Abstract
This study aims to assess the global prevalence of kinesiophobia and the potential influencing factors among patients with heart disease. A comprehensive search was conducted in PubMed, Embase, Web of Science, PsycINFO, and Scopus databases to identify studies reporting on the prevalence of kinesiophobia and its influencing factors in heart disease patients up to January 2024. A random-effects model was employed to aggregate prevalence rates. Heterogeneity sources were investigated through subgroup analysis, while differences in the prevalence of kinesiophobia across regions, types of heart disease, and gender were evaluated. Additionally, a qualitative analysis of the factors influencing kinesiophobia was performed. This research incorporated 15 studies from six countries, with 14 providing data on the prevalence of kinesiophobia and nine exploring its potential influencing factors. The findings indicated that the overall prevalence of kinesiophobia among heart disease patients was 61.0% (95% CI 49.4–72.6%). Subgroup analysis revealed that the prevalence in upper-middle-income countries was 71.8% (95% CI 66.2–77.4%), while it stands at 49.9% (95% CI 30.2–69.5%) in high-income countries. The prevalence rates among patients with coronary artery disease, heart failure, and atrial fibrillation were 63.2% (95% CI 45.2–81.3%), 69.2% (95% CI 57.6–80.8%), and 71.6% (95% CI 67.1–76.1%), respectively. Gender-wise, no significant difference was observed in the prevalence of kinesiophobia between men and women (52.2% vs. 51.8%). A total of 24 potential influencing factors of kinesiophobia were identified, with education level, monthly income, anxiety, and exercise self-efficacy being the most recognized. The prevalence of kinesiophobia in patients with heart disease is notably high and is influenced by a multitude of factors. Early implementation of targeted preventive measures is imperative to mitigate the incidence of kinesiophobia in this population.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is a major global health burden. According to the World Heart Federation (WHF), CVD caused 20.5 million deaths globally in 2021, with ischemic heart disease being the leading cause of premature death for both men and women in several countries, contributing to more than half of CVD deaths1. Reports from the American Heart Association (AHA) also indicate that in the United States, coronary artery disease (CAD) and heart failure (HF) accounted for 41% and 10% of CVD deaths, respectively, in 20192. Heart disease not only has a high mortality rate, but its prevalence is also on the rise with the accelerated aging of the global population and the impact of poor lifestyles. According to surveys, about 34.66 million Chinese suffer from heart disease, representing 10% of the CVD population3, and the prevalence of all types of heart disease in the United States is 11.2% of the population4. It is evident that heart disease poses a significant public health challenge that requires prompt action.
The European Association for the Prevention of Heart Disease (EAPC) emphasizes physical activity as a pivotal component in the secondary prevention of CVD, exercise-based cardiac rehabilitation (CR) which has been shown to decrease morbidity and mortality in cardiac patients5. It modulates disease progression by improving cardiovascular symptoms, cardiac function, and psychological well-being6. Studies have shown that exercise-based CR decreases cardiac mortality by 26% and recurrent hospitalization by 18% in patients with acute coronary syndromes (ACS), and is also effective for patients with chronic heart failure (CHF) and after cardiac surgery5,7. Multiple guidelines recommend exercise-based CR for all patients with CHF, irrespective of left ventricular ejection fraction8,9.
However, many patients have low adherence and engagement in CR due to fear of cardiac distress or heart attack induced by exercise10. This phenomenon is termed kinesiophobia, an excessive and irrational fear of physical activity that may cause harm or re-injury to the body11. The term was initially coined and used by Kori11 for chronic pain patients, and later adapted by Bäck12 for cardiac patients in 2012, after refining its meaning. The cognitive-behavioral-avoidance model suggests that kinesiophobia leads to catastrophic thinking and exercise avoidance, which impair the rehabilitation outcomes and quality of life of cardiac patients13,14.
