Abstract
CT and MR tools are commonly used to diagnose lumbar fractures (LF). However, numerous limitations have been found in practice. The aims of this study were to innovate and develop a spinal disease-specific neural network and to evaluate whether synthetic MRI of the LF affected clinical diagnosis and treatment strategies. A total of 675 LF patients who met the inclusion and exclusion criteria were included in the study. For each participant, two mid-sagittal CT and T2-weighted MR images were selected; 1350 pairs of LF images were also included. A new Self-pix based on Pix2pix and Self-Attention was constructed. A total of 1350 pairs of CT and MR images, which were randomly divided into a training group (1147 pairs) and a test group (203 pairs), were fed into Pix2pix and Self-pix. The quantitative evaluation included PSNR and SSIM (PSNR1 and SSIM1: real MR images and Pix2pix-generated MR images; PSNR2 and SSIM2: real MR images and Self-pix-generated MR images). The qualitative evaluation, including accurate diagnosis of acute fractures and accurate selection of treatment strategies based on Self-pix-generated MRI, was performed by three spine surgeons. In the LF group, PSNR1 and PSNR2 were 10.884 and 11.021 (p < 0.001), and SSIM1 and SSIM2 were 0.766 and 0.771 (p < 0.001), respectively. In the ROI group, PSNR1 and PSNR2 were 12.350 and 12.670 (p = 0.004), and SSIM1 and SSIM2 were 0.816 and 0.832 (p = 0.005), respectively. According to the qualitative evaluation, Self-pix-generated MRI showed no significant difference from real MRI in identifying acute fractures (p = 0.689), with a good sensitivity of 84.36% and specificity of 96.65%. No difference in treatment strategy was found between the Self-pix-generated MRI group and the real MRI group (p = 0.135). In this study, a disease-specific GAN named Self-pix was developed, which demonstrated better image generation performance compared to traditional GAN. The spine surgeon could accurately diagnose LF and select treatment strategies based on Self-pix-generated T2 MR images.
Similar content being viewed by others
Introduction
Lumbar fracture (LF) is the most common type of spinal fracture and is more likely to occur in elderly individuals1. LF is classified into sub-acute fracture (SF) and acute fracture (AF).The characteristics of SF include chronic lower back pain, vertebral height loss and even lumbar kyphosis deformity, which are often caused by microfractures due to poor bone quality in elderly individuals. The characteristics of AF include severe lower back pain, lower limb paralysis and even urinary and faecal incontinence, which are often caused by severe trauma2,3. Computerized tomography (CT) and magnetic resonance imaging (MRI) are standard methods for diagnosing LF. Although CT scans are fast and suitable for high spatial resolution, they have a limited capacity to distinguish between the characteristics of SF and AF4, which is very important for selecting treatment strategies. MR excels in diagnosing AF, but it is costly, time-consuming and inconvenient in actual clinical situations5. For example, MR examination cannot be performed for emergencies that occur at night, which may delay the diagnosis and treatment of trauma patients and adversely affect neurological recovery6. In addition, some patients are not suitable for MR examinations, such as those with metal implants or who experience claustrophobia. Furthermore, MR machines are not available in some developing countries and remote regions. Therefore, exploring new techniques to convert CT images to MR images holds significant practical relevance.
Artificial intelligence, particularly deep learning (DL), has made significant progress in medical imaging in recent years. For normal medical images, some studies have shown that DL can help to accomplish complicated tasks, such as recognition and segmentation of features in two-dimensional (2D) or three-dimensional (3D) images7,8, image transformation and simulation9,10, and reconstruction of three-dimensional images8,11. Most of these findings are limited to technological innovations. If these techniques are used for clinically specific diseases, it is essential to accurately process medical images depicting abnormalities, which places greater demands on DL and researchers due to the complexity of the disease ___location and degree. Previous studies have reported automatic disease classification based on ___location and features12, but these investigations were confined to the images themselves and were not associated with clinical diagnosis or treatment. The most recent studies focused on single-modal disease image synthesis and its clinical diagnostic value13,14, indicating that mutual transformation of different models may be feasible, such as CT images synthesized from MR images. The generative adversarial network (GAN), including an image generation network (G) and an image discrimination network (D)15, is a promising approach in the field of image transformation. Many complicated tasks can be achieved through the constant confrontation between G and D, such as image enhancement and denoising16,17, image alignment18,19, image synthesis14 and image transformation20,21. In studies of multimodal medical image transformation, a GAN was used to synthesize CT data from head and neck MR images to guide tumour radiotherapy dosing20, which is critical for accurately eradicating tumours and minimizing patient harm. Weijuan et al. reported that a GAN can be used to diagnose lumbar AF via digital radiography (DR)21, which is helpful for improving the efficiency of LF diagnosis.
