Abstract
Recently, in Hepatocellular carcinoma (HCC) setting, the use of metformin has been associated to a trend toward worse response rate, overall survival and progression free survival in patients who received immunotherapy. The study population included individuals from both Eastern and Western regions with a confirmed diagnosis of HCC and receiving first line treatment with Atezolizumab plus bevacizumab or Lenvatinib. Univariate and multivariate analyses were performed by Cox proportional. For the analysis, patients were stratified based on their use of concomitant medication or not. At the time of database lock, 319 deaths were observed: 209 in the Lenvatinib cohort, 110 in the Atezolizumab plus bevacizumab cohort. In the Atezolizumab plus Bevacizumab arm, 50 (16.5%) patients were on chronic metformin use. At the univariate analysis for OS, patients who used metformin showed significantly shorter OS compared to patients who did not use metformin (HR 1.9, 95% CI 1.1–3.2). Multivariate analysis confirmed that patients in metformin group had significantly shorter OS compared to patients in no-metformin group (HR 1.9; 95% CI 1.1–3.1). At the univariate analysis for PFS, patients in metformin group had significantly shorter PFS compared to patients in no-metformin group (HR 1.6, 95% CI 1.0–2.6). Multivariate analysis confirmed that patients in metformin group had significantly shorter PFS compared to patients in no-metformin group (HR 1.7; 95% CI 1.1–2.7; p = 0.0147). No differences were reported in terms of ORR and DCR between patients in metformin group and those in no-metformin group. In the Lenvatinib cohort, 65 (15%) patients were recorded to chronically use metformin. No statistically significant differences in terms of both OS and PFS were found between patients in metformin group and patients in no-metformin group. This analysis unveils a negative prognostic role associated with metformin use specifically within the Atezolizumab plus Bevacizumab group.
Similar content being viewed by others
Introduction
Hepatocellular Carcinoma (HCC) is currently a global health challenge and represents the sixth most common cancer and the third leading cause of cancer-related death worldwide1. Recently, immunotherapy has become an important part of the therapeutic armamentarium for advanced HCC. The combination of Atezolizumab and bevacizumab has been settled as the new first-line standard of care, along with Lenvatinib and Sorafenib, for patients affected by advanced HCC2. Furthermore, the dual immune checkpoint inhibitors blockade has been recently tested in the HIMALAYA trial, thus leading to positive results3. In all the aforementioned studies, no preplanned subgroup analyses highlighted different efficacy basing on clinical factors, including etiology. In recent years, a growing body of evidence has emerged that suggest that patients affected by advanced HCC arising from metabolic dysfunction related steatosis liver disease (MASLD) and metabolic dysfunction related steatohepatitis (MASH) may be less responsive to immunotherapy4,5,6,7. However, MASLD/MASH is considered a frequent manifestation of metabolic disease, and therefore several comorbidities need to be taken into account in the clinical decision-making process for these patients. Among others, diabetes is a frequent manifestation and metformin is one of the most commonly used drugs for this type of disease. Discordant evidences about the metformin’s antineoplastic properties have been highlighted8,9,10. Recently, Kang and collaborators conducted a retrospective analysis on 111 patients affected by advanced HCC who received immune checkpoint inhibitors (ICIs) and demonstrated that the use of metformin was associated with a trend towards worse objective response rates (ORR), overall survival (OS), and progression-free survival (PFS), even without reaching statistical significance11. Building upon these findings, the aim of the present study was to investigate the potential prognostic role of metformin use and other concomitant medications (such as statins, insulin, and aspirin) in a cohort of advanced HCC patients who received Lenvatinib or Atezolizumab plus Bevacizumab as first-line treatment.