At present, local research on the prevalence of kinesiophobia in patients with heart disease has been conducted, but comprehensive global research is lacking. Moreover, the prevalence of kinesiophobia varies considerably across studies due to different sample sizes, study populations, and study regions, and no qualitative and comprehensive study on its influencing factors exists. Therefore, this study aims to summarize the current evidence and provide a quantitative and qualitative synthesis of the prevalence and influencing factors of kinesiophobia in cardiac patients worldwide, with the aim of helping researchers, clinicians, and policymakers to understand the current situation better, and to provide strong evidence to support early screening and effective prevention of kinesiophobia.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines15. This study is registered in PROSPERO under registration number CRD42024501028.
Search strategies
Two authors (WZ and SS) conducted a computerized search of databases including PubMed, Embase, PsycINFO, Web of Science, and Scopus, using targeted terms related to heart diseases (e.g. "cardiac disease," "cardiovascular disease," "coronary heart disease," "heart failure" etc.) and kinesiophobia (e.g. "exercise phobia," "Fear of movement," "Fear of exercise," "Fear of physical activity" etc.). We conducted the literature search using a combination of subject terms and free words. The search covered the period from the inception of each database until January 2024. The specific search strategy can be found in the supplementary files.
Study selection and data extraction
The inclusion criteria for our systematic review were: (1) Participants were heart disease patients aged 18 years or older. (2) The study designs were observational, encompassing cohort, case–control, or cross-sectional studies. For longitudinal studies reporting varied prevalence rates over time, only baseline prevalence data were considered. (3) The studies needed to report the prevalence of kinesiophobia in heart disease patients or its influencing factors. To minimize confounding effects, only studies conducting multifactorial analyses of influencing factors were included. (4) The assessment tool for kinesiophobia was the Tampa Scale for kinesiophobia Heart (TSK-SV Heart). Adapted by Bäck12 in 2012 from the Tampa Scale for kinesiophobia originally developed for pain patients16, this scale is tailored for individuals with heart disease. It comprises 17 items across four dimensions, with a total scoring range of 17 to 68. A score above 37 is indicative of kinesiophobia, with higher scores reflecting increased levels of fear. This scale has been translated and utilized by scholars in various countries. Our exclusion criteria were: (1) Literature with duplicate publications or identical original data. (2) Non-English articles, conference abstracts, reviews, case reports, and related types of publications. (3) Studies where the full text was unavailable.
The retrieved results were managed using Endnote software. Following the removal of duplicates, two authors (LL and LZ) independently screened the studies according to predefined inclusion and exclusion criteria. This process involved an initial screening by reviewing titles and abstracts to identify potentially relevant studies, followed by a detailed examination of the complete texts to confirm final inclusion and extract pertinent data. The following data were extracted: general information (authors, publication year, country of study), sample size, participant characteristics (age, type of heart disease, TSK-SV heart score), study design, and primary outcomes (prevalence or influencing factors of kinesiophobia). In instances of disagreement, the third author (WZ) was consulted for resolution through discussion. We conducted preliminary pilot testing on PubMed to calibrate our study selection criteria and data extraction protocols. The outcomes of these pilot tests informed refinements in our methodology, ensuring robustness and reliability in our systematic review and meta-analysis.
Quality assessment
The risk of bias in the included studies was independently assessed by two trained researchers (LL and LZ). In divergent opinions, a third author (WZ) was consulted to reach a consensus. Case–control and cohort studies were evaluated using the Newcastle–Ottawa Scale (NOS)17, which comprises eight items across three domains: study population selection (0–4 points), group comparability (0–2 points), and exposure factor measurement (0–3 points), culminating in a maximum score of 9. The cross-sectional studies used the Agency for Healthcare Research and Quality (AHRQ) methodology checklist18. This tool comprises 11 criteria, each scored 1 for a 'yes' response and 0 for a 'no' or 'uncertain' response, resulting in a maximum possible score of 11.