Compared to DR and CT, MRI is the gold standard for diagnosing AF due to its detailed damage characteristics and lack of organ interference5. To the best of our knowledge, no studies on CT-to-MR conversion have specifically focused on LF.
The primary objectives of this study were twofold:
-
To innovate and develop a spine disease-specific GAN and verify the quality of synthetic images.
-
To evaluate whether synthetic MRI of LF affects the clinical diagnosis and treatment strategies using large samples.
Methods and materials
Patients and images
From January 2017 to July 2023, 2902 patients with LF were treated in our hospital. Patients whose CT and MR images were obtained before surgery and within one week of each other were included. The exclusion criteria included significant differences in lumbar spine curvature between CT and MRI, metal artefacts, pathological fractures (spinal tuberculosis and spinal tumours), non vertebral fractures (vertebral arch, transverse process, spinous process), and other diseases (spondylolisthesis, disc herniation). Ultimately, 675 LF participants met the inclusion and exclusion criteria (Fig. 1). Moreover, 55 participants with non lumbar fracture (NLF) were randomly included to examine the potential impacts of LF on the quantitative evaluation. Patient details, including age, sex, fracture ___location, and symptoms, are shown in Table 1.
For each participant included, two mid-sagittal CT and T2-weighted MR images were selected. Ultimately, the study included 1350 pairs of LF CT and MR images, along with 110 pairs of NFL CT and MR images (Fig. 2). This study was approved by the review board of China Three Gorges University. All the study methods were conducted in accordance with the China Three Gorges University guidelines and regulations, and all the experimental protocols were approved by the China Three Gorges University committee. The requirement for informed consent was waived by the China Three Gorges University committee because retrospective data were used.
Overview of the research pipeline. LFP = lumbar fracture participant, NLFP = non lumbar fracture participant, Self-pix = Pix2pix with Self-Attention, LF = lumbar fracture, ROI = region of interest, NF = no fracture, PSNR = peak signal-to-no ratio, SSIM = structural similarity, R = real MRI, S = synthetic MRI, AF = acute fracture, NAF = no acute fracture, ConT = conservative treatment, PKP/PVP = percutaneous vertebroplasty/percutaneous kyphoplasty, MIF = minimally invasive fixation, ODF = open decompression and fixation.
CT and MR scans
The vast majority of participants were scanned using a 64-row multidetector CT scanner (LightSpeed VCT; GE Medical System; GE: General Electric Company) and dual-source CT scanners (SOMATOM Definition Flash; SIEMENS Healthineers). Few patients received a 16-row multidetector CT scanner (SOMATOM SCOPE, Siemens Healthineers) in the emergency department. All CT images were reconstructed using GE ADW 4.6 (Advantage workstation). All MR scans were acquired using 3.0 T MR scanners (GE MR750) (Tables 2, 3).
Paired data processing
The images were cropped manually to focus precisely on the lumbar spine: the top was the T12 upper edge, the bottom was the S1 lower edge, the front was the L4 or L5 anterior edge, and the rear was the final edge of the lumbar spinous process. The brightness and contrast of the images were adjusted to accentuate the features of the vertebrae, and the images were further subjected to a noise reduction algorithm (Fig. 2). By splicing cropped CT and MR images, 1350 matched LF images and 110 matched NLF images were obtained. These images were randomly divided into 1,147 training, 203 test, 89 training and 21 test datasets.
Model architecture
A new model called Self-pix was constructed that introduces Self-Attention22. The Residual Network23 (Resnet) was used as the generator of Self-pix, and the Markovian discriminator24 (PatchGAN) was used as the discriminator (Fig. 3).
Generator
The Pix2pix25generator used the Unet26 structure, but Self-pix utilised the Resnet23 structure (Fig. 3). Resnet has advantages over Unet in dealing with complex images and is more suitable for our subject. Self-pix contains 9 ResNet_blocks, which are built by adding a shortcut every two layers of the network (shortcut connections); these can deepen the network model: the deeper the network, the better is the representation ability. However, an increase in depth can cause problems, such as elevation dissipation and gradient explosion. The emergence of residual blocks effectively solves the above problems.
Discriminator
Self-pix’s discriminator is PatchGAN24 (Fig. 3). The difference between the PatchGAN and the general GAN is that the PatchGAN outputs an N*N matrix (N = 70 in Self-pix). All the elements in this matrix have only two values, true or false, which increase the receptive field of the model to the original image.
Objective function
Self-pix is a variant of the conditional GAN27 (cGAN), the objective function of which can be expressed as follows:
G wants to reduce this objective function, and D wants to play against G. Self-pix chooses the objective function of the L1 distance because L1 can promote less fuzzy generation; its objective function is expressed as follows:
Self-pix’s objective function is described as follows:
where \(\lambda\) is a weight parameter that is used to balance the two objective functions L1 and cGAN.
