Abstract
MRI-guided targeted biopsy (MRGB) was recommended as part of biopsy paradigm of prostate cancers by current guidelines. This study aimed to analyze the diagnostic efficacy of MRGB and systemic biopsy (SB), and to compare diagnostic capabilities within subgroups of MRGB: MRI-cognitive biopsy (MRCB) and MRI-fusion biopsy (MRFB). We retrospectively enrolled patients who underwent MRGB for suspicious malignant lesion(s) identified on MRI in a single tertiary center, sample size was 74 patients. An mpMRI was performed prior to biopsy and reviewed by an experienced radiologist specialized in prostate cancer. Per-person results of MRGB and each concomitant SB were analyzed as independent biopsies for its positive biopsy rate and positive core percentage. Per-lesion results of MRFB and MRCB were compared for the detection rate. Variables of interest were analyzed with t-test, chi-squared test, and logistic regression analysis. Statistical analyses were performed with IBM Statistical Product and Service Solutions (SPSS), Version 23 (IBM, Armonk, New York). Total of 74 patients fulfilled the inclusion criteria and were enrolled. MRFB had higher PCa detection rate comparing to both MRCB and SB (56.1%, 30.3%, and 33.9% respectively, p value = 0.036); clinically significant prostate cancer (csPCa) detection rate was also significantly higher in MRFB group (43.9%, 24.2%, and 16.9% in each group respectively, p value = 0.011). In per-lesion analysis, MRCB and MRFB had no significant difference in PCa and csPCa detection rate (41.0% vs. 26.2% and 29.5% vs. 16.7% respectively, p value = 0.090 and 0.103). In the lesion ≦ 1.3 cm group, MRFB could achieve higher PCa detection rate, comparing to MRCB (36.4% vs. 14.3%, p value = 0.047); there were also higher positive rates for PCa and csPCa per biopsied cores (22.1% vs. 6.8% and 15.6% vs. 2.7%, p value = 0.029 and 0.028, respectively). Further logistic regression of multi-variate analysis in subgroup of lesion ≦ 1.3 cm revealed that PIRADS score and biopsy method were significant predictors of positive biopsy result for PCa (p value = 0.045 and 0.026, respectively) and for csPCa (p value = 0.043 and 0.025, respectively). In patients receiving trans-perineal prostate biopsy, MRFB had higher cancer detection rate than MRCB and SB. In per lesion comparison, MRFB and MRCB had similar diagnostic accuracy. However, in lesions with diameter less than 1.3 cm, MRFB can provided better diagnose value for PCa and csPCa than MRCB.
Similar content being viewed by others
Introduction
As the 2nd most prevalent male malignancy, prostate cancer (PCa) caused over 30,000 deaths in the United States in 20221. Moreover, PCa was also estimated to have a 12% increment from 2001 to 20352 and about 15% male would be diagnosed to have PCa throughout their lifetimes3.
Despite the high prevalence, a considerable population of PCa is indolent and rarely symptomatic or with metastatic potentials; therefore, definite therapies are not routinely acquired in these patients2. Whereas certain population may demonstrate highly metastatic potentials and eventually proven fatal if left untreated, being clinically significant PCa. How to differentiate the former from the more life-threatening and malignant PCa, which warranted essential intervention, is an issue of importance in order to avoid overdiagnosis and over-treatment in the clinical insignificant group of PCa. Clinical significance is defined as ≥ grade group 2 according to the International Society of Urological Pathology (ISUP)2.
To date, transrectal ultrasonography-guided systemic biopsy of prostate (TRUS-SB, short as SB) remained as the standard diagnostic tool for patients with suspicion for PCa, either due to elevated prostate-specific antigen (PSA) levels or abnormal findings on digital examination (DRE). The standard procedure had been adopted for past decades, and there had only been minor modifications throughout the years, such as the number of biopsy cores, biopsy preparation, ___location of biopsy, and principle for re-biopsy4.
It was not until recent years with the breakthrough of high tesla (3 T) multiparametric MRI (mpMRI), had there been any major changes to the diagnostic process of prostate cancer. The dynamic contrast-enhancing (DCE) and diffusion weighted imaging (DWI) had further improved the ability to identify prostatic malignancy with the Prostate Imaging-Reporting and Data System (PI-RADS); it was soon incorporated into lesion-oriented TRUS biopsy to increase the diagnostic accuracy of PCa5. Nowadays, MRI-guided targeted biopsy (MRGB) is recommended for patients with previous negative biopsy findings yet still suspicious of PCa, and some may even suggest for prostate biopsy candidates who are biopsy naïve6.