Statistical analysis
Statistical analysis was conducted using Stata 15.0. We calculated the prevalence of kinesiophobia and its 95% confidence interval as the effect size. Heterogeneity among studies was assessed using the Q test, with the I2 test providing quantification (low: 25–50%; medium: 50–75%; high: > 75%), a fixed-effect model was applied for meta-analysis when P > 0.1 or I2 < 50%, indicating acceptable heterogeneity. Conversely, for significant heterogeneity (P ≤ 0.1 or I2 ≥ 50%), a random-effect model was used. Sensitivity analysis was conducted to evaluate the robustness of the results. Additionally, Begg's and Egger's tests were applied to investigate the presence of publication bias in the studies. To elucidate the sources of heterogeneity and to explore the variations in the prevalence of kinesiophobia across different regions and populations, subgroup analyses were conducted based on income levels, types of cardiac diseases, and gender. Income levels were categorized according to the Gross National Income per capita as published by the World Bank19. Due to the diverse influencing factors and statistical method discrepancies among the studies, a qualitative approach was adopted to analyze influencing factors instead of a meta-analysis of OR values.
Results
Study selection
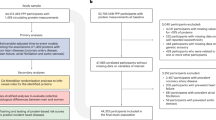
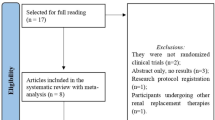
A comprehensive and systematic search yielded 1132 original studies, of which 236 were removed due to duplication. After screening the titles and abstracts, 48 studies were selected for full-text reading, and 15 original studies were finally included in the systematic review (Fig. 1).
Characteristics of the included studies
This meta-analysis synthesized findings from 15 studies14,20,21,22,23,24,25,26,27,28,29,30,31,32,33 across six countries in Europe and Asia. Seven studies14,24,29,30,31,32,33 were conducted in European nations (the Netherlands, Poland, Sweden, Italy), five from China21,22,23,25,26, and three from Turkey20,27,28. The majority were cross-sectional studies (n = 12, 80%)20,21,22,23,25,26,27,28,29,31,32,33, while the remainder were cohort studies (n = 3, 20%)14,24,30, totaling 2973 participants. Of these, eight studies14,20,23,27,28,29,31,33 involved subjects with CAD, three focused on HF patients21,22,26, one study32 included patients with both CAD and HF, one targeted atrial fibrillation (AF) patients25, and two studies24,30 did not differentiate between types of heart disease. Fourteen studies14,20,22,23,24,25,26,27,28,29,30,31,32,33 reported the prevalence of kinesiophobia using the TSK-heart scale, with 1610 cases identified. Of the nine studies14,21,22,23,24,25,26,27,33 that examined the factors influencing kinesiophobia, five used multiple linear regression22,23,25,26,27, two used binary logistic regression24,33, one used mixed linear modeling14, and one used structural equation modeling21 as the statistical methods. Table 1 shows the main characteristics of the included studies.
Quality assessment
Quality assessment scores exhibited variation across the studies, with cross-sectional studies achieving scores ranging from 5 to 9, and cohort studies scoring between 5 and 7. The 15 studies were assessed to be of medium to high quality after a rigorous literature quality assessment, as delineated in Table 1. Furthermore, a detailed checklist of quality assessment and the corresponding tables for each study are provided in the supplementary files.
Prevalence of kinesiophobia among patients with heart disease
Overall pooled prevalence of kinesiophobia
The prevalence of kinesiophobia among patients with heart disease ranged from 20 to 83%, and the overall pooled prevalence of kinesiophobia was 61.0% (95% CI 49.4–72.6%). The results of the heterogeneity test showed significant heterogeneity among the studies (I2 = 97.8%, P < 0.0001), so the random effects model was used for meta-analysis (Fig. 2). The sensitivity analysis indicated that the meta-analysis results were robust, as the pooled effect size remained stable after omitting each study sequentially (Fig. 3). Egger’s test (t = 0.57, P = 0.580) and Begg’s test (z = -0.27, P = 0.784) both indicated that the possibility of potential publication bias was minimal. Regarding national trends, Turkey exhibited the highest kinesiophobia prevalence at 76.7% (95% CI 70.5–82.8%), succeeded by China with 69.4% (95% CI 62.2–76.5%), and Sweden reported the lowest at 21.5% (95% CI 16.5–26.5%). The Italian data was omitted from this comparative analysis due to the single study’s insufficient representativeness, rendering it unsuitable for meta-analysis.