Self-attention
Self-Attention22 has been widely used in NLP since its inception. Parmar et al.28 proposed a model that adds Self-Attention to the image field to generate images. Han Zhang et al.29 proposed a model in which Self-Attention is added to GAN to capture the query position of an image through Self-Attention. Self-Attention computes three matrices, Query, Key, and Value, by the elements of the input sequence X. The corresponding attention score is obtained by dot multiplication of Q and K. Then, the Softmax activation function is used to change its value to 0–1 to obtain the corresponding attention weight. We further obtained the output by multiplying the dot by V and summing it.
Self-Attention was added to the generator's first downsampling layer, the first upsampling layer and Resblocks to find the best-optimised model. This process is conducive for the model to capture the features in the input image and improve the model's performance and imaging effect. To further improve the model's performance and reduce the computational overhead, we added spectral normalisation30 in the convolutional layer of D.
Quantitative image analysis
The mean square error (MSE), peak signal-to-noise ratio (PSNR), and structural similarity (SSIM)31 were calculated to evaluate the differences between the original MR image and the synthetic MR image. Pix2pix-generated and Self-pix-generated MR images were compared with the original MR images using the SSIM and PSNR.
The calculation formula for MSE is as follows: M and N are the number of rows and columns in an image, Xij is the pixel intensity in the real MR image, and Yij is the pixel intensity in the synthesized MR image.
The PSNR is an error-sensitive image quality evaluation tool based on errors between corresponding pixel points. It does not consider the visual characteristics of human eyes. The calculation formula for the PSNR is as follows: MAXi is the maximum in an image. To ensure the accuracy of the calculation, we converted the image type from 8 to 64. In this case, MAXi = 1.
The SSIM is applied to measure the structural similarity of two images, and its values range from 0 to 1. When two images are infinitely identical, the SSIM is close to 1. The following formula was used to calculate the SSIM between two images at a given pixel: where µx and µy are the mean values of the two images within a small window around a fixed pixel point;ðx and ðy are the standard deviations of the two images computed within the same window; ðxy is the covariance between the two images computed within the same window; and C1 and C2 e regularisation constants to prevent denominators of zero.

Qualitative image analysis
Three practised spine surgeons with more than ten years of professional experience who were blinded to the acquisition protocols were invited to independently conduct the qualitative evaluation independently, which consisted of 203 original MRIs and 203 Self-pix-generated MRIs. First, the surgeons identified whether patients had acute fractures and recorded the fractured vertebrae if confirmed. In addition, all surgeons discussed and then chose an appropriate treatment strategy based on the patient's basic information and imaging findings: conservative treatment (ConT), percutaneous vertebroplasty (PVP)/percutaneous kyphoplasty (PKP), minimally invasive surgery (MIS), and open decompression and fixation (ODF). Finally, the overall similarity was evaluated using the following four-point scale: 1 = undiagnosable, 2 = acceptable for diagnosis, 3 = good and 4 = excellent.
Statistical analysis
Normally distributed continuous variables are presented as the mean ± standard deviation (SD). Otherwise, variables are presented as medians with the corresponding 25th and 75th percentiles. Categorical variables are expressed as absolute numbers with percentages. Both Pix2pix and Self-pix utilised the same datasets, so the statistical method of paired validation was adopted to check the difference between the two GANs. The paired-sample ttest was used for quantitative analysis if the data were normally distributed. Otherwise, the Wilcoxon signed-rank test was used. In the qualitative analysis, McNemar's test was used to assess the accuracy of Self-pix in diagnosing AF, and the McNemar-Bowker test was used to assess treatment strategy consistency. All statistical analyses were performed using IBM SPSS Statistics software (version 25.0, IBM Corp). A two-tailed P value less than 0.05 indicated a statistically significant difference.
Results
Quantitative assessment
The PSNR1 and SSIM1 were compared between real MR images and Pix2pix-generated MR images. The PSNR2 and SSIM2 were compared between real MR images and Self-pix-generated MR images. The region of interest (ROI) was defined as the fractured vertebra (Fig. 4).
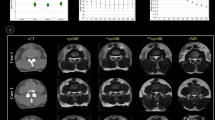
The PSNR and SSIM of the different groups are displayed in Fig. 5. In the LF group, the PSNR1 and PSNR2 were 10.884 and 11.022 (Wilcoxon signed-rank test, Z = − 6.603, p < 0.001), and the SSIM1 and SSIM2 were 0.767 and 0.771 (paired-sample t test, t = − 5.546, p < 0.001), respectively. In the ROI group, the PSNR1 and PSNR2 were 12.351 and 12.670 (paired-sample t test, t = − 2.937, p = 0.004), and the SSIM1 and SSIM2 were 0.817 and 0.832 (paired-sample t test, t = − 2.822, p = 0.005), respectively. In the NLF group, the PSNR1 and PSNR2 were 17.254 and 17.709 (Wilcoxon signed-rank test, Z = − 2.485, p = 0.013), and the SSIM1 and SSIM2 were 0.933 and 0.939 (Wilcoxon signed-rank test, Z = − 2.659, p = 0.008), respectively. The Self-pix-generated MRI parameters were greater than the Pix2pix-generated MRI parameters in all groups. The SSIM and PSNR of the ORI group with Self-pix increased by 7.91% and 14.91%, respectively, compared with those of the LF group, but Pix2pix improved by only 6.5% and 13.48%, respectively (Fig. 5C).
