14 days. The mouse was gently grasped by the scruff of its neck and then positioned on its back57. The EV was diluted with DPBS to a concentration of 1 µg/µl, and 10 µl of this solution was administered intranasally to the mice using a pipettor. The mouse inhaled 5 µl of the droplet in each nostril. All mice were weighed and scored twice a daily according to clinical signs of EAE. Their scores were evaluated using a scale ranging from 0 to 5, using the following system: grade 0, no obvious clinical symptoms; grade 0.5, partial tail paralysis; grade 1, tail paralysis or waddling gait; grade 1.5, partial tail paralysis and waddling gait; grade 2, tail paralysis and waddling gait; grade 2.5, partial limb paralysis; grade 3, paralysis of one limb; grade 3.5, paralysis of one limb and partial paralysis of another; grade 4, paralysis of two limbs; grade 4.5, moribund state; and grade 5, death. In the active EAE mouse model, since partial recovery appeared on days 18 to 23, EV was administered until day 24, symptoms were monitored until day 25, and euthanasia was performed. Before that, euthanasia was performed with a clinical score of 4.5 or higher.
Three different investigators were involved in injection and evaluating clinical score: a first investigator injected emulsification and pertussis toxin (Sigma-Aldrich), a second investigator evaluated clinical score and weight, a third investigator performed the euthanasia and tissue collection procedure.
Histological analysis
Hematoxylin and eosin (H&E) staining was used to evaluate the degree of accumulation of inflammatory cells, and Luxol Fast Blue (LFB) staining was used to evaluate demyelination. On day 25 post-EAE induction, the mice were euthanized by exposure to CO2, and the spleen, brain, and spinal cord were removed from all mice. Paraffin-embedded spinal cords were prepared, and 5-μm sections of each sample were prepared and used for staining. Cell counting was performed using CaseViewer (Version 1.4.0.50094 and 2.0, 3DHISTECH Ltd, Budapest, Hungary) at 20× and 40× magnification. For semiquantitative assessment of the infiltration of inflammatory cells in the spinal cord, we evaluated the scores (based on the scoring system in58) as follows: 0, no inflammation; 1, localized infiltration of a small number of inflammatory cells; 2, extensive infiltration of inflammatory cells, concentrated in specific areas of the spinal cord.; 3, widespread infiltration of inflammatory cells throughout the entire spinal cord, covering the neural tissue.. The percentage of demyelination was calculated as the demyelinated area compared to the total area of white matter. Quantification was performed using the ImageJ software (National Institutes of Health, Bethesda, MD).
Immunofluorescence analysis
Paraffin-embedded spinal cord sections were deparaffinized and rehydrated with ethyl alcohol, and antigen retrieval was performed in 10 mM citrate buffer. The sections were then washed with Dulbecco’s phosphate-buffered saline (DPBS; Welgene) and blocked with a blocking buffer containing 1% bovine serum albumin and 0.1% Tween 20 for 30 min. After the blocking procedure, the sections were incubated overnight at 4 ℃ with mouse monoclonal PE-conjugated anti-CD11b (1:100; BioLegend, CA, USA) as macrophage marker, mouse monoclonal FITC-conjugated anti-CD206 (1:100; BioLegend) as M2 phase macrophage marker, and mouse monoclonal FITC-conjugated anti-Foxp3 (1:100; eBioscience, USA) as regulatory T cell. All samples were mounted with Vectashield mounting medium containing 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). Samples were observed under an EVOS FL microscope (Life Technologies, Darmstadt, Germany).
RNA extraction, cDNA synthesis, and real-time PCR
Total RNA was extracted from cells or tissues using an Easy-Blue RNA extraction kit (iNtRON Biotechnology, Korea) according to the manufacturer’s instructions. cDNA was synthesized using the Cell Script All-in-One 5X First Strand cDNA Synthesis Master Mix (Cell Safe, Korea). The samples were analyzed using AMPIGENE qPCR Green Mix Hi-ROX with SYBR Green dye (Enzo Life Sciences, Farmingdale, NY, USA) and 400 nM forward and reverse primers (Bionics, Korea) in a qRT-PCR thermal cycler. The expression level of each gene was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the relative expression was calculated against the contrasting control group. Primer sequences used in this study are listed in Table 1.