Subgroup analysis
We performed subgroup analyses based on study characteristics to account for the sizeable inter-study heterogeneity (Table 2). In the subgroup analysis by income level, the prevalence of kinesiophobia in upper-middle-income countries (Turkey, China) was 71.8% (95% CI 66.2–77.4%), higher than that in high-income countries (Netherlands, Poland, Sweden, Italy) at 49.9% (95% CI 30.2–69.5%). Subgroups were analyzed based on different types of heart disease, the prevalence of kinesiophobia was lowest in patients with CAD at 63.2% (95% CI 45.2–81.3%), followed by patients with HF at 69.2% (95% CI 57.6–80.8%). Patients with atrial fibrillation (AF) had the highest prevalence of kinesiophobia at 71.6% (95% CI 67.1–76.1%). Gender-wise, there was no significant difference in the prevalence of kinesiophobia between men and women (52.2% versus 51.8%).
Factors influencing kinesiophobia in patients with heart disease
We conducted a qualitative analysis of 9 studies on kinesiophobia influences and identified 24 factors, of which 4 were reported by over two studies and 20 were reported by only one study. Several studies have shown that lower education level25,26, lower monthly income23,25,26, and anxiety22,24,33 are risk factors for kinesiophobia in patients with heart diseases, while exercise self‐efficacy21,25 is a protective factor.
In concrete terms, Qin21 suggested that symptom status of HF and avoidance coping were risk factors for kinesiophobia (β = 0.24, 0.21, p < 0.1), while exercise self‐efficacy and social support were protective factors (β = − 0.32, − 0.35, p < 0.1); Zhang22 indicated that cardiac anxiety, depressive symptoms and employment were risk factors for kinesiophobia (β = 0.25, 0.31, 0.19, p < 0.001), while subjective social status was a protective factor (β = − 0.10, p = 0.038). These four variables explained 30.3% of the variance in the model. Shen23 indicated that higher NYHA classification (β = 1.43, p = 0.027) and pain intensity (β = 0.94, p = 0.037) were associated with more severe kinesiophobia, while higher pain resilience (β = − 3.38, p < 0.001) and monthly income (β = − 1.56, p = 0.003) had the opposite effect. These four variables explained a total of 30.2% of the variance in the model. Ter Hoeve24 showed cardiac anxiety (OR = 1.12, 95% CI = 1.06–1.19) and generic anxiety (OR = 1.26, 95% CI = 1.11–1.42) to be risk factors for kinesiophobia. Ding25 indicated that older age, higher resting heart rate, and higher severity of self-conscious symptoms were risk factors for kinesiophobia in patients with atrial fibrillation(β = 2.54, 3.15, 3.96, p < 0.001). At the same time, higher education, exercise self‐efficacy(β = − 3.24, − 0.13, p < 0.001), higher monthly income(β = − 2.04, p = 0.007), and physical function (β = − 0.074, p = 0.028) were protective factors. The above variables explained 49.4% of the variance in the study. Qin26 suggested that patients with disease course (≥ 5 years) and fatigue had higher scores of kinesiophobia (β = 2.02, 0.23, p < 0.05), in contrast, those with higher education and higher monthly income had lower scores of kinesiophobia (β = − 3.98, − 2.45, p < 0.05). These factors explained 41% of the variance. Çakal27 suggested that angina was a risk factor for kinesiophobia(β = 0.35, p = 0.014) , the variable explained 21% of the variance in the model. Bäck14 reported that women (p = 0.045) were more susceptible to kinesiophobia. In their previous study33, they also found that patients with comorbid HF (OR = 5.19, 95% CI = 1.71–15.74) and anxiety (OR = 1.19, 95% CI = 1.08–1.32) were more likely to have kinesiophobia, while health-related quality of life (HRQoL) (OR = 0.96, 95% CI = 0.94–0.98) and physical activity (OR = 0.19, 95% CI = 0.06–0.63) were protective factors against kinesiophobia.