When using MRI image as guidance, the lesion targeting can be performed by in-bore MRI biopsy, MRI-cognitive biopsy (MRCB) and MRI-fusion biopsy (MRFB). Among the techniques for MRGB, the use of in-bore MRI biopsies was limited due to the requirement of MRI expertise on real-time interpretation, and the lengthy occupation of MRI facility and personnel4. Thus, MRCB and MRFB were the 2 most performed modalities of biopsy for MRGB.
In cognitive biopsy (MRCB), the physician conducting prostate biopsy would preview the MRI image first with recognition and localization of suspicious lesion, and subsequently perform precise biopsy on the corresponding ___location on ultrasound image guidance. The accuracy of MRCB varied greatly from inter-physician variability of biopsy experience and the difficulty of correlating MRI prostatic anatomical landmarks on ultrasound4. In MRI and ultrasound fusion biopsy (MRFB), after fusion of MRI with ultrasound with image software, the suspicious lesions were marked on real-time ultrasound image as desired targets for biopsy. The maturation of image fusion technique had prompted the more widespread practice of MRFB instead of MRCB4. However, despite the short learning curve and theoretically more precise lesion localization, the application of MRFB was still unavailable in resource scarce regions due to higher medical cost and requirement of specific device and software.
Whether MRCB and MRFB had comparable diagnostic power under different circumstances remained in question and yet to be validated. In this study, we aimed to compare the prostate cancer detection efficacy of MRGB to conventional systemic biopsy (SB), and the diagnostic capabilities between MRCB and MRFB.
Materials and methods
Patient selection
We retrospectively enrolled patients in a single tertiary center since Jan 2020 to Oct 2022. Patients who underwent MRI-guided targeted prostate biopsy due to suspicious lesion identified on MRI were enrolled. All clinical data and image files were collected by reviewing electrical medical records. The study was approved by the institutional review board of Chang Gung Medical Foundation (IRB number: 202300663B0) and was conducted in accordance with the ethical principles of the Declaration of Helsinki (2013). The requirement for informed consent was waived by the IRB due to the retrospective design of the study.
MRI and fusion technique
All mpMRI examinations were performed on the 3 Tesla (T) MRI scanners with phased array body surface coils. The mpMRI protocols included T2-weighted imaging (T2WI) in sagittal, coronal, and axial planes, axial T1-weighted imaging (T1WI), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) sequence, complied with the Prostate Imaging-Reporting and Data System version 2.1 (PI-RADS v2.1) recommendations. All MRI images were reviewed by a junior genitourinary radiologist with 9-year-experience in mpMRI interpretation. A senior genitourinary radiologist with 30-year-experience supervised the interpretation process and served as a consultant for ambiguous cases. Both radiologists followed the European Society of Urogenital Radiology/European Association of Urology Section of Urological Imaging consensus, with a minimum interpretation of 1000 mpMRI and an annual read of over 200 cases. However, the radiologists were not blinded to clinical data, in line with clinical routine. The PI-RADS v2.1 scoring system was used to score suspicious prostatic lesions, and in the case of multiple lesions on a mpMRI scan, the highest PI-RADS score was used for further analysis. All consecutive positive and negative MRGB cases were retrospectively enrolled, excluding patients who underwent MRGB via the transrectal approach and those with incomplete data during the medical chart review.
Before undergoing MRFB, the radiologists segmented the prostate gland and delineated suspicious lesions using the MrDraw workstation (Koelis, Grenoble, France). The labeled MR images were then exported for use in MRFB. In MRFB, the urologists utilized the Trinity Image-Fusion system (Koelis, Grenoble, France) to integrate the labeled MR and real-time transrectal ultrasound (TRUS) images. After image fusion, the targeted biopsy was performed with the help of a brachytherapy grid.
In MRCB, the urologists recognized suspicious lesions on MR images based on the mpMRI reports before biopsies. These MRI-revealed lesions were then targeted for biopsies under TRUS guidance cognitively. In both MRFB and MRCB, all biopsies were performed via a trans-perineal approach, and the urologists determined whether to perform a simultaneous SB based on their clinical judgment.