Methods
Patients and procedures
The study encompassed a diverse population drawn from both Eastern and Western regions, spanning Japan, Portugal, Germany, and Italy. Participants were required to have a confirmed diagnosis of hepatocellular carcinoma (HCC), validated either through histological examination or appropriate imaging studies, in accordance with international guidelines. These individuals were classified as being at stage B or C according to the Barcelona Clinic Liver Cancer (BCLC) staging system and were considered unsuitable candidates for loco-regional therapies.
Baseline characteristics were documented by each participating institution and subsequently verified through centralized review. Patients were assigned to receive either Atezolizumab plus Bevacizumab, administered from August 2018 to March 2023, or Lenvatinib, administered from November 2017 to April 2023, as their primary treatment. Lenvatinib dosing followed the protocol established by the REFLECT trial: patients received a daily oral dose of 12 mg if their baseline body weight was ≥ 60 kg or 8 mg if it was < 60 kg. Atezolizumab plus Bevacizumab treatment adhered to the regimen outlined in the IMbrave150 trial: patients received intravenous infusions of 1200 mg of atezolizumab alongside 15 mg/kg of body weight of bevacizumab every 3 weeks. Treatment response was evaluated using computed tomography or magnetic resonance imaging scans and categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) based on local review, following modified Response Evaluation Criteria in Solid Tumors (mRECIST) 1.1 guidelines. Patients continued treatment until either clinical benefit was observed as determined by the treating physician or until unacceptable toxicity occurred.
Adverse events (AEs) were assessed and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0. Management of AEs allowed for treatment interruptions and/or dose reductions as deemed necessary.
Ethical approval for the study was obtained from the respective Ethics Committees at each participating center, and the study was conducted in compliance with Good Clinical Practice guidelines, the Declaration of Helsinki, local laws, and Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 concerning the protection of natural persons with regard to the processing of personal data. The protocol number assigned by the ethics committee was 113/INT/2021.
Statistical analysis
Demographic, clinical, and pathological characteristics of the patients were gathered and summarized utilizing descriptive statistics. Categorical variables underwent comparison via the Fisher exact test, while continuous variables were compared using the t-test. Survival curves for overall survival (OS) and progression-free survival (PFS) were generated using Kaplan–Meier estimates.
Univariate and multivariate analyses were conducted utilizing Cox proportional hazards models to examine potential associations between patients' baseline characteristics and survival outcomes (OS and PFS). Overall response and objective response rate were computed. Objective response rate (ORR) was defined as the proportion of patients achieving complete response (CR) and partial response (PR), while disease control rate (DCR) encompassed ORR plus the proportion of stable disease (SD).
For analysis purposes, patients were stratified based on their use of concomitant medication. In particular, patients were categorized into either the metformin group or the no-metformin group. Concomitant medication usage was determined at baseline before initiating first-line treatment. A significance level of p < 0.05 was considered statistically significant.
Statistical analysis was conducted using the MedCalc package (MedCalc® version 16.8.4).
Results
Study population
Overall, 730 consecutive patients with HCC met inclusion criteria and were included in the analysis. Among them, 430 (59%) patients received Lenvatinib and 300 (41%) patients received Atezolizumab plus Bevacizumab.
At the time of database lock, 319 deaths were observed: 209 in the Lenvatinib cohort, 110 in the Atezolizumab plus bevacizumab cohort. The median follow-up was 14.7 months (95% CI 12.4–51.1) for Atezolizumab plus Bevacizumab patients and 21.0 months (95% CI 18.4–55.3) for Lenvatinib patients. The two cohorts of patients were almost homogeneous, except for the proportion of patients with diabetes (42.5% in Lenvatinib cohort vs. 35% in Atezolizumab plus Bevacizumab cohort), BCLC B (45% in Lenvatinib cohort vs. 38% in Atezolizumab plus Bevacizumab cohort), portal vein’s involvement (15.5% in Lenvatinib cohort vs. 26.5% in Atezolizumab plus Bevacizumab cohort), and NLR ≥ 3 (24% in Lenvatinib cohort vs. 14% in Atezolizumab plus Bevacizumab cohort).