Discussion
The meta-analysis incorporated 15 studies from six countries, offering insights into the prevalence of kinesiophobia and its associated factors among heart disease patients. The study indicated that the prevalence of kinesiophobia among heart disease patients was 61%, signifying that a majority—more than half—of these individuals harbor a fear of physical activity. This rate is notably higher compared to those with other conditions, such as total knee arthroplasty at 24.4%34 and chronic low back pain at 46.3%35. This variation may be attributed to the different perceived threats among these patient groups. For those with arthroplasty or chronic pain, fear of movement is often linked to catastrophic pain perceptions. In contrast, cardiac patients’ fear is primarily driven by concerns over survival, including the fear of death or experiencing another cardiac event14.
Subgroup analyses indicate a significant variation in kinesiophobia prevalence across diverse countries and regions. Notably, upper-middle-income countries exhibit a higher prevalence compared to high-income countries. This discrepancy may be attributed to economic disparities that influence healthcare quality and the extent of CR adoption and practice. Research demonstrates that approximately 75% of cardiovascular diseases globally manifest in low-middle-income countries, whereas high-income countries are witnessing concentrated advancements in cardiovascular health1. Considering China, the largest developing country in the world, the situation of CR implementation is not optimistic. Survey data indicates that a mere 24% of hospitals offer CR services3, and only a quarter of clinicians are proficient in administering professional CR. Furthermore, a staggering 83% of the general population lacks awareness of CR36. In the management of heart disease, insufficient knowledge among medical staff regarding CR and inadequate patient health education can contribute to or exacerbate patients’ misconceptions about exercise37. It is imperative for hospitals to enhance CR services, deliver evidence-based rehabilitation programs, and prioritize patient education. Such measures are essential to empower patients with a comprehensive understanding of exercise’s benefits, thereby mitigating kinesiophobia.
The study findings indicate that atrial fibrillation patients exhibit the highest kinesiophobia rates among cardiac conditions. This heightened prevalence is likely due to the abrupt onset of atrial fibrillation, which instills a sense of unpredictability and distressing symptoms in patients, fostering a fear of exercise38. Postoperatively, this apprehension persists, with patients often avoiding physical activity to prevent disease recurrence25. In terms of gender, the prevalence of kinesiophobia among male and female heart disease patients does not significantly differ, suggesting that gender may not be a critical determinant in the development of kinesiophobia. Consequently, this implies that equal support should be extended to both genders during patient rehabilitation to address the challenges presented by kinesiophobia. Meta-regression analyses were not conducted due to a constrained dataset comprising a limited number of studies and the reporting inconsistencies of pivotal variables, which precluded a robust meta-regression approach.
In addition, we have synthesized the factors influencing kinesiophobia in cardiac patients, encompassing both risk factors and protective factors. The more recognized factors are education level, monthly income, anxiety and exercise self-efficacy, paralleling Keessen’s path analysis study findings39. Patients’ capacity to proactively acquire disease-specific knowledge and their self-care awareness vary according to education levels40. Consequently, individuals with lower literacy are less cognizant of the critical role of exercise rehabilitation in cardiac health and exhibit greater kinesiophobia, corroborating the findings of Aily41. In terms of monthly income, cardiac patients often face substantial economic burdens due to long-term medical expenses42. Consequently, those with lower incomes exercise increased caution regarding behaviors that may pose cardiac risks to avoid additional healthcare expenses, potentially heightening their level of kinesiophobia. Research posits that anxiety is a primary emotional component of phobias, with fear and avoidance tendencies being mediated to some extent by sensitivity to anxiety43. Patients with heightened cardiac anxiety remain in a state of tension and hypersensitivity, potentially exacerbating their vigilance and avoidance of exercise22. Exercise self-efficacy is a protective factor against kinesiophobia, denoting an individual’s confidence or belief in their physical activity or exercise capabilities44. Patients with high self-efficacy can overcome fear and engage in active exercise, while those with lower self-efficacy tend to adopt passive coping strategies, avoiding exercise. This insight prompts healthcare professionals to prioritize enhancing patients’ exercise self-efficacy in clinical settings. Through education and psychological support, healthcare providers can assist patients in developing confidence in exercise, thereby mitigating the impact of kinesiophobia.