Quantitative and qualitative analyses. In the quantitative image evaluations (A, B), the median and IQR are represented as box plots for Pix2pix’s PSNR and Self-pix’s SSIM in the region of interest (ROI). All other box plots are presented as the means ± SDs. (C) Improvement due to Self-Attention according to the comparison of metrics between the ROI and LF. In the qualitative images (D), the bar charts represent the number of each score. (1 = undiagnosable, 2 = acceptable, 3 = good, 4 = excellent). None of the scores were 1.
Qualitative assessment
A total of 3045 (203*5*3) pairs of vertebrae were used to assess the consistency of the diagnosis of acute lumbar fractures. Among the pairs of vertebrae, 518 were diagnosed with AF and 2527 were normal (including SF) using real-MRI (R-MRI), and 512 were diagnosed with AF and 2533 were normal (including SF) using synthetic-MRI (S-MRI). No statistical difference was found between R-MRI and S-MRI (Mcnemar’s test, p = 0.689). The sensitivity was 84.36% (95% CI 80.98–87.24%), and the specificity was 96.65% (95% CI 96.29–97.63%).The consistency between Self-pix-generated MRI and real MRI for diagnosing acute lumbar fracture was excellent (Cohen’s kappa test, κ = 0.818, p < 0.001) (Table 4).
After removing patients with false positive and false negative findings based on synthetic MRI, 197 LFPs were included to evaluate treatment strategy consistency, and only four patients received different treatments (Fig. 6). The four patients underwent ConT with synthetic MRI; however, two patients were treated with PKP/PVP, and the others were treated with MIF. These findings revealed no significant difference (Mcnemar-Bowker test, χ2 = 4, p = 0.135) and excellent consistency (Cohen’s kappa test, κ = 0.932, p < 0.001) between Self-pix-generated MRI and real MRI in terms of treatment strategy (Table 5). The overall similarity scores were as follows: none of the images were undiagnosable, 44 (7.22%) were acceptable, 232 (38.1%) were good, and 333 (54.68%) were excellent; the average score was 3.47 (Fig. 5D).
The same treatment strategy based on R-MRI and S-MRI. A man (35 y, BMI = 20.7) who fell off a horse was diagnosed with an L3 fracture and received ConT (A). A man (70 y, BMI = 20.7) who fell was diagnosed with an L2 fracture and underwent PVP (B). A man (54 y, BMI = 24.2) who fell from a height of 2 m was diagnosed with an L1 fracture and underwent percutaneous pedicle screw fixation (C). A man (55 y, BMI = 17.9) who fell was diagnosed with an L3 fracture and underwent posterior open decompression, bone graft fusion and internal fixation (D). ConT = conservative treatment; PKP/PVP = percutaneous vertebroplasty/percutaneous kyphoplasty; MIF = minimally invasive fixation; ODF = open decompression and fixation, R-MRI = real MRI, S-MRI = synthetic MRI, BMI = body mass index.
Discussion
The results of this study showed that synthetic T2 MRI achieved the same effect as real MRI in identifying AF, indicating its significant importance for precision medicine and promoting patient recovery. Although X-ray and CT examinations can better display longitudinal cracks in the vertebral body, the display is not ideal for horizontal vertebral fractures and mild compression fractures4. These fractures may only manifest as abnormalities in vertebral morphology on X-rays and CT scans, making their diagnosis highly dependent on the surgeon's experience. Although dual-energy computed tomography can provide information about bone marrow oedema, it has not been widely adopted in clinical practice due to its high cost and steep learning curve. In comparison, MR is not only widely used, but it also has significant advantages in terms of resolution, contrast, imaging sequence, soft tissue lesions, and bone marrow oedema5. In addition, MRI can reveal not only soft tissue conditions, such as blood and oedema, but also fracture lines, which can comprehensively determine the duration of damaged lesions4. T1-weighted sequences provide excellent contrast for bone, muscle and fat, which is beneficial for identifying normal and abnormal anatomical structures. T2-weighted sequences are highly sensitive to fluid and oedema, making them more effective for observing features such as bone marrow extravasation, blood and oedema around fractured vertebrae, which are crucial for diagnosing AF. Moreover, severe AF fragments may compress the spinal cord, and T2-weighted sequences provide superior visualization of the spinal cord, aiding in the selection of surgical strategies and improving patient prognosis. After vertebral AF, the T1-weighted and T2-weighted signals are irregular, with sharp edges and clearly visible fracture lines, and the high-signal fracture line on T2 is as clear as that on T1. SF of the vertebral body has undergone basic repair and reconstruction due to injury, with changes only in appearance. Therefore, the signals presented at T1 and T2 are similar to those of normal vertebral bodies32. Based on the above characteristics, T2-weighted sequences are often preferred by spinal surgeons for evaluating lumbar fractures. Although fat suppression T2 sequences indeed enhance the identification of soft tissue lesions by suppressing fat signals, they are unsuitable for learning and training in deep learning due to noticeable and uncontrollable noise.