The complete baseline characteristics in the two cohorts of patients are reported in Table 1.
Use of statins, aspirin, insulin and metformin and clinical outcome in Atezolizumab plus Bevacizumab group
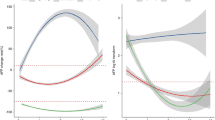
In the Atezolizumab plus bevacizumab cohort, there were no statistically significant differences observed in overall survival (OS) and progression-free survival (PFS) between patients who chronically used statins, aspirin, or insulin compared to those who did not. Within the Atezolizumab plus bevacizumab arm, 50 (16.5%) patients were on chronic metformin therapy. Baseline characteristics were similar between patients in the metformin and no-metformin groups, except for etiology (viral etiology: 24% vs. 58%, p = 0.000011; MASH etiology: 50% vs. 18%, p = 0.000005; in the metformin and no-metformin groups, respectively), statin use (36% vs. 9% in metformin vs. no-metformin groups, p = 0.000007), aspirin use (26% vs. 12.5% in metformin vs. no-metformin groups, p = 0.026006), and insulin use (22% vs. 8.5% in metformin vs. no-metformin groups, p = 0.009734). Univariate analysis revealed that patients using metformin had significantly shorter OS [14.9 months (95% CI 6.4–16.3) vs. 19.7 (95% CI 16.0–30.4); HR 1.87 (95% CI 1.08–3.24) p = 0.0248] (Fig. 1A) and PFS [4.5 months (95% CI 2.9–14.2) vs. 5.8 (95% CI 4.1–34.0); HR 1.61 (95% CI 0.99–2.62) p = 0.0212] (Fig. 1B) compared to those not using metformin. Multivariate analysis confirmed that patients in the metformin group had significantly shorter OS (HR 1.79; 95% CI 1.10–3.12; p = 0.035) (Table 2) and PFS (HR 1.78; 95% CI 1.13–2.77; p = 0.014) (Table 3) compared to those in the no-metformin group. To exclude the bias of diabetes, it is included in the multivariate analysis.
There were no differences in terms of objective response rate (ORR) and disease control rate (DCR) between the metformin and no-metformin groups (p = 0.722399 and p = 0.866298, respectively). Additionally, no significant differences in adverse events were detected between the two groups (Supplementary table).
Use of statins, aspirin, insulin and metformin and clinical outcome in Lenvatinib group
In the Lenvatinib group of patients, no statistically significant differences in terms of both OS and PFS were observed in patients who were on chronic use of statins, aspirin, or insulin compared to those who were not.
In the Lenvatinib cohort, 65 (15%) patients were recorded to chronically use metformin.
At the univariate analysis, no statistically significant differences in terms of OS were found between patients in metformin group and patients in no-metformin group [respectively, 16.6 months (95% CI 14.8–51.3) vs. 19.5 (95% CI 15.1–36.2); HR 1.2 (95% CI 0.8–1.8) p = 0.3164] (Table 4).
The multivariate analysis confirmed that the use of metformin was not a prognostic factor for OS in the cohort of patients who received Lenvatinib (Table 4).
At the univariate analysis, no statistically significant differences in terms of PFS were found between patients in metformin group and patients in no-metformin group [respectively, 4.7 months (95% CI 3.7–24.2) vs. 4.4 months (95% CI 3.8–41.8); HR 1.0 (95% CI 0.8–1.4) p = 0.8542] (Table 5).
The multivariate analysis confirmed that the use of metformin was not a prognostic factor for PFS in the cohort of patients who received Lenvatinib (Table 5).
No differences were reported in terms of ORR and DCR between patients in metformin group and those in no-metformin group (p = 0.661410 and p = 0.669873, respectively) (Supplementary table).