Moreover, we have identified and described various factors pertinent to disease or symptomatology, including the symptom status of HF, NYHA classification, pain intensity and resilience, resting heart rate, angina, and severity of self-conscious symptoms, alongside HF comorbidities and the disease course. Additionally, we have described a range of sociodemographic variables, namely age, gender, HRQoL, physical activity levels, physical function, social support, employment status, and subjective social status, all of which exhibit a significant correlation with the emergence of kinesiophobia. Beyond anxiety, psychological constructs like avoidance coping strategies, fatigue, and depressive symptoms have also been demonstrated to exert a considerable impact on the development of kinesiophobia. These influencing factors have been substantiated by individual quantitative studies, and additionally, some qualitative research45,46 has provided supporting evidence. Nevertheless, there is an imperative for ongoing high-quality research to further validate these findings and establish a consensus.
In this study, we systematically searched and screened multiple databases to ensure the comprehensiveness and diversity of the data. Rigorous inclusion and exclusion criteria were applied, and the recruited studies underwent meticulous quality assessment. A variety of analytical methods were employed to synthesize the extracted data and information, affirming the authenticity and reliability of our findings. However, certain limitations are present: (1) Despite subgroup analyses, unexplained heterogeneity persists, possibly due to variations in sample sizes, ethnicities, and disease durations across studies; (2) Most influencing factors have been corroborated by single studies, limiting their practical guidance, necessitating further validation by more high-quality research; (3) The literature predominantly comprises studies from China, Turkey, and some European regions, lacking a global perspective, which may affect the representativeness of the results. (4) Furthermore, it is crucial to acknowledge that, despite our efforts to reduce bias, the variability in disease progression and treatment stages among participants—some of whom may have undergone CR interventions prior to study enrollment—could have influenced the estimated prevalence of kinesiophobia. This variability represents a potential source of the observed heterogeneity in our combined prevalence findings. Future research should consider this source of bias. We recommend that subsequent research adopt a more standardized approach to assessing the prevalence of kinesiophobia and incorporate data from various time points into the study design to determine its trends and influencing factors more accurately.
Our study underscores the importance of preventing or promptly intervening in cases of kinesiophobia among patients with heart disease. In nations with underdeveloped economies and healthcare resources, it is imperative to actively promote science education to enhance the public’s comprehension of cardiac exercise rehabilitation. There should also be an increase in policy support for the professional practice of CR in medical institutions. Clinicians, while advancing their professional capabilities in CR, should pay special attention to patients with heart disease who have lower levels of education, lower monthly incomes, and anxiety. Measures to enhance their exercise self-efficacy are essential. For instance, developing tailored exercise plans, providing positive feedback, and establishing a supportive environment are effective strategies to boost patients’ exercise self-efficacy. Additionally, regular assessments of patients’ psychological states and levels of exercise self-efficacy can lead to the timely identification of issues and interventions, further promoting the rehabilitation process.
Conclusion
In conclusion, this study provides compelling evidence that the prevalence of kinesiophobia among patients with cardiac diseases is alarmingly high. Factors such as educational level, monthly income, anxiety, and exercise self-efficacy are closely linked to kinesiophobia in these patients. Our findings offer insights for clinicians and policymakers, underscoring the need to focus on kinesiophobia in cardiac patients, enhance early identification of risk factors, and actively leverage protective factors against the disease. This is particularly pertinent in economically underdeveloped countries, where tailored healthcare policies are needed to mitigate the levels of kinesiophobia among cardiac patients.
Data availability
The datasets used or analysed during the current study available from the corresponding author on reasonable request.
References
Di Cesare, M., Bixby, H., Gaziano, T. et al. World Heart Report 2023: Confronting the World’s Number One Killer (2023).
Tsao, C. W. et al. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation. 145(8), e153–e639 (2022).
The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Chin. Circ. J. 6(37), 553–578 (2022).
Centers for Disease Control and Prevention and National Center for Health Statistics. Summary health statistics: National Health Interview Survey, 2018: table A-1 (2021).
Ambrosetti, M., Abreu, A., Corrà, U. et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 28(5), 460–495 (2021).
Price, K. J., Gordon, B. A., Bird, S. R. & Benson, A. C. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus?. Eur. J. Prev. Cardiol. 23(16), 1715–1733 (2016).
