Abstract
Spontaneous intracerebral hemorrhage (ICH) is a very serious kind of stroke. If the outcome of patients can be accurately assessed at the early stage of disease occurrence, it will be of great significance to the patients and clinical treatment. The present study was conducted to investigate whether non-contrast computer tomography (NCCT) models of hematoma and perihematomal tissues could improve the accuracy of short-term prognosis prediction in ICH patients with conservative treatment. In this retrospective analysis, a total of 166 ICH patients with conservative treatment during hospitalization were included. Patients were randomized into a training group (N = 132) and a validation group (N = 34) in a ratio of 8:2, and the functional outcome at 90 days after clinical treatment was assessed by the modified Rankin Scale (mRS). Radiomic features of hematoma and perihematomal tissues of 5 mm, 10 mm, 15 mm were extracted from NCCT images. Clinical factors were analyzed by univariate and multivariate logistic regression to identify independent predictive factors. In the validation group, the mean area under the ROC curve (AUC) of the hematoma was 0.830, the AUC of the perihematomal tissue within 5 mm, 10 mm, 15 mm was 0.792, 0.826, 0.774, respectively, and the AUC of the combined model of hematoma and perihematomal tissue within 10 mm was 0.795. The clinical-radiomics nomogram consisting of five independent predictors and radiomics score (Rad-score) of the hematoma model were used to assess 90-day functional outcome in ICH patients with conservative treatment. Our findings found that the hematoma model had better discriminative efficacy in evaluating the early prognosis of conservatively managed ICH patients. The visual clinical-radiomics nomogram provided a more intuitive individualized risk assessment for 90-day functional outcome in ICH patients with conservative treatment. The hematoma could remain the primary therapeutic target for conservatively managed ICH patients, emphasizing the need for future clinical focus on the biological significance of the hematoma itself.
Similar content being viewed by others
Introduction
Cerebral hemorrhage is a highly morbid and fatal disease, accounting for 15–30% of stroke cases worldwide1. Early surgery for supratentorial cerebral hemorrhage (STICH and STICH II)2,3, as demonstrated in two large clinical randomized controlled trials, did not yield significant benefit compared to conservative treatment. There has been significant improvement in the clinical management of acute cerebral hemorrhage, but currently, there is no research that conclusively demonstrates that surgery can improve the prognosis of ICH patients4. This study focused on ICH patients who were treated conservatively throughout their hospitalization. The mRS5 was used to categorize the functional outcome of patients at 90 days post-discharge into good outcome (mRS ≤ 2) and poor outcome (mRS ≥ 3). NCCT of the head is the most important and widely used diagnostic examination for ICH.
Survival and functional recovery in ICH patients are related to the direct injury produced by the hematoma and are also inextricably linked to the development of secondary perihematomal edema (PHE)6 which can further lead to elevated intracranial pressure and neurological deterioration7. Within the first few hours after ICH onset, PHE begins to develop, and it rapidly increases within the first 48 h after ICH onset8. The development of PHE is commonly attributed to several factors, including direct compression of surrounding tissues by the hematoma, disruption of the blood–brain barrier, erythrocyte lysis, activation of the coagulation cascade, and inflammation9. In recent years, there has been growing recognition of PHE-induced secondary brain injury as a promising target for therapeutic interventions.
Radiomics is a new field that leverages high throughput computing to extract many features from conventional medical images, providing non-invasive disease information for quantitative analysis10,11. Although radiomics is widely used in tumor diseases, it is also increasingly being applied in the research of cerebral hemorrhage hematomas and prognosis.
According to previous studies12, we hypothesize that the combined hematoma and perihematomal tissue model has the best predictive efficacy, and establish several radiomics models based on the NCCT hematoma and perihematomal tissue13, and construct a clinical-radiomics nomogram14 to assess the 90-day functional outcome in ICH patients with conservative treatment by incorporating radiographic features and clinical characteristics.