Discussion
This analysis has highlighted, for the first time, the use of metformin as a negative prognostic factor in a cohort of patients who received atezolizumab plus bevacizumab for advanced HCC. Conversely, the utilization of metformin was found to have no prognostic impact in a cohort of patients with advanced HCC who received Lenvatinib as a first-line treatment. Recently, Kang and colleagues performed an analysis on a cohort of patients treated with immunotherapy for advanced HCC and highlighted worse survival outcomes in patients included in the metformin group compared to those in the no-metformin groups, even without reaching the statistical significance11. Our study encompassed a larger patient sample receiving first-line treatment for advanced HCC (Atezolizumab plus Bevacizumab or Lenvatinib), whereas the previous study focused exclusively on patients who received immunotherapy in either the first or subsequent lines of treatment. However, our findings provide substantial support, based on a larger patient cohort, to the notion that individuals receiving immunotherapy for advanced HCC and using metformin as a chronic medication exhibit inferior survival outcomes when compared to those not taking metformin chronically. Preclinical evidence provides a biological rationale for the anticancer properties of metformin. Metformin has been demonstrated to improve the restoring of CD8 + tumor infiltrating lymphocytes (TILs) from immune exhaustion12and to reduce hypoxic status in tumor microenvironment and improve intra-tumoral T cell function13. In addition, metformin could inhibit the differentiation of naïve CD4 + T cells into regulatory T cells (Tregs), thus blocking the activation of myeloid-derived suppressor cells (MDSCs) and reverting the M2-like polarization of tumor associated macrophages (TAMs)14. These evidence seems to suggest that the combination of immunotherapy and metformin could act in synergism and potentially enhancing the anticancer response. In the field of HCC, additional factors contribute to shaping the overall scenario. Unlike other oncologic diseases, HCC develops within the context of hepatopathy, which can have various underlying etiologies. These different etiologies have been shown to exert distinct effects on the immune microenvironment, resulting in diverse carcinogenic pathways4,5,6,7. In addition both preclinical and retrospective clinical data support the hypothesis that patients with MASH-related HCC could be less responsive to immune checkpoint inhibitors7. Wabitsch and colleagues recently confirmed that patients with MASH-related HCC are less responsive to immunotherapy, due to aberrant activation and exhaustion of CD8 + T cells15. In the same work, authors demonstrated that metformin treatment restores the motility and metabolism of CD8 + T cells, thus enhancing the anti-tumor immune responses15, which is inconsistent with the present analysis. As expected, in our analysis the proportion of MASH-related HCC in the metformin-group of patients is higher, which could have influenced the results reported. Nevertheless, after correction for etiology, the multivariate analysis confirmed the negative prognostic factor of the chronic use of metformin in the cohort of patients who received Atezolizumab plus Bevacizumab. Several further speculations on the negative prognostic impact of the chronic use of metformin could be done. First of all, a possible explanation could be based on the changes in gut microbiota induced by metformin. Indeed, previous, extensive researches underscored the critical role of gut microbiota in the efficacy of ICIs in several oncologic settings. More specifically, strong evidence indicates that metformin exposure significantly interferes with human intestinal microbiota and gut metabolome, even if the specific mechanisms are not completely highlighted yet16,17,18,19.
Another consideration is that metformin has been shown to negatively modulate the immune system by increasing the peripheral proportions of CD4 + and CD8 + regulatory T cells while decreasing CD4 + T helper cell 17 levels20. In the context of ICI immunotherapy, metformin may unintentionally dampen the desired anti-tumor immune response elicited by ICIs. Therefore, this interaction could potentially contribute to poorer outcomes in patients using metformin concurrently with ICIs.
Additionally, studies suggest that metformin may have proangiogenic effects in hypoxic conditions21. It is also important to note that, in diabetic contexts, metformin has been reported to enhance the angiogenic function of endothelial progenitor cells22. This dual role of metformin introduces the possibility that its proangiogenic effect might contribute to the adverse association between metformin use and less favorable prognoses in patients undergoing ICI therapy. However, further research is needed to confirm this speculative link. Finally, we have to consider that it is convincible that the underlying diabetes’s biological mechanisms could have contributed to the survival outcomes, which constitutes a bias difficult to eliminate, since deeper investigations focused on that are needed in order to clarify the complex link between HCC, immune response to immune checkpoint inhibitors, diabetes and chronic use of metformin.