Anderson, L. et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J. Am. Coll. Cardiol. 67(1), 1–12 (2016).
Seferovic, P. M. et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 21(10), 1169–1186 (2019).
Atherton, J. J. et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of heart failure 2018. Med. J. Aust. 209(8), 363–369 (2018).
Campkin, L. M., Boyd, J. M. & Campbell, D. Coronary artery disease patient perspectives on exercise participation. J. Cardiopulm. Rehabil. Prev. 37(5), 305–314 (2017).
Kori, S. H., Miller, R. P. & Todd, D. D. Kinesiophobia: A new view of chronic pain. Pain Manag. 3, 35–43 (1990).
Bäck, M., Jansson, B., Cider, A., Herlitz, J. & Lundberg, M. Validation of a questionnaire to detect kinesiophobia (fear of movement) in patients with coronary artery disease. J. Rehabil. Med. 44(4), 363–369 (2012).
van Ittersum, M. et al. Fear of exercise and health-related quality of life in patients with an implantable cardioverter defibrillator. Int. J. Rehabil. Res. 26(2), 117–122 (2003).
Bäck, M., Lundberg, M., Cider, Å., Herlitz, J. & Jansson, B. Relevance of kinesiophobia in relation to changes over time among patients after an acute coronary artery disease event. J. Cardiopulm. Rehabil. Prev. 38(4), 224–230 (2018).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 18(3), e1003583 (2021).
Miller, R. P., Kori, S. H. & Todd, D. D. The Tampa Scale: A measure of kinisophobia. Clin. J. Pain. 7(1), 51 (1991).
Margulis, A. V. et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: The Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol. 6, 359–368 (2014).
Zeng, X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 8(1), 2–10 (2015).
The World Bank. World Bank Country and Lending Groups. (Accessed 1 Feb 2024) https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (2024).
Yakut Ozdemir, H., Ozalevli, S., Felekoglu, E., Baskurt, A. A. & Dursun, H. Kinesiophobia and associated factors in patients with myocardial infarction. Percept. Motor Skill. 315125231204059 (2023).
Qin, J. et al. Influencing factors of kinesiophobia in older patients with chronic heart failure: A structural equation model. Clin. Cardiol. 46(7), 729–736 (2023).
Zhang, X., Zhao, Q., Wang, M., Yang, M. & Fan, X. Fear of movement and its associated psychosocial factors in heart failure patients: A cross-sectional study. Eur. J. Cardiovasc. Nurs. 22, 273–281 (2022).
Shen, Y. et al. Kinesiophobia in patients with angina pectoris of coronary artery disease: A cross-sectional survey. Heart Lung. 57, 7–11 (2022).
Ter Hoeve, N. et al. Assessing changes in fear of movement in patients attending cardiac rehabilitation: Responsiveness of the TSK-NL Heart Questionnaire. J. Rehabil. Med. 54, jrm00328 (2022).
Ding, Y. et al. Factors influencing kinesiophobia during the “blanking period” after radiofrequency catheter ablation in patients with atrial fibrillation by the fear-avoidance model. Int. J. Cardiol. 363, 49–55 (2022).
Qin, J., Xiong, J., Wang, X., Gao, Y. & Gong, K. Kinesiophobia and its association with fatigue in CHF patients. Clin. Nurs. Res. 31(7), 1316–1324 (2022).
Çakal, B., Yildirim, M. & Emren, S. V. Kinesiophobia, physical performance, and health-related quality of life in patients with coronary artery disease. Postep Kardiol. Int. 18(3), 246–254 (2022).
Sahin, H. B., Kalaycioglu, E. & Sahin, M. The effect of cardiac rehabilitation on kinesiophobia in patients with coronary artery disease. Turk. J. Ph. Med. Rehab. 67(2), 203–210 (2021).
Dąbek, J. et al. Fear of movement (kinesiophobia)—an underestimated problem in Polish patients at various stages of coronary artery disease. Ann. Agric. Environ. Med. 27(1), 56–60 (2020).
Keessen, P. et al. fear of movement in patients attending cardiac rehabilitation: A validation study. J. Rehabil. Med. (Stiftelsen Rehabiliteringsinformation). 52(2), 1–7 (2020).