Materials and methods
Patients
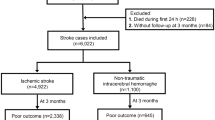
We retrospectively analyzed 166 ICH patients with conservative treatment from January 2019 to June 2022 in the Second Affiliated Hospital of Dalian Medical University. The Ethics Committee of the Second Affiliated Hospital of Dalian Medical University approved this study and waived the requirement for informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
All patients with ICH over 18 years old who within 24 h performed baseline NCCT examination were eligible for the study. The ___location of the ICH was supratentorial and patients received conservative treatment throughout their hospitalization. All included patients had a pre-morbid mRS score of 0. And the exclusion criteria for this study were as follows: those who refused surgery; those with intracranial hemorrhage due to rupture of tumors, arteriovenous malformations, cerebral aneurysms, or trauma; those with primary intraventricular hemorrhage; those with subarachnoid hemorrhage; those who had incomplete clinical and radiographic data; and those who had been absent from the clinic for 90 days after ICH onset. The flowchart of study enrollment as shown in Fig. 1. The mRS was utilized to assess the clinical functional prognosis of ICH patients by reviewing follow-up medical records at 90 days after onset. Consistent with previous studies5, a mRS score of 0–2 was considered good outcome, while a mRS score of 3–6 was considered poor outcome. The baseline NCCT images and clinical data of the patients at admission were reviewed from the electronic medical record system of the hospital.
Image acquisition
All NCCT images were scanned according to a standardized protocol. Further details can be found in the Supplementary Material 1.
Image analysis
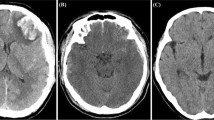
Two radiologists with 5 and 8 years of clinical experience in diagnostic central nervous system (CNS) imaging were unaware of the patients' clinical outcome and performed semi-automatic segmentation of the NCCT images and labeled the ROI using 3D Slicer software15 (version 4.11.0, https://www.slicer.org), which is an open source software. We calculated the baseline hematoma volume using 3D Slicer software, and it did not include the volume of intraventricular hemorrhage. Morotti13 investigated the relationship between perfusion of perihematomal tissues of 10 mm and the prognosis of ICH by CT perfusion. They suggested that reduced cerebral blood flow around the hematoma was associated with poor outcome, further highlighting the close correlation between perihematomal tissue and the outcome of ICH.
Therefore, we used the margin module to select the tissues within 5 mm, 10 mm, and 15 mm around the hematoma for analysis, as shown in Fig. 2. If the expanded ROI involved the ventricle or skull, these areas were manually removed. All segmentations were reviewed by another radiologist with 12 years of diagnostic experience, and the final ROI was determined through overlapping segmentation by the two radiologists, both of whom were blinded to any clinical information.
There were 944 radiomics features extracted from hematoma and perihematomal tissue images by 3D Slicer radiomics. Respectively, including (1) 14 shape Features (Least Axis Length, Surface Area, etc.); (2) 18 First Order Features (first-order. mean, Entropy, skewness, kurtosis, etc.); (3) 75 texture features to quantify internal lesion heterogeneity: these include 24 Gray Level Co-occurrence Matrix (GLCM. contrast, correlation, Cluster Prominence, etc.), 14 Gray Level Dependence Matrix features (GLDM. Dependence Entropy, etc.), 16 Gray Level Run Length Matrix features (GLRLM. High Gray Level Run Emphasis, Short Run Emphasis, etc.), 16 Gray Level Size Zone Matrix features (GLSZM. Small Area Emphasis, Gray Level Variance); 5 Neighboring Gray Tone Difference Matrix (NGTDM); (4) 837 Filter characteristics (including wavelet filter, Laplacian of Gaussian filter).
Intra-observer agreement
Two radiologists, with 5 and 8 years of clinical experience in diagnostic imaging of the CNS, respectively, and who were unaware of patient information, randomly selected 20 samples from the collected patient images for re-delineation. The inter-observer consistency of radiomic features was evaluated by the intra-class correlation coefficient (ICC). An ICC score greater than 0.75 was considered indicative of satisfactory agreement.