In synthetic MRI using a Self-pix network, the AF of the vertebrae displayed a high signal intensity with high sensitivity and specificity, which is extremely beneficial for clinical applications. For trauma patients with severe spinal fractures in the emergency department, recovery is closely related to rapid diagnosis and surgical decompression. An optimal postoperative spinal cord recovery requires completion of the operation decompression within 8 h6, which not only tests the surgeon’s skill but also requires the diagnostic efficiency of the radiology department. A fast spine MR examination takes at least 10 to 15 min, during which patients are required to remain motionless in a confined and enclosed space. To some extent, this immobility prolongs the patient's physical pain and may also cause psychological stress. Moreover, MRI scans are not available at night in most hospitals, resulting in extended time for the referral process and delaying optimal decompression and recovery. These conditions can cause irreversible damage to the spinal cord and have a serious impact on future quality of life. Moreover, MRI is not available for some patients, such as those with metal implants or claustrophobia. MR machines are not available in certain poor developing countries or remote areas. A lack of MR support can lead to insufficient understanding of fracture conditions, such as vertebral body information around obvious fractures, among surgeons and patients. An inappropriate treatment strategy might result in potentially exacerbated injuries. Based on the various limitations mentioned above, in the present study, high-quality synthesis MR images of lumbar fractures obtained from CT images will help surgeons and patients make accurate diagnoses as early as possible and then formulate treatment strategies.
In recent years, networks such as GAN have made significant advancements in the field of clinical medical imaging, including within specialised areas such as spinal surgery. Jiawei et al. developed a fully automated procedure to segment and quantitatively measure lumbar vertebrae and intervertebral discs33. Ki-Taek et al.34 used a GAN to synthesize lumbar MR images from CT images. Gao et al. achieved 3D reconstruction of the spine using 2D orthogonal X-ray images11. While these studies have achieved major breakthroughs in technology, their application remains limited to normal vertebrae and has not yet been implemented in clinical practice. In addition, Li et al. explored the feasibility of automatically detecting vertebral fractures using DL35, and Weijuan et al. employed a GAN to distinguish lumbar AF from DR21. Although these studies were relevant to the disease, the results were not in line with clinical practice. Spine surgeons are more concerned about the diagnosis and treatment of AF rather than LF detection, which is closely related to the prognosis of patients. MRI is the gold standard for diagnosing AF due to the abovementioned inconveniences. In an emergency setting, X-ray and CT serve as standard methods for diagnosing vertebral fractures, but there may be risks of missed diagnoses and delayed treatment, which can worsen the prognosis. To solve this problem, this study proposed a new disease-specific GAN for CT images-to-MR images.
In the present study, a new model named Self-pix is proposed, which is based on Pix2pix and introduces Self-Attention into the generator and Resnet blocks. Generating diseased MR images is more difficult than generating normal MR images due to the random area and degree of the disease. In addition, the learning and output of traditional GANs are too generalised to focus on diseased areas. Therefore, Self-Attention was introduced to emphasise areas with larger changes, which can be reflected in higher similarity values between the Query and the Key, leading togreater attention on these areas and the subsequent output of more realistic diseased MR images. This model was applied to LF for the first time. Comparing ROI with LF, Self-pix showed an increase of 14.91% in PSNR and 7.91% in SSIM, significantly surpassing the 13.48% and 6.50% of Pix2pix, which confirmed the consistency and enhanced image quality of the Self-Attention mechanism. This improvement further demonstrated that the Self-Attention mechanism mainly focused on the fractured vertebrae, aligning closely with our study's clinical goal. However, the PSNR averaged between 10 and 12 in the LF ROI, thus markedly deviating from visual perception. To address this issue, NFL MR images were specially trained and tested, where the PSNR reached approximately 18. This result aligned more closely with human visual perception. This discrepancy might be due to differences in brightness between CT and MR images. GAN learns CT and MR features simultaneously and transforms these features between the two when they are output. Although we tried to unify the brightness of CT and MR images, some systematic errors persisted. Therefore, the pixel value in synthetic MR images was affected by CT, and the high complexity of fractured vertebrae magnified this difference. As the PSNR was defined to calculate the difference in pixel values, synthetic MRI of LF had lower PSNR values than synthetic MRI of NLF. Based on the above findings and our experimental results, we believe that the PSNR may be unsuitable for assessing the similarity between synthetic LF MR images and their original MR images.