Covariates and outcomes
Each MRGB (MRFB and MRCB) with the concomitant SB were compared as independent biopsies for its positive biopsy rate and positive core percentage. For each suspicious lesion identified on MRI, MRFB and MRCB were also compared for the positive detection rate. In addition to positive biopsy rate, we also compared the rate of clinically significant prostate cancer (csPCa). CsPCa was defined as International Society of Urological Pathology (ISUP) 2 group or higher. Logistic regression was then performed to analyze the potential factors influencing the cancer detection rate of PCa and csPCa.
Statistical analysis
Categorical variables were reported as frequencies, while continuous variables were reported as mean value, standard deviation, upper and lower limits. Chi-square test was used to assess differences between categorical variables. Differences between continuous variables were assessed by independent T-test or Mann–Whitney test, as appropriate. Logistic regression was used in multi-variate analysis for factors influencing cancer detection rate. Significance for all tests was set at p value < 0.05. Analyses were performed using Statistical Product and Service Solutions (SPSS), Version 23 (IBM, Armonk, New York).
Ethics approval and consent to participate
This study has received approval from the Chang Gung Medical Foundation Institutional Review Board that also waived the participants' informed consent (IRB no.202300663B0).
Results
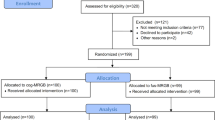
Among 1661 patients who received prostate biopsy with suspicious prostate lesions during Jan 2020 to October 2022, 74 patients fulfilled the inclusion criteria and were enrolled for analysis. The flowchart of patient selection was illustrated in Fig. 1. Forty-one patients with MRFB and 33 patients with MRCB were reviewed respectively, among them 59 patients had concomitant SB and 15 patients only received MRGB target biopsies. Total 103 lesions identified on MRI were included for per-lesion analysis. Total 33 patients (44.6%) had positive biopsy results as prostate adenocarcinoma, while 39 patients (52.7%) had negative biopsy results. CsPCa accounted for 90.5% of all prostate cancer cases. The detailed general data of patients were listed in Table 1. There had been no severe adverse events noted in both groups and summarize of events showed only Clavien-Dindo grade 1 and 2 adverse events with majority being hematuria (42%); and only 3 patients had clinically significant urinary tract infection requiring antibiotics of which 1 had urosepsis. (Fig. 2).
We then compared the different biopsy methods in each patient. All biopsies were divided into three groups: MRFB, MRCB, and paired SB. Patients in MRFB group had significant higher PSA and PSA density before biopsy comparing to patients in MRCB group. Patients in MRFB group also had higher PCa detection rate comparing to those in MRCB and SB group (56.1%, 30.3%, and 33.9% respectively, p value = 0.036). The csPCa detection rate was also significantly higher in MRFB group (43.9%, 24.2%, and 16.9% in each group respectively, p value = 0.011). In addition to significantly more total biopsy cores in SB group, patients in SB group had lowest PCa and csPCa positive biopsy core percentage. Detailed analysis result is listed in Table 2.
Per lesion analysis was further performed between targeted biopsies between MRFB and MRCB groups. In overall per lesion analysis, MRFB group still had significantly higher PSA and PSA density before biopsy. Patients in both groups had similar prostate volume, lesion size, and PIRADS score on MRI. As for PCa and csPCa detection rate, the two methods had no significant difference (41.0% vs. 26.2% and 29.5% vs. 16.7% respectively, p value = 0.090 and 0.103). Similar results were noted in the lesion > 1.3 cm group in the further subgroup analysis. However, in the lesion ≦ 1.3 cm group, MRFB could achieve higher PCa detection rate comparing to MRCB (36.4% vs. 14.3%, p value = 0.047). With MRFB, there were also higher positive rates for PCa and csPCa per biopsied cores (22.1% vs. 6.8% and 15.6% vs. 2.7%, p value = 0.029 and 0.028, respectively). The results were listed in Table 3.
Logistic regression of multi-variate analysis for positive biopsy result revealed that PIRADS score (p = 0.010), PSA (p = 0.022), PSA volume (p = 0.005) and PSA density (p = 0.012) were significant predictors for overall lesions biopsy result. The subgroup multi-variate analysis revealed that in lesions ≦ 1.3 cm, PIRADS and biopsy method were both significant predictors of positive biopsy result for PCa (p value = 0.045 and 0.026, respectively) and for csPCa (p value = 0.043 and 0.025, respectively). Detailed results were listed in Table 4.