Protein extraction and western blotting
Protein samples were obtained from preconditioned cAT-MSC-derived EVs, mouse splenocytes, and mouse brains using a Pro-Prep protein extraction solution (Intron Biotechnology, Korea). The concentration of protein samples was analyzed using a DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Nuclear proteins were isolated using the Cell Fractionation Kit Standard (Abcam, Cambridge, MA, USA). For western blot assays, 25 μg of protein was loaded and separated using SDS-PAGE. SDS-PAGE bands were transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA), which were then blocked with 5% non-fat dry milk and Tris-buffered saline. Membranes were incubated with primary antibodies against COX-2 (1:1000; Santa Cruz Biotechnology, TX, USA), HIF-1α (1:500; LifeSpan BioSciences, Seattle, WA, USA), STAT3 (1:500; LifeSpan BioSciences), pSTAT3 (Tyr705) (1:500; LifeSpan BioSciences), lamin A (1:500; Santa Cruz Biotechnology), and β-actin (1:1000; Santa Cruz Biotechnology) at 4 °C overnight. The membranes were subsequently incubated with the appropriate secondary antibody for 1 h. Using a chemiluminescence detection kit (Advansta, Menlo Park, CA, USA), immunoreactive bands were detected and normalized to the housekeeping protein β-actin. To ensure quantitative accuracy, we applied multiple antibodies to a single blot. During this process, the blot membrane was edited before antibody hybridization. As a result, full-length membranes are not provided for some samples.
Isolated splenocytes and activation
EAE mice were euthanized 25 days after induction. Splenocytes were isolated using a 100 μm cell strainer (SPL Life Science, Pocheon, Korea). Red blood cells (RBCs) were eliminated using RBC lysis buffer, and splenocytes were cultured in Roswell Park Memorial Institute-1640 (RPMI-1640; Welgene) supplemented with 10% FBS (Gibco, USA), 100 units/mL penicillin G (Sigma-Aldrich), and 100 μg/mL streptomycin (Sigma-Aldrich). To evaluate antigen-specific reactions, splenocytes were activated using 10 μg/mL MOG35-55 (Prospecbio) for 48 h.
Cytokine assay
A cell culture medium was used to measure cytokine production from activated splenocytes. The concentrations of IL-1β and IL-6 were measured using commercial ELISA kits for mouse IL-1β (Abbkine, CA, USA) and mouse IL-6 (Invitrogen, MA, USA) according to the manufacturer’s instructions.
Flow cytometry analysis of the Treg cell population
To measure the changes in the Treg cell population after EV treatment, mouse splenocytes were stained with a Treg Detection Kit (CD4/CD25/FoxP3) (Miltenyi Biotech, Germany) according to the manufacturer’s instructions, and the Treg population was analyzed using flow cytometry. Briefly, isolated splenocytes from the mice (1 × 106) were washed with staining buffer and stained with the appropriate concentration of specific monoclonal antibodies—FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 antibodies—incubated with the cells at 4 °C for 30 min, followed by washing steps. Cells were then fixed and permeabilized for intracellular staining of Foxp3 using an APC-conjugated anti-Foxp3 antibody. The size and granularity of the cells were analyzed via flow cytometry using the side and forward scatter signals. First, CD4+ lymphocytes were gated, among which CD25+FoxP3+ cells were selected as Treg cells. The results were analyzed using FlowJo™ 10.8.1 software (Treestar, Ashland, OR).
Obtaining PBMCs and treatment with EVs
PBMCs were obtained from the debris of blood donations, which were collected into sterile CPDA packs and centrifuged to separate the red blood cells and plasma. Separated plasma containing the buffy coat was collected and diluted with an equal volume of PBS. The diluted samples were layered over Ficoll-Paque PLUS (GE Healthcare Life Sciences, Piscataway, NJ, USA) in a conical tube. After centrifugation at 400×g for 30 min, the buffy coat layer was separated by pipetting. The collected buffy coat-layer sample was treated with the RBC lysis buffer and centrifuged again at 400×g for 10 min. PBMCs were seeded at a density of 1 × 106 cells/well in six-well plates (SPL Life Science), resuspended in DMEM containing 10% FBS and 1% penicillin–streptomycin (PS), and stimulated with 5 μg/mL concanavalin A (Con A; Sigma-Aldrich) for 6 h before further experiments. After stimulation, the medium was removed and replaced with a medium containing EVs or EVDFO for 24 h.
Statistical analyses
Data are shown as the means ± standard deviation. Mean values from different groups were compared using the Mann–Whitney t-test, Wilcoxon-Man-Whitney, and one-way analysis of variance with post-hoc Tukey’s test using GraphPad Prism v.7.01 software (GraphPad Software, La Jolla, CA, USA). Statistical significance was set at P < 0.05.