Knapik, A., Dąbek, J., Gallert-Kopyto, W., Plinta, R. & Brzęk, A. Psychometric features of the Polish version of TSK heart in elderly patients with coronary artery disease. Medicina 56(9), 467 (2020).
Brunetti, N. D. et al. Scared for the scar: Fearsome impact of acute cardiovascular disease on perceived kinesiophobia (fear of movement). Clin. Cardiol. 40(7), 480–484 (2017).
Bäck, M., Cider, Å., Herlitz, J., Lundberg, M. & Jansson, B. The impact on kinesiophobia (fear of movement) by clinical variables for patients with coronary artery disease. Int. J. Cardiol. 167(2), 391–397 (2013).
Cai, L. et al. Incidence and risk factors of kinesiophobia after total knee arthroplasty in Zhengzhou, China: A cross-sectional study. J. Arthroplasty. 33(9), 2858–2862 (2018).
Pan, L. & Shi, B. Prevalence of kinesiophobia in patients with chronic low back pain and its influential factors. Chin. Gen. Pract. 08(22), 978–982 (2019).
Cardiac preventive rehabilitation knowledge urgently needed. Prev. Treat. Cardiovasc. Dis. (21), 5 (2017).
Chen, H. Study on the Correlation Between Kinesiophobia and Quality of Life in Patients with Coronary Heart Disease after PCI (Guangxi University of Chinese Medicine, 2022).
Kang, Y. The relationships between uncertainty and its antecedents in Korean patients with atrial fibrillation. J. Clin. Nurs. 20(13–14), 1880–1886 (2011).
Keessen, P. et al. Impact of kinesiophobia on initiation of cardiac rehabilitation: A prospective cohort path analysis. BMJ Open. 12(11), e066435 (2022).
Finney, R. L. et al. Cancer-related information seeking among cancer survivors: Trends over a decade (2003–2013). J. Cancer Educ. 31(2), 348–357 (2016).
Aily, J. B., de Almeida, A. C., Ramírez, P. C., Da, S. A. T. & Mattiello, S. M. Lower education is an associated factor with the combination of pain catastrophizing and kinesiophobia in patients with knee osteoarthritis?. Clin. Rheumatol. 40(6), 2361–2367 (2021).
Gheorghe, A. et al. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: A systematic review. BMC Public Health. 18(1), 975 (2018).
Vlaeyen, J. W. & Linton, S. J. Fear-avoidance and its consequences in chronic musculo-skeletal pain: A state of the art. Pain. 85, 317–332 (2000).
Zelle, D. M. et al. Fear of movement and low self-efficacy are important barriers in physical activity after renal transplantation. PLoS ONE. 11(2), e0147609 (2016).
Keessen, P. et al. Factors related to fear of movement after acute cardiac hospitalization. BMC Cardiovasc. Disord. https://doi.org/10.1186/s12872-020-01783-9 (2020).
Keessen, P. et al. Fear of movement after an acute cardiac event, experiences, beliefs, barriers and support needs in patients and their caregiver. Eur. J. Prev. Cardiol. 27(1 Suppl 4), S56 (2020).
Funding
This work was supported by the Sichuan Science and Technology Program under Grant No. 2020YFQ0060.
Author information
Authors and Affiliations
Contributions
LL and HX conceptualized and designed the study. LL, LZ, ZW, and SS were involved in the extraction, analysis, and interpretation of all data. LL and BZ drafted the initial manuscript. LT and QY critically revised the manuscript. All authors have read and approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, L., Yang, Q., Li, T. et al. Prevalence and influencing factors of kinesiophobia in patients with heart disease: a meta-analysis and systematic review. Sci Rep 14, 18956 (2024). https://doi.org/10.1038/s41598-024-69929-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69929-9
Keywords
This article is cited by
-
Summary of the best evidence for the management of kinesiophobia in patients after cardiac surgery
BMC Cardiovascular Disorders (2025)
-
Identifying potential action points for reducing kinesiophobia among atrial fibrillation patients: a network and DAG analysis
Quality of Life Research (2025)