Discussion
Conventional random biopsy had been the standard procedure for diagnosis of prostate cancer for decades, and it was not until recent years that MRGB was introduced into the paradigm of prostate biopsy with the advancement of mpMRI and fusion technique. Aside from the in-bore MRI targeted biopsy, which was less performed due to inconvenience, MRCB and MRFB are currently the two most performed methods for MRI-guided biopsies.
Reviewing previous literatures, few studies had performed comparison between the different methods of MRGB. The FUTURE trial had compared the 3 methods by multi-center randomized controlled trial7 and revealed that in-bore MRI biopsy had slightly higher PCa overall detection rate, while MRFB had higher csPCa detection rate. However, all the three biopsy methods showed no significantly difference among both PCa and csPCa detection rate. Another trial, the SmartTarget biopsy trial, which was a blinded, within-person randomized paired trial that compared the biopsy efficacy of MRCB and MRFB. The results from SmartTarget showed no difference between the two biopsy strategies and each of the biopsy method would miss 14% of total detected clinically significant cancers. Combining of the two biopsy methods could provide the highest diagnostic efficacy8. The PICTURE trial also compared the MRGB with transperineal template prostate mapping biopsies. The PICTURE trial concluded that MRGB can detect more csPCa with nearly tenfold less biopsy cores. Simmons et al. also compared the MRCB and MRFB methods and found that although MRFB diagnosed slightly more csPCa than MRCB, there was no statistically difference9. Although similar diagnostic accuracy of MRCB and MRFB were reported in among the three trials, there were absence of any subgroup analysis investigating further possibilities.
In our study, we performed per-lesion analysis of MRCB and MRFB instead of within person comparison due to study design. In resemblance with the previous studies comparing the two methods, we also found no significant difference between MRCB and MRFB in PCa and csPCa diagnostic rate, biopsy Gleason score, and positive cores percentage in overall study population. Furthermore, in the subgroup analysis allocated by lesion size, we discovered that in the subgroup with lesion size less than 1.3 cm, MRFB diagnosed significantly more PCa than MRCB with significant higher positive biopsy rates for PCa and csPCa. The same difference was not observed in subgroup with larger lesion size and in overall population.
After searching through previous publications, we found no study discussing the impact of lesion size on the diagnostic accuracy of MRGB. Barrett et al. had compared the histological outcomes in patients undergoing MRFB and background systemic biopsy10 and found that the incremental benefit of MRFB was limited in patients with larger lesion size (short axis diameter > 1.0 cm). Although this study only examined the diagnostic value of MRGB in contrast with background SB and concluded that in larger sized lesions the efficacy of additional MRGB was limited, and implied that lesion size may also impact the diagnostic accuracy of MRGB, including MRCB and MRFB. The theorem was to some extent endorsed by our study which demonstrated MRCB would diagnose less lesions comparing to MRFB when target lesion size was less than 1.3 cm and had lesser biopsy positive rates. Since MRCB is relied on both the visual cognition and mental correlation of the ___location, it is considered to be more operator dependent and would task individual surgeon’s experience. On the other hand, MRFB is a software-based fusion approach which, according to studies, may improve cancer detection and reduce the learning curve necessary for visual cognition. In sum, the benefit of MRFB is more prominent when targeting small lesions and/or conducting biopsy of a larger prostate, while MRCB can be adopted for those larger lesions as a comparable alternative11.
Although the correlation of lesion size and diagnostic capabilities of MRGB had not been clearly established, the relationship of csPCa and lesion volume has been described. Suspicious PIRADS 3 lesions are subclassified according to volume threshold of 0.5 ml; lesions < 0.5 ml are classified as PIRADS 3a (indolent or low-risk) and those ≥ 0.5 ml as PIRADS 3b (significant or high-risk)12. Rico et al. revealed in a retrospective study encompassing 99 patients that no csPCa cases were observed in the 3a group, while 17.8% of the 3b group presented csPCa13. When incorporating volume size for detecting csPCa, 3b lesions demonstrated sensitivity and specificity of 100% and 48.3%, respectively, with negative predicting value (NPV) of 17.8% andmpositive predicting value (PPV) of 100%13. The effect of volume on diagnosing csPCa aligns with our study, as the effect size of volume would theoretically proportionally magnify the effect of lesion diameter on diagnosing csPCa. Another parameter associating with lesion volume is PSA density (PSAd). Rico et al. also disclosed 62.5% of 3b lesions with PSAd > 0.15 showed csPCa, whereas those with PSAd ≤ 0.15 did not. Integrating PSAd with 3b lesions would increase specificity and NPV to 86.9% and 62.5%, respectively13. However, we did not investigate the effect of PSAd and further investigations may be warranted.