Ethics approval and consent to participate
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University, Korea. The study was confirmed and followed in accordance with ARRIVE guidelines. All authors were personally and actively involved in the substantial work leading to this paper, taking public responsibility for its content.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Raposo, G. & Stahl, P. D. Extracellular vesicles: A new communication paradigm?. Nat. Rev. Mol. Cell Biol. 20(9), 509–510 (2019).
Konoshenko, M. Y. et al. Isolation of extracellular vesicles: General methodologies and latest trends. BioMed Res. Int. 2018, 1–27 (2018).
Robbins, P. D. & Morelli, A. E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14(3), 195–208 (2014).
Li, X. et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine 8, 72–82 (2016).
Liu, R. et al. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Sci. Rep. 5(1), 1–11 (2015).
Lo Sicco, C. et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl. Med. 6(3), 1018–1028 (2017).
Kahmini, F. R. & Shahgaldi, S. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders. Exp. Mol. Pathol. 118, 104566 (2021).
Lu, M. et al. Functionalized extracellular vesicles as advanced therapeutic nanodelivery systems. Eur. J. Pharm. Sci. 121, 34–46 (2018).
Bister, N. et al. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. Journal of extracellular vesicles 10(1), e12002 (2020).
Ejtehadifar, M. et al. The effect of hypoxia on mesenchymal stem cell biology. Advanced pharmaceutical bulletin 5(2), 141 (2015).
Madrigal, M., Rao, K. S. & Riordan, N. H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 12(1), 1–14 (2014).
Zhang, H.-C. et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem cells and development 21(18), 3289–3297 (2012).
Tchanque-Fossuo, C. et al. Deferoxamine: Potential novel topical therapeutic for chronic wounds. Br. J. Dermatol. 176, 1056–1059 (2017).
Park, S.-M. et al. Extracellular vesicles derived from DFO-preconditioned canine AT-MSCs reprogram macrophages into M2 phase. PLoS ONE 16(7), e0254657 (2021).
Dobson, R. & Giovannoni, G. Multiple sclerosis—a review. Eur. J. Neurol. 26(1), 27–40 (2019).
Constantinescu, C. S. et al. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164(4), 1079–1106 (2011).
Hauser, S. L. & Cree, B. A. Treatment of multiple sclerosis: A review. Am. J. Med. 133(12), 1380-1390.e2 (2020).
Kurschus, F. T cell mediated pathogenesis in EAE: Molecular mechanisms. Biomedical J. https://doi.org/10.4103/2319-4170.155590 (2015).
Chu, F. et al. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 318, 1–7 (2018).
Loma, I. & Heyman, R. Multiple sclerosis: Pathogenesis and treatment. Curr. Neuropharmacol. 9(3), 409–416 (2011).
Vogel, D. et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflammation 10(1), 1–12 (2013).
Liu, X. et al. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 180(9), 6070–6076 (2008).
Yu, C.-R. et al. Therapeutic targeting of STAT3 (signal transducers and activators of transcription 3) pathway inhibits experimental autoimmune uveitis. PLoS ONE 7(1), e29742 (2012).
Aqel, S. I. et al. A STAT3 inhibitor ameliorates CNS autoimmunity by restoring Teff: Treg balance. JCI Insight https://doi.org/10.1172/jci.insight.142376 (2021).
Durant, L. et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32(5), 605–615 (2010).
Egwuagu, C. E. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine 47(3), 149–156 (2009).
Park, S.-M. et al. Preconditioning of canine adipose tissue-derived mesenchymal stem cells with deferoxamine potentiates anti-inflammatory effects by directing/reprogramming M2 macrophage polarization. Vet. Immunol. Immunopathol. 219, 109973 (2020).
Gao, F. et al. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 7, e2062 (2016).
Song, W.-J. et al. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res. Ther. 9(1), 91 (2018).
Li, Q. et al. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates severe acute pancreatitis via ER stress downregulation in mice. Stem Cell Res. Ther. 9(1), 1–13 (2018).
de Jong, O. G. et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracellular Vesicles 1(1), 18396 (2012).
Wen, S. W. et al. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics 19(8), 1800180 (2019).
Lai, R. C. et al. Proteolytic potential of the MSC exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics 2012, 1–14 (2012).