Dimensionality reduction and radiomics feature selection
To avoid the curse of dimensionality during modeling and reduce bias in radiomic features, redundant features were removed through correlation testing. Mann–Whitney U test was performed on the features extracted from the training set, and important features were selected from the primary cohort. Then, the least absolute shrinkage and selection operator (LASSO) was employed to select features to improve the prediction accuracy and interpretability of the model16. Further details can be found in the Supplementary Material 2.
Model construction and validation
The radiomics score (Rad-score) was calculated for each patient by combining selected radiomics features with their respective coefficients. The calculation followed the formula: Rad score = \({\Sigma }_{1}^{i}{\beta }_{i}{x}_{i}+{\beta }_{0}\), where β0 represented the constant term, xi denoted the selected radiomics feature, and βi represented the coefficient determined by LASSO regression.
Random forest algorithm performed well in prognostic evaluation. The stability of the model was validated through ten-fold cross-validation. The Receiver Operating Characteristic (ROC) curve was used to evaluate the performance of the model. The calibration curve was used to evaluate or interpret the nomogram model.
Statistical analysis
SPSS software (version 25.0) was used to analyze the clinical characteristics. The data were presented as mean ± standard deviation, median and interquartile range (IQR), or percentages, depending on whether the data were normally distributed or categorical variables. Independent t-test or Mann–Whitney U test was applied for continuous variables between two groups, and chi-square test was used for categorical variables between the two cohorts. A two-sided p < 0.05 was considered statistically significant.
Results
This study included 166 ICH patients with conservative treatment. There were 78 patients with good outcome (mRS ≤ 2) and 88 patients with poor outcome (mRS ≥ 3). All demographic information and clinical characteristics of the patients were shown in Table 1. There were 118 male patients and 48 female patients. The average age at onset was 59.94 years for males and 63.60 years for females, with females showing a higher average onset age than males. After multivariate analysis (Table 2), combined ventricular hemorrhage (OR, 4.452; 95% CI 1.404–14.122; p = 0.011), gender (OR, 0.371; 95% CI 0.172–0.803; p = 0.012), baseline ICH volume (OR, 1.048; 95% CI 1.009–1.087; p = 0.014), CT time from ICH onset (OR, 0.920; 95% CI 0.867–0.976; p = 0.005), and AST/ALT (OR, 2.618; 95% CI 1.146–5.981; p = 0.022) were statistically significant as independent predictors for poor 90-day prognosis in conservatively treated patients with ICH.
Based on these clinical predictive factors, we established a clinical factor model to evaluate the 90-day functional outcome of ICH patients with conservative treatment. However, the discriminative performance of this clinical model did not reach optimal level, with an AUC of 0.760 in the validation cohort (Fig. 3). This result indicated that while the clinical model can identify conservatively treated ICH patients with poor prognosis, evaluating patients’ prognosis based solely on these clinical predictive factors was insufficient.
After eliminating redundant and irrelevant features, all features showed good consistency in hematoma, perihematoma-5 mm, perihematoma-10 mm, and perihematoma-15 mm segmentation. Eventually, the LASSO algorithm selected 19, 19, 16, and 12 most valuable features for these respective segmentations.
It provided the optimal radiomic features by LASSO, and four radiomic models were established for hematoma, perihematomal region within 5 mm, 10 mm, and 15 mm. The predictive accuracy of the four sets of radiomic models was compared by ROC curves.
The AUC values obtained in the validation cohort for the four models were 0.830 (95% CI 0.809–0.854) for the hematoma model, 0.792 (95% CI 0.766–0.815) for the perihematomal region within 5 mm model, 0.826 (95% CI 0.795–0.854) for the perihematomal region within 10 mm model, and 0.774 (95% CI 0.742–0.797) for the perihematomal region within 15 mm model (Fig. 4). The AUC of the hematoma model was superior to that of the other three models.