Another issue for prostate biopsy is the route for biopsy, including the trans-rectal approach (TR) and the trans-perineal approach (TP). Generally, TP approach is correlated with less complications, especially infectious events and are recommended by many clinical guidelines over TR approach recently. However, it is still debatable to date whether one approach is superior to the other with various clinical factors including: diagnostic accuracy in certain tumor ___location, deliverability in the outpatient clinic setting, and steepness of learning curve to be taken into consideration6. In a recent multicenter retrospective cohort study, Zattoni et, al suggested that TP approach could improve csPCa detection comparing to TR approach, especially in the transition zone, anterior zone, central zone, and apex6. In our study, we selected only patients with TP approach for analysis, thus we could exclude the potential bias from different biopsy routes.
Retrospective study design is the main limitation of this study, and this caused the selection bias of patients who received MRCB and MRFB with heterogenous baseline characteristics. Per lesion analysis for overall lesions disclosed significant difference of PSA prior to biopsy and subsequently significant difference in PSA density between Fusion biopsy (MRFB) and cognitive biopsy (MRCB) groups. Potential selection bias may be accounted for this phenomenon as MRFB may be offered specifically for patients who had persistent abnormal high PSA despite previously negative systemic biopsy or even multiple negative biopsies in real world setting. This group may encompass prostate cancer patients with failed systemic biopsies who would benefit from utilization of MRFB or other MRGB. In real world setting, economical barrier of MRFB may also contribute to potential selection bias which was not analyzed in our study. The small sample size is also an important limitation due to relative late introduction of MRI Fusion biopsy into our institution and preliminary results would serve as steppingstone and guidance for designing future studies to validate and investigate the effect of lesion size on MRI-pathway diagnosis of clinically significant prostate cancer. Furthermore, another limitation was from the paired study method, by which a portion of the patients would receive both MRGB and SB at the same time. Paired biopsies would be prone to bias as the surgeon had already identified the ___location of suspicion on MRI image and the selection of SB ___location may no longer be systemically randomized.
To date, current guideline from European Association of Urology (EAU) stated no obvious superiority of one MRI-pathway biopsy from another, including MRFB, MRCB and direct in-bore guidance (Strength of Recommendation: Weak), from current literature review of systemic reviews and meta-analysis14. However, recent guideline statement (July 2023) from American Urology Association (AUA) suggested otherwise that clinicians may use software registration of MRI and ultrasound images during fusion biopsy, when available (Expert Opinion), especially for small MRI lesions15. Results from our study would agree with the latter, and with further recommendation of lesion size at cut-off of 1.3 cm with utilization of MRFB for smaller lesions. However, further investigations are warranted for confirmation of our study findings of superiority of MRFB over MRCB in lesions with diameter less than 1.3 cm. Multiple factors may contribute to variability and difficult reproductivity of our study; including higher requirement of clinical experience for optimal MRCB performance, cost barrier of MRFB and patient’s preference. In our daily practices, only systemic biopsy is reimbursed by national health insurance; however, patients would likely choose MRFB over MRCB when personal insurances provide coverage. The size effect of economical barrier may vary across different healthcare systems. We aim to verify and also investigate how lesion size affects the diagnostic capabilities of MRI-pathway prostate biopsy.
Conclusion
MRGB with TP approach is becoming the standard diagnostic procedure for prostate cancer. Comparing to MRFB, MRCB can be performed without specific software and device but may require longer learning curve and experienced hands. MRCB and MRFB had similar diagnostic accuracy in overall patients and were superior to SB only. However, in patients with lesion diameter less than 1.3 cm, MRFB can provided better cancer detection rate for PCa and superior positive biopsy rates for PCa and csPCa than MRCB. MRCB could be an alternative in those with larger lesions (greater than 1.3 cm) and when MRFB was not available. Large prospective trial is required to validate this finding.