Our synthetic MR image shows promise for clinical application. AF occurs within a short period and is often characterised by severe pain and signs of acute injury. The fracture is unstable at this stage, and the vertebra may further collapse or even compress the spinal cord after movement4. As a result, more aggressive treatments are often used to relieve spinal cord compression or prevent further vertebrae collapse36. SF can occur within several months or more prior to diagnosis. At this point, the vertebra has stabilised, but sequelae such as chronic pain and malunionmay continue4. Patients are prioritised for conservative therapies such as bed rest, pain management, brace fixation, and physical therapy37. There are significant differences in disease progression and treatment strategies between patients with AF and SF, so it is important to distinguish between them. In this study, surgeons accurately identified SF and AF based on our synthetic MR images, with a sensitivity of 84.36% and a specificity of 96.65%. In AF, different surgical procedures are chosen depending on the degree of vertebral destruction. PKP/PVP is an interventional procedure in which bone cement is injected into the fractured vertebra through small incisions in the skin to stabilise the vertebra and reduce pain38. Surgical injury is mild, and postoperative recovery is rapid. This procedure is suitable for compression fractures and currently stable fractures39. MIF involves a relatively large area and incision and requires the insertion of internal fixation screws40. The damage degree is greater, and the recovery is slower. This procedure suitable for treating fractures with severe vertebral collapse38. The ODF is the most damaged, has the slowest recovery, and is suitable for burst fractures or patients with spinal cord compression39,41,42,43. Surgeons choose the most beneficial operation for patients according to the degree of vertebral destruction and the patient’s physical situation. Among the 197 patients accurately diagnosed using synthetic MRI in the present study, 193 received the same treatment strategies as those used in the real-world study. Based on the results of this study, our synthetic MRI does not affect the diagnosis or treatment strategy of LF patients.
This was a large-sample study, starting from a clinical question and ultimately contributing to clinical practice. Our study has several limitations. First, CT scans were obtained from three different machines with various imaging styles. Despite our best efforts to standardise the data, unavoidable systematic error may have been introduced. Second, only the MR T2-weighted sequence was included, but in certain cases, it is challenging to distinguish between acute and sub-acute fractures without the support of T1 or fat-suppressed sequences, T1 and T2 with fat suppression will be investigated in future research.
Conclusion
In this study, we developed a disease-specific GAN with better image generation performance than the traditional GAN. The spine surgeon can correctly diagnose LF and select a treatment strategy based on Self-pix-generated T2 MR images. The PSNR may be unsuitable for assessing the similarity between synthetic MRI and original MRI in LF.
Data availability
All data generated or analyzed during this study were deposited at correspondence author’s ___location.
Abbreviations
- LF:
-
Lumbar fracture
- SF:
-
Sub-acute fracture
- AF:
-
Acute fracture
- CT:
-
Computerized tomography
- MRI:
-
Magnetic resonance image
- DL:
-
Deep learning
- DR:
-
Digital radiography
- GAN:
-
Generative adversarial network
- NLF:
-
Non lumbar fracture
- LFP:
-
Lumbar fracture participant
- NLFP:
-
Non lumbar fracture participant
- Mul:
-
Multiple fracture
- NF:
-
No fracture
- ADW:
-
Advantage workstation
- MSE:
-
Mean square error
- PSNR:
-
Peak signal-to-no ratio
- SSIM:
-
Structural similarity
- NAF:
-
No acute fracture
- ConT:
-
Conservative treatment
- PKP/PVP:
-
Percutaneous vertebroplasty/percutaneous kyphoplasty
- MIF:
-
Minimally invasive fixation
- ODF:
-
Open decompression and fixation
- Resnet:
-
Residual network
- PatchGAN:
-
Markovian discriminator
- cGAN:
-
Conditional GAN
- ROI:
-
Region of interest
- R-MRI:
-
Real-MRI
- S-MRI:
-
Synthetic-MRI
- BMI:
-
Body mass index
References
Alsoof, D. et al. Diagnosis and management of vertebral compression fracture. Am. J. Med. 135(7), 815–821 (2022).
Jin, C., Xu, G., Weng, D., Xie, M. & Qian, Y. Impact of magnetic resonance imaging on treatment-related decision making for osteoporotic vertebral compression fracture: A prospective randomized trial. Med. Sci. Monit. 24, 50–57 (2018).