Concerning the absence of prognostic impact of metformin use in patients who received Lenvatinib, several considerations could be done. In a previous work, the concomitant use of metformin and sorafenib was associated with worse OS and PFS in a cohort of patients affected by advanced HCC, due to a competitive action on PI3K and MAPK signaling exerted by metformin, which leads to the development of resistance to sorafenib23,24. Although belonging to the same class of drugs, Lenvatinib and Sorafenib present different target spectra, which could explain the varying results when combined with metformin. In a recent work, Chen and colleagues showed that Lenvatinib and Metformin both suppress the activation of AKT signaling pathway thus leading to the nuclear aggregation of downstream effector FOXO3. Finally, interactions between metformin and oncologic treatments depend also to the timing, since the pathways and, consequently, the biological behavior of an HCC arising in a patient already treated with metformin is different from that of an HCC arising in a patient who, at some point, undergoes treatment with metformin.
The present study has several limitations, primarily stemming from its retrospective and multicenter nature. Selection bias among patients cannot be entirely ruled out, and it's important to consider the absence of centralized imaging review for the evaluation of PFS. Finally, data about doses and schedule of use of metformin as well as data on concomitant medications for diabetes and cardiovascular comorbidity were unavailable, due to the large retrospective and multicentric design of the study. Thus, bias related to the prognostic incidence of diabetes as well as other cardiovascular disease has not been included in the analysis, which means that come biases could not be completely excluded. Further investigations and prospective validations on external cohort are needed in order to verify our results. Nevertheless the present study represents the first analysis focusing on the role of metformin in a large cohort of patients with advanced HCC, who underwent first-line therapy with either Lenvatinib or Atezolizumab plus Bevacizumab. This analysis unveils a negative prognostic role associated with metformin use specifically within the Atezolizumab plus Bevacizumab group. Our findings corroborated in a larger sample size the earlier study by Kang and colleagues adding a crucial piece to the complex puzzle of the interaction between metformin and immunotherapy for patients dealing with advanced HCC.
Data availability
Data available on request from the authors (contact: [email protected]).
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382(20), 1894–1905. https://doi.org/10.1056/NEJMoa1915745 (2020).
Abou-Alfa, G. K. et al. Phase 3 randomized, open-label, multicenter study of tremelimumab and durvalumab as first-line therapy in patients with unresectable hepatocellular carcinoma: HIMALAYA. J. Clin. Oncol. 40, 379. https://doi.org/10.1200/JCO.2022.40.4_suppl.379- (2022).
Pfister, D. et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592(7854), 450–456. https://doi.org/10.1038/s41586-021-03362-0 (2021).
Rimini, M. et al. Nonalcoholic steatohepatitis in hepatocarcinoma: New insights about its prognostic role in patients treated with Lenvatinib. ESMO Open. 6(6), 100330. https://doi.org/10.1016/j.esmoop.2021.100330 (2021).
Rimini, M. et al. Atezolizumab plus Bevacizumab versus Lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open. 7(6), 100591. https://doi.org/10.1016/j.esmoop.2022.100591 (2022).
Rimini, M. et al. Real-world data for atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma: How does adherence to the Imbrave150 trial inclusion criteria impact prognosis?. Target Oncol. 18(2), 221–233. https://doi.org/10.1007/s11523-023-00953-x (2023).
Mei, Z. B. et al. Survival benefits of metformin for colorectal cancer patients with diabetes: A systematic review and meta-analysis. PLoS One. 9(3), e91818. https://doi.org/10.1371/journal.pone.0091818. Erratum in: PLoS One. 2014;9(7):e103652
Sonnenblick, A. et al. Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: analysis from the ALTTO phase III randomized trial. J. Clin. Oncol. 35(13), 1421–1429. https://doi.org/10.1200/JCO.2016.69.7722 (2017).