When the hematoma model was combined with the best-performing perihematomal region model, a combined hematoma and perihematomal region within 10 mm model was constructed. However, this combined model (Fig. 5) still exhibited a lower AUC of 0.795 (95% CI 0.761–0.818) in the validation set compared to the hematoma model alone.
The clinical-radiomic nomogram shown in Fig. 6a was used to assess the 90-day prognosis of ICH patients with conservative treatment. The AUC of this clinical-radiomic nomogram (Fig. 6c) was 0.910 in the training set and 0.840 in the validation set. The high-risk probability calibration curve (Fig. 6b) of this nomogram showed good consistency between the predicted and actual probabilities of prognosis at 90 days.
(a) The clinical-radiomic nomogram for assessing 90-day clinical functional outcome in ICH patients with conservative treatment. The clinical-radiomic nomogram, combining baseline ICH volume, CT time from ICH onset, combined ventricular hemorrhage, gender, AST/ALT and Rad-score developed for the validation cohort. (b) The calibration curve for the clinical-radiomic nomogram in the validation cohort. (c) The clinical-radiomic nomogram ROC curve for assessing 90-day clinical functional outcome in ICH patients with conservative treatment.
Discussion
In this study, we established five models including the traditional hematoma model, the 5 mm perihematomal tissue model, the 10 mm perihematomal tissue model, the 15 mm perihematomal tissue model, and the combined model of hematoma and 10 mm perihematomal tissue based on the NCCT radiomics features of hematoma and surrounding tissues to evaluate the 90-day functional prognosis of ICH patients with conservative treatment. Contrary to the initial hypothesis, the traditional hematoma model demonstrated better prognostic discriminatory ability in this study.
The main objective of this study was to investigate the impact of hematoma combined with perihematomal tissue on the prognosis of ICH patients with conservative treatment. The study found that the combined model of hematoma and perihematomal tissue had lower efficacy compared to the individual hematoma model. This could be attributed to the differences in inclusion criteria in our study compared to previous studies. Specifically, this study included patients with ICH who received conservative treatment throughout their hospitalization and excluded patients who met surgical criteria but opted not to undergo surgery. In clinical practice, patients who selected for conservative treatment generally had milder conditions compared to those who underwent surgery. Therefore, the inclusion criteria of this study helped to some extent mitigate the influence of large hematoma volumes on patient outcome. This also reduced the compression of surrounding tissues by the hematoma and minimized the formation of perihematomal edema, since the occurrence of PHE was highly dependent on the initial hematoma volume17. As a result, the combined model incorporating perihematomal edema did not provide better predictive performance for conservatively treated ICH patients.
This study found that hematoma could provide more information about patient prognosis than hemorrhage combined with the surrounding tissue in ICH patients with conservative treatment. When evaluating hemorrhage, more objective and accurate radiological features were used. 944 features were extracted from the hemorrhage and LSSSO dimensionality reduction was used to ultimately determine 19 features, including 1 shape feature, 2 primary features, and 16 texture features, which described the heterogeneity, shape, and density of the hemorrhage. Previous studies have shown that irregular hemorrhage shapes and uneven density were closely related to poor prognosis in ICH patients18,19.