Cunningham, C. & Mc Laughlin, H. Physiotherapy post vertebral fragility fracture: A scoping review. Physiotherapy 119, 100–116 (2023).
Wood, K. B., Li, W., Lebl, D. R. & Ploumis, A. Management of thoracolumbar spine fractures. Spine J. 14(1), 145–164 (2014).
Lenski, M., Büser, N. & Scherer, M. Concomitant and previous osteoporotic vertebral fractures. Acta Orthop. 88(2), 192–197 (2017).
Kato, S. et al. Does surgical intervention or timing of surgery have an effect on neurological recovery in the setting of a thoracolumbar burst fracture?. J. Orthop. Trauma 31(Suppl 4), S38-s43 (2017).
Hallinan, J. et al. Deep learning model for automated detection and classification of central canal, lateral recess, and neural foraminal stenosis at lumbar spine MRI. Radiology 300(1), 130–138 (2021).
Wu, W., Pan, J., Wang, Y., Wang, S. & Zhang, J. Multi-channel optimization generative model for stable ultra-sparse-view CT reconstruction. IEEE Trans. Med. Imag. https://doi.org/10.1109/TMI.2024.3376414 (2024).
Zhao, M., Liu, X., Liu, H. & Wong, K. K. L. Super-resolution of cardiac magnetic resonance images using laplacian pyramid based on generative adversarial networks. Comput. Med. Imaging Graph. 80, 101698 (2020).
Xiao, Y. et al. A novel hybrid generative adversarial network for CT and MRI super-resolution reconstruction. Phys. Med. Biol. 68(13), 135007 (2023).
Gao, Y. et al. 3DSRNet: 3D spine reconstruction network using 2D orthogonal X-ray images based on deep learning. IEEE Trans. Instrum. Meas. https://doi.org/10.1109/TIM.2023.3296838 (2023).
Qiu, S. et al. Multimodal deep learning for Alzheimer’s disease dementia assessment. Nat. Commun. 13(1), 3404 (2022).
Jiang, B. et al. Deep learning reconstruction shows better lung nodule detection for ultra-low-dose chest CT. Radiology 303(1), 202–212 (2022).
Lyu, J. et al. Generative adversarial network-based noncontrast CT angiography for aorta and carotid arteries. Radiology 309(2), e230681 (2023).
Goodfellow, I., Pouget-Abadie, J., Mirza, M et al. Generative adversarial nets. 2014; 27.
Halupka, K. J. et al. Retinal optical coherence tomography image enhancement via deep learning. Biomed. Opt. Express. 9(12), 6205–6221 (2018).
Ma, Y. et al. Structure and illumination constrained GAN for medical image enhancement. IEEE Trans. Med. Imaging 40(12), 3955–3967 (2021).
Zhou, H., Ma, J., Tan, C. C., Zhang, Y. & Ling, H. Cross-weather image alignment via latent generative model with intensity consistency. IEEE Trans. Image Process. 29, 5216 (2020).
Huang, S., Sun, L., Yousefnezhad, M., Wang, M. & Zhang, D. Functional alignment-auxiliary generative adversarial network-based visual stimuli reconstruction via multi-subject fMRI. IEEE Trans. Neural Syst. Rehabil. Eng. 31, 2715–2725 (2023).
Touati, R., Le, W. T. & Kadoury, S. A feature invariant generative adversarial network for head and neck MRI/CT image synthesis. Phys. Med. Biol. 66(9), 095001 (2021).
Chen, W. et al. A deep-learning model for identifying acute vertebral compression fractures on digital radiography. Eur. Radiol. 32(3), 1496–1505 (2022).
Vaswani, A., Shazeer, N., Parmar, N., et al. Attention is all you need. In Proceedings of the 31st International Conference on Neural Information Processing Systems. Long Beach, California, USA: Curran Associates Inc.; 2017:6000–6010.
He, K., Zhang, X., Ren, S., Sun, J. Deep residual learning for image recognition. In Paper presented at: 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 27–30 June 2016, 2016:770–778.
Zhu, J.Y., Park, T., Isola, P., Efros, A.A. Unpaired image-to-image translation using cycle-consistent adversarial networks. In Paper presented at: 2017 IEEE International Conference on Computer Vision (ICCV); 22–29 Oct. 2017, 2017:2242–2251.
Isola, P., Zhu, J.Y., Zhou, T., Efros, A.A. Image-to-image translation with conditional adversarial networks. In Paper presented at: 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 21–26 July 2017, 2017:5967–5976.
Ronneberger, O. & Fischer, P. Brox T Convolutional Networks for Biomedical Image Segmentation 234–241 (Springer, 2015).
Mirza, M., Osindero, S. Conditional Generative Adversarial Nets.