Data availability
All research data are derived from the Chang Gung Hospital medical record system. Due to the inclusion of personal information, it is not convenient to provide. The data from this study can be requested by contacting the corresponding author, See-Tong Pang. The entire study, including conception, design, and manuscript writing, was conducted by See-Tong Pang.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Ippoliti, S. et al. Optimal biopsy approach for detection of clinically significant prostate cancer. Br. J. Radiol. 95(1131), 20210413 (2022).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Yamada, Y. et al. Moving away from systematic biopsies: image-guided prostate biopsy (in-bore biopsy, cognitive fusion biopsy, MRUS fusion biopsy)-literature review. World J. Urol. 39(3), 677–686 (2021).
Klotz, L. et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: A phase 3 randomized clinical trial. Erratum in: JAMA Oncol. 2021 Apr 1;7(4):639. Erratum in: JAMA Oncol. 2021;7(7):1074. JAMA Oncol. 7(4), 534–542. https://doi.org/10.1001/jamaoncol.2020.7589 (2021).
Zattoni, F. et al. The detection of prostate cancer with magnetic resonance imaging-targeted prostate biopsies is superior with the transperineal vs the transrectal approach. A European Association of Urology-Young Academic Urologists Prostate Cancer Working Group multi-institutional study. J. Urol. 208(4), 830–837 (2022).
Wegelin, O. et al. The FUTURE Trial: A multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur. Urol. 75(4), 582–590 (2019).
Hamid, S. et al. The SmartTarget biopsy trial: A prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for prostate cancer risk stratification. Eur. Urol. 75(5), 733–740 (2019).
Simmons, L. A. M. et al. Accuracy of transperineal targeted prostate biopsies, visual estimation and image fusion in men needing repeat biopsy in the PICTURE trial. J. Urol. 200(6), 1227–1234 (2018).
Barrett, T. et al. Targeted transperineal biopsy of the prostate has limited additional benefit over background cores for larger MRI-identified tumors. World J. Urol. 34(4), 501–508 (2016).
Marra, G. et al. Controversies in MR targeted biopsy: alone or combined, cognitive versus software-based fusion, transrectal versus transperineal approach. World J. Urol. 37(2), 277–287 (2019).
Scialpi, M. et al. Score 3 prostate lesions: A gray zone for PI-RADS v2. Turk. J. Urol. 43(3), 237 (2017).
Rico, L. et al. PI-RADS 3 Lesions: Does the association of the lesion volume with the prostate-specific antigen density matter in the diagnosis of clinically significant prostate cancer. Urol. Oncol. 39(7), 431.e9-431.e13 (2021).
EAU Guidelines. Edn. Presented at the EAU Annual Congress Paris. ISBN 978-94-92671-23-3 (2024).
Wei, J. T. et al. Early detection of prostate cancer: AUA/SUO guideline Part II: Considerations for a prostate biopsy. J. Urol. 210(1), 54–63 (2023).
Acknowledgements
The authors express their gratitude to the entire team at the Cancer Center in Chang Gung Memorial Hospital for their indispensable assistance.
Funding
The present study was supported by grants from The Chang Gung Memorial Hospital Research Foundation (Grant Nos. CORPG3H0051).
Author information
Authors and Affiliations
Contributions
Conception and design: See-Tong Pang, I-Hung Shao Provision of study materials or patients: I-Hung Shao, Fan-Ting Liao; Chun-Bi Chang; Ying-Hsu Chang; Li-Jen Wang; Liang-Kang Huang; Hung-Cheng Kan; Po-Hung Lin; Kai-Jie Yu; Cheng-Keng Chuang; Chun-Te Wu; See-Tong Pang Collection and assembly of data: Fan-Ting Liao, I-Hung Shao Data analysis and interpretation: I-Hung Shao, Chun-Bi Chang, Li-Jen Wang Data Availability: See-Tong Pang Manuscript writing: I-Hung Shao, See-Tong Pang All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shao, IH., Liao, FT., Chang, CB. et al. Lesion size may affect diagnostic capabilities of MRI-guided ultrasound fusion biopsy and cognitive targeted biopsy for clinically significant prostate cancer. Sci Rep 14, 20173 (2024). https://doi.org/10.1038/s41598-024-69661-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69661-4