By performing univariate and multivariate logistic regression analysis on admission clinical factors of patients, baseline ICH volume, CT time from ICH onset, combined ventricular hemorrhage, gender, and AST/ALT were identified as predictors of poor 90-day outcome for patients. Previous studies have shown that larger hematoma volume and shorter CT time from ICH onset were associated with poor 90-day outcome in patients with ICH12,20, which is consistent with our findings that larger hematoma volume can cause severe direct compressive damage to the brain parenchyma. A shorter time from ICH onset to first CT may be related to the severity of neurological dysfunction due to early hematoma expansion or edema formation after symptom onset. Additionally, the incidence of hematoma expansion decreases as the time interval from onset to baseline CT increases21. Intraventricular haemorrhage (IVH) complicating ICH was an independent predictor of poor outcome22, with the degree of IVH closely associated with mortality rates, and the secondary IVH being closely related to altered consciousness at presentation23. According to reports, the incidence and prevalence of ICH were higher in males compared to females24, which may be related to higher rates of smoking and alcohol consumption among males25. In this study, the prevalence of ICH was significantly higher in males than females, but males had slightly lower risk of poor prognosis compared to females, which may be associated with the older age of onset in females. A higher AST/ALT ratio in patients upon admission is associated with poor outcome in ICH patients, as the liver is responsible for synthesizing and metabolizing various coagulation factors, including fibrinolytic factors and fibrinogen26,27. However, in this study, neither has shown correlation with prognosis, which contradicts previous research. The most likely reason could be that the patients in our study only had mild liver function changes, in which case there was no substantial alteration in the synthesis of coagulation factors and fibrinogen. Some research28,29 indicated that after ICH, glutamate accumulated, leading to neuronal excitotoxicity, while aminotransferases protected brain tissue by reducing glutamate levels. Kim30 found a negative correlation between AST and functional recovery in 90 days of ICH. Currently, there is limited research on the association between aminotransferases and ICH prognosis, necessitating further investigation and discussion.
A visual clinical-radiomics nomogram was constructed by combining the rad-score based on hemorrhage and independent clinical predictors to evaluate the 90-day functional prognosis of ICH patients with conservative treatment. The nomogram can intuitively estimate the 90-day functional prognosis of ICH patients with conservative treatment and can further provide assistance in precision medicine and individualized risk assessment.
This study has a few limitations. Firstly, it is a single-center retrospective study with a relatively small sample. Further validation of the radiomics model would require a larger sample size and data from multiple centers. Additionally, our study only focused on the hemorrhage and surrounding tissue in ICH patients with conservative treatment, without including patients who underwent surgery. Furthermore, we only examined short-term functional outcome of the patients and did not conduct long-term follow-up, which requires further investigation and research.
Conclusion
The radiomics analysis of hematoma and perihematomal tissue based on non-contrast computed tomography (NCCT) scans in ICH patients with conservative treatment showed that the hematoma model had better discriminative efficacy in evaluating the early prognosis of conservatively managed ICH patients. The visual clinical-radiomics nomogram provided a more intuitive individualized risk assessment for 90-day functional outcome in ICH patients with conservative treatment. The hematoma could remain the primary therapeutic target for conservatively managed ICH patients, emphasizing the need for future clinical focus on the biological significance of the hematoma itself.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wan, Y. et al. ADVISING score: A reliable grading scale based on injury and response for intracerebral haemorrhage. Stroke Vasc. Neurol. 8, 111–118 (2023).
Mendelow, A. D. et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet 382, 397–408 (2013).
Mendelow, A. D. et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet 365, 387–397 (2005).
Moullaali, T. J. et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: A preplanned pooled analysis of individual participant data. Lancet Neurol. 18, 857–864 (2019).
Haggag, H. & Hodgson, C. Clinimetrics: Modified Rankin Scale (mRS). J. Physiother. 68, 281 (2022).
Cordonnier, C., Demchuk, A., Ziai, W. & Anderson, C. S. Intracerebral haemorrhage: Current approaches to acute management. Lancet 392, 1257–1268 (2018).
Zheng, H., Chen, C., Zhang, J. & Hu, Z. Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovasc. Dis. 42, 155–169 (2016).
Venkatasubramanian, C. et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke 42, 73–80 (2011).
Wan, Y., Holste, K. G., Hua, Y., Keep, R. F. & Xi, G. Brain edema formation and therapy after intracerebral hemorrhage. Neurobiol. Dis. 176, 105948 (2023).
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 278, 563–577 (2016).
Lambin, P. et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 48, 441–446 (2012).