Katsuda, T. et al. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 13(10–11), 1637–1653 (2013).
Pulido-Escribano, V. et al. Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J. Stem Cells 14(7), 453 (2022).
Zhu, L.-P. et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 8(22), 6163 (2018).
Sangolkar, S. et al. Particle size determination of nasal drug delivery system: A review. Int. J. Pharm. Sci. Rev. Res. 17, 66–73 (2012).
Bitsch, A. et al. Acute axonal injury in multiple sclerosis: Correlation with demyelination and inflammation. Brain 123(6), 1174–1183 (2000).
Eggenhuizen, P. J., Ng, B. H. & Ooi, J. D. Treg enhancing therapies to treat autoimmune diseases. Int. J. Mol. Sci. 21(19), 7015 (2020).
Smigiel, K. S. et al. Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunol. Rev. 259(1), 40–59 (2014).
Guo, S. et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano 13(9), 10015–10028 (2019).
Long, Q. et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. 114(17), E3536–E3545 (2017).
Lu, H. C. et al. STAT3 signaling in myeloid cells promotes pathogenic myelin-specific T cell differentiation and autoimmune demyelination. Proc. Natl. Acad. Sci. 117(10), 5430–5441 (2020).
Xia, Y. et al. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res. Ther. 11(1), 1–17 (2020).
He, N. et al. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis. 9(10), 1–14 (2018).
Sugimoto, K. Role of STAT3 in inflammatory bowel disease. World J. Gastroenterol. 14(33), 5110 (2008).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in crohn disease and experimental colitis in vivo. Nat. Med. 6(5), 583–588 (2000).
Chen, C.-L., Hsieh, F.-C. & Lin, J. Systemic evaluation of total Stat3 and Stat3 tyrosine phosphorylation in normal human tissues. Exp. Mol. Pathol. 80(3), 295–305 (2006).
Hosono, M. et al. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis 168(1), 73–80 (2003).
Campbell, D. J. & Koch, M. A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11(2), 119–130 (2011).
Yuan, X.-L. et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin. Immunol. 134(3), 277–288 (2010).
Trinath, J. et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2–dependent prostaglandin E2 in human dendritic cells. Blood J. Am. Soc. Hematol. 122(8), 1419–1427 (2013).
Sharma, S. et al. Tumor cyclooxygenase-2/prostaglandin E2–dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 65(12), 5211–5220 (2005).
Han, C. et al. Modulation of Stat3 activation by the cytosolic phospholipase A2α and cyclooxygenase-2-controlled prostaglandin E2 signaling pathway. J. Biol. Chem. 281(34), 24831–24846 (2006).
Lotvall, J. & Valadi, H. Cell to cell signalling via exosomes through esRNA. Cell Adhesion Migr. 1(3), 156–158 (2007).
Gao, W. et al. Hypoxia and STAT3 signalling interactions regulate pro-inflammatory pathways in rheumatoid arthritis. Ann. Rheum. Diseases 74(6), 1275–1283 (2015).
Hanson, L. R. et al. Intranasal administration of CNS therapeutics to awake mice. JoVE (J. Vis. Exp.) 74, e4440 (2013).
Okuda, Y., Okuda, M. & Bernard, C. C. The suppression of T cell apoptosis influences the severity of disease during the chronic phase but not the recovery from the acute phase of experimental autoimmune encephalomyelitis in mice. J. Neuroimmunol. 131(1–2), 115–125 (2002).
Acknowledgements
We are grateful to the Research Institute for Veterinary Science at Seoul National University. We also wish to thank the staff and crew of the Seoul National University FACS Facility for their tireless efforts and perseverance.
Funding
This study was partially supported by the Research Institute for Veterinary Science, Seoul National University.
Author information
Authors and Affiliations
Contributions
Su-Min Park: conception and design, provision of study material, data analysis and interpretation, manuscript writing. Yong-Hun Oh, Ga-Hyun Lim, Ga-Hee Yun: collection and/or assembly of data. Kyung-Bo Kim: data analysis and interpretation. Ju-Hyun An: conception and design. Kyung-Won Seo: administrative support. Hwa-Young Youn: administrative support, final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, SM., Oh, YH., Lim, GH. et al. Deferoxamine preconditioning of canine stem cell derived extracellular vesicles alleviates inflammation in an EAE mouse model through STAT3 regulation. Sci Rep 14, 19273 (2024). https://doi.org/10.1038/s41598-024-68853-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68853-2