Reni, M. et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: An open-label, randomized phase II trial. Clin. Cancer Res. 22(5), 1076–1085. https://doi.org/10.1158/1078-0432.CCR-15-1722 (2016).
Kang, S. et al. Impact of metformin on clinical outcomes in advanced hepatocellular carcinoma treated with immune checkpoint inhibitors. Liver Cancer Int. 4, 77–88 (2023).
Eikawa, S. et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl. Acad. Sci. U. S. A. 112(6), 1809–1814. https://doi.org/10.1073/pnas.1417636112 (2015).
Scharping, N. E., Menk, A. V., Whetstone, R. D., Zeng, X. & Delgoffe, G. M. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol. Res. 5(1), 9–16. https://doi.org/10.1158/2326-6066.CIR-16-0103 (2017).
Li, L. et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 78(7), 1779–1791. https://doi.org/10.1158/0008-5472.CAN-17-2460 (2018).
Wabitsch, S. et al. Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J. Hepatol. 77(3), 748–760. https://doi.org/10.1016/j.jhep.2022.03.010 (2022).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Mirji, G. et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci Immunol. 7, eabn0704 (2022).
Forslund, K. et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266 (2015).
Wu, H. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858 (2017).
Lee, S. K. et al. Combination treatment with metformin and tacrolimus improves systemic immune cellular homeostasis by modulating treg and Th17 imbalance. Front Immunol. 11, 581728 (2021).
Ruan, Z. et al. Metformin accelerates bone fracture healing by promoting type H vessel formation through inhibition of YAP1/TAZ expression. Bone Res. 11(1), 45 (2023).
Yu, J. W. et al. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 15, 88 (2016).
Casadei Gardini, A. et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert. Opin. Pharmacother. 16(18), 2719–2725. https://doi.org/10.1517/14656566.2015.1102887 (2015).
Casadei Gardini, A. et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur. J. Cancer. 86, 106–114. https://doi.org/10.1016/j.ejca.2017.09.003 (2017).
Author information
Authors and Affiliations
Contributions
Conception and design: A.C.G., J.P., M.R. Acquisition of data (acquired and managed patients): M.R., M.M., E.A., F.V., M.K., T.T., G.S., S.S., S.L., F.F., F.S., L.A., FM., M.I., G.C., F.G.F., M.S., R.S., I.G.R., M.S., P.N., L.A., M.P., S.C., F.R., S.F., T.K., A.H., H.I., M.D.R., V.H., G.M., M.C., C.C., C.F. G.L.F., S.C., A.C.-G., J.P. Analysis and interpretation of data: A.C.-G, J.P., M.R. Writing, review, and/or revision of the manuscript: A.C.-G, J.P., M.R. Final approval of manuscript: M.R., M.M., E.A., F.V., M.K., T.T., G.S., S.S., S.L., F.F., F.S., L.A., F.M., M.I., G.C., F.G.F., M.S., R.S., I.G.R., M.S., P.N., L.A., M.P., S.C., F.R., S.F., T.K., A.H., H.I., M.D.R.8, V.H., G.M., M.C., C.C., C.F., G.L.F., S.C., A.C.-G., J.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board and informed consent
The Ethical Review Board of each Institutional Hospital approved the present study. This study was performed in line with the principles of the Declaration of Helsinki. Written informed consent for treatment was obtained for all patients.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rimini, M., Montes, M., Amadeo, E. et al. Impact of metformin, statin, aspirin and insulin on the prognosis of uHCC patients receiving first line Lenvatinib or Atezolizumab plus Bevacizumab. Sci Rep 14, 20200 (2024). https://doi.org/10.1038/s41598-024-70928-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70928-z