Qi, X., Hu, G., Sun, H., Chen, Z. & Yang, C. Machine learning-based perihematomal tissue features to predict clinical outcome after spontaneous intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 31, 106475 (2022).
Morotti, A. et al. Association between perihematomal perfusion and intracerebral hemorrhage outcome. Neurocrit. Care 33, 525–532 (2020).
Song, Z. et al. A clinical-radiomics nomogram may provide a personalized 90-day functional outcome assessment for spontaneous intracerebral hemorrhage. Eur. Radiol. 31, 4949–4959 (2021).
Fedorov, A. et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging 30, 1323–1341 (2012).
Huang, Y.-Q. et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J. Clin. Oncol. 34, 2157–2164 (2016).
Chen, Y. et al. Perihematomal edema after intracerebral hemorrhage: An update on pathogenesis, risk factors, and therapeutic advances. Front. Immunol. 12, 740632 (2021).
Delcourt, C. et al. Significance of hematoma shape and density in intracerebral hemorrhage: The intensive blood pressure reduction in acute intracerebral hemorrhage trial study. Stroke 47, 1227–1232 (2016).
Barras, C. D. et al. Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke 40, 1325–1331 (2009).
Flemming, K. D., Wijdicks, E. F. & Li, H. Can we predict poor outcome at presentation in patients with lobar hemorrhage?. Cerebrovasc. Dis. 11, 183–189 (2001).
Liu, J. et al. Prediction of hematoma expansion in spontaneous intracerebral hemorrhage using support vector machine. EBioMedicine 43, 454–459 (2019).
Mustanoja, S. et al. Extent of secondary intraventricular hemorrhage is an independent predictor of outcomes in intracerebral hemorrhage: Data from the Helsinki ICH Study. Int. J. Stroke 10, 576–581 (2015).
Hanley, D. F. Intraventricular hemorrhage: Severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 40, 1533–1538 (2009).
Wang, S.S.-Y. et al. Sex-related differences in patients’ characteristics, provided care, and outcomes following spontaneous intracerebral hemorrhage. Neurocrit. Care 37, 111–120 (2022).
Roquer, J. et al. Sex-related differences in primary intracerebral hemorrhage. Neurology 87, 257–262 (2016).
Niizuma, H., Suzuki, J., Yonemitsu, T. & Otsuki, T. Spontaneous intracerebral hemorrhage and liver dysfunction. Stroke 19, 852–856 (1988).
Parikh, N. S. et al. Liver fibrosis indices and outcomes after primary intracerebral hemorrhage. Stroke 51, 830–837 (2020).
Tan, G. et al. Subclinical change of liver function could also provide a clue on prognosis for patients with spontaneous intracerebral hemorrhage. Neurol. Sci. 37, 1693–1700 (2016).
Campos, F. et al. High blood glutamate oxaloacetate transaminase levels are associated with good functional outcome in acute ischemic stroke. J. Cereb. Blood Flow Metab. 31, 1387–1393 (2011).
Kim, K.-H. Predictors of 30-day mortality and 90-day functional recovery after primary intracerebral hemorrhage: Hospital based multivariate analysis in 585 patients. J. Korean Neurosurg. Soc. 45, 341–349 (2009).
Author information
Authors and Affiliations
Contributions
Conception and design: C.Y., X.S. Acquisition, analysis, or interpretation of data: H.Z., Y.H. Drafting of the manuscript: C.Y., X.S. Critical revision of the manuscript for important intellectual content: S.L., E.Z., Y.D. Statistical analysis: X. S., H.Z. All authors reviewed the manuscript. Co-first authors: X.S., H.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, X., Zhang, H., Han, Y. et al. Based on hematoma and perihematomal tissue NCCT imaging radiomics predicts early clinical outcome of conservatively treated spontaneous cerebral hemorrhage. Sci Rep 14, 18546 (2024). https://doi.org/10.1038/s41598-024-69249-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69249-y