Parmar, N., Vaswani, A., Uszkoreit, J., et al. Image Transformer. In Paper presented at the International Conference on Machine Learning, Stockholm, Sweden, July 10–15, 2018. Proceedings of Machine Learning Research; 80:4055–4064.
Zhang, H., Goodfellow, I., Metaxas, D. & Odena, A. Self-attention generative adversarial networks. Int. Conf. Mach. Learn. 97, 7354–7363 (2019).
Miyato, T., Kataoka, T., Koyama, M., & Yoshida, Y. Spectral normalization for generative adversarial networks. In Proceedings of the 6th International Conference on Learning Representations (ICLR), Vancouver, Canada, April 30–May 3, 2018.
Chow, L. S. Paramesran RJBsp, control. Rev. Med. Image Qual. Assess. 27, 145–154 (2016).
Duy, P. Q., Ikuta, I., Johnson, M. H., Davis, M. & Zohrabian, V. M. MRI in Spine Trauma. In MRI of the Spine: A guide for orthopedic surgeons (eds Morrison, W. B. et al.) 31–86 (Springer, 2020).
Huang, J. et al. Spine explorer: A deep learning based fully automated program for efficient and reliable quantifications of the vertebrae and discs on sagittal lumbar spine MR images. Spine J. 20(4), 590–599 (2020).
Hong, K. T. et al. Lumbar spine computed tomography to magnetic resonance imaging synthesis using generative adversarial network: Visual turing test. Diagnostics 12(2), 530 (2022).
Li, Y. C. et al. Can a deep-learning model for the automated detection of vertebral fractures approach the performance level of human subspecialists?. Clin. Orthop. Relat. Res. 479(7), 1598–1612 (2021).
Muratore, M., Ferrera, A., Masse, A. & Bistolfi, A. Can we predict the progression of vertebral collapse in conservative treatment of osteoporotic vertebral fractures? A 3-year retrospective study of 180 patients from the emergency department. Int. J. Spine Surg. 14(4), 641–648 (2020).
Spiegl, U. J. et al. The conservative treatment of traumatic thoracolumbar vertebral fractures. Dtsch. Arztebl. Int. 115(42), 697–704 (2018).
Zhu, D. et al. A comparison between modified unilateral extrapedicular and bilateral transpedicular percutaneous kyphoplasty in the treatment of lumbar osteoporotic vertebral compression fracture. World Neurosurg. 166, e99–e108 (2022).
Drazin, D. et al. Outcomes and national trends for the surgical treatment of lumbar spine trauma. Biomed. Res. Int. 2016, 3623875 (2016).
Caruso, G. et al. Minimally invasive fixation techniques for thoracolumbar fractures: Comparison between percutaneous pedicle screw with intermediate screw (PPSIS) and percutaneous pedicle screw with kyphoplasty (PPSK). Eur. J. Orthop. Surg. Traumatol. 28(5), 849–858 (2018).
Zeng, Z., Zhang, D., Zeng, F. L. & Ao, J. Posterior unilateral small fenestration of lamina combined with a custom-made Y-shaped fracture reduction device for the treatment of severe thoracolumbar burst fracture: A prospective comparative study. J. Orthop. Surg. Res. 18(1), 529 (2023).
Pan, J. et al. Iterative residual optimization network for limited-angle tomographic reconstruction. IEEE Trans. Image Process. 33, 910–925 (2024).
Wu, W., Wang, Y., Liu, Q., Wang, G. & Zhang, J. Wavelet-improved score-based generative model for medical imaging. IEEE Trans. Med. Imag. 43(3), 966–979 (2024).
Acknowledgements
Thank Yong Zong and Song Chen for qualitative assessment of all images in the study.
Funding
The study was supported by National Natural Science Foundation of Hubei province (2023AFB1006) and Hubei Provincial Health Commission Young Talent Programme (WJ2023Q020).
Author information
Authors and Affiliations
Contributions
The authors report no conflict of interest concerning the materials or methods used in this study or findings specified in this paper. Author contributions to the study and manuscript preparation include the following. Conception and design: WF W. clinical assessment: WF W, K S, B L, ML Z, J L. Neural network development: WD Z, ZX Z,TL T. Data measurement and analysis: K S,WD Z,ZX Z. Drafting the article: K S,WD Z,ZX Z,B L,ML Z ,TL T ,J L,WF W. Critically revising the article: WF W. Reviewed final version of the manuscript and approved it for submission: all authors. Study supervision: WF W. The authors report no conflict of interest concerning the materials or methods used in this study or findings specified in this paper. The authors have reviewed the final version of the manuscript and approve it for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The present study was approved by the review boards of the Three Gorges University.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, K., Zhu, W., Zhang, Z. et al. Synthetic lumbar MRI can aid in diagnosis and treatment strategies based on self-pix networks. Sci Rep 14, 20382 (2024). https://doi.org/10.1038/s41598-024-71288-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71288-4