Abstract
This single-arm multi-institutional prospective study aimed to evaluate the 10-year outcomes of sublobar resection for small-sized ground-glass opacity-dominant lung cancer. Among 73 patients prospectively enrolled from 13 institutions between November 2006 and April 2012, 53 ground-glass opacity-dominant lung cancer patients underwent sublobar resection with wedge resection as the first choice. The inclusion criteria were maximum tumor size of 8–20 mm; ≥ 80% ground-glass opacity ratio on high-resolution computed tomography; lower 18F-fluorodeoxyglucose accumulation than the mediastinum; intraoperative pathological diagnosis of adenocarcinoma in situ; and no cancer cells on intraoperative cut margins. The primary endpoint was a 10-year disease-specific survival. The 53 eligible patients had a mean tumor size of 14 ± 3.4 mm and a mean ground-glass opacity ratio of 95.9 ± 7.2%. Wedge resection and segmentectomy were performed in 39 and 14 patients, respectively. The final pathological diagnoses were adenocarcinoma in situ in 47 patients (88.7%) and adenocarcinoma with mixed subtype in 6 patients (11.3%). The 10-year disease-specific survival and overall survival were 100% and 96.2%, respectively, during a median follow-up period of 120 months (range, 37–162 months). Ground-glass opacity-dominant small lung cancer is cured by sublobar resection when patients are strictly selected by the inclusion criteria of this study.
Similar content being viewed by others
Introduction
The early detection rate of small-sized lung nodules with ground-glass opacity (GGO) has recently increased owing to the widespread use of high-resolution (HR) computed tomography (CT)1. Most of these nodules are known to be early-stage lung cancer. Among these, GGO-dominant peripheral small lung nodules were shown to have a favorable prognosis after lobectomy2 and the feasibility of sublobar resections including wedge resection for such nodules had been pursued even before the publication of the results of a nation-wide single-arm trial to evaluate the efficacy of sublobar resection with wedge resection as the first choice (JCOG0804/WJOG4506L) in 20223.
We have previously reported excellent 5-year disease-specific survival (DSS) in a single-arm multi-institutional prospective study evaluating the validity of sublobar resection for small-sized GGO-dominant pulmonary adenocarcinoma (JNETS 0601)4. To our knowledge, this was the first multi-institutional prospective study to have shown a 5-year outcome of sublobar resection with wedge resection as the first choice for GGO-dominant pulmonary adenocarcinoma. Based on the background at that time when the validity of sublobar resection was not well established, the inclusion criteria were defined very strictly and included the following: a maximum tumor diameter (MTD) of 8–20 mm, GGO ratio of ≥ 80%, clinical T1N0M0 (UICC TNM classification, 6th edition), 18F-fluorodeoxy- glucose (FDG) accumulation lower than that in the mediastinum in positron emission tomography imaging (PET), bronchioalveolar carcinoma (adenocarcinoma in situ [AIS] in the present 5th revised WHO classification) in the intraoperative pathological examination, and an intraoperative cytological/ histological examination of the surgical margin negative. This study finally included 53 patients who met these criteria and no recurrence of the original lung cancer was found during an average observation period of 72.8 months (60.0–126.3 months) after surgery. The 5-year DSS and overall survival (OS) were 100% and 98.1%, respectively. However, GGO-dominant adenocarcinoma is usually slow-growing and recurrences of this type of lung cancer were reported during a long-term follow-up after sublobar resection5,6. Therefore, to evaluate the feasibility of sublobar resections for such adenocarcinoma, long-term outcomes over 5 years must be verified.
We conducted the present study to evaluate the 10-year outcomes of patients who underwent sublobar resection (wedge resection as the first choice) for GGO-dominant small-sized lung cancers using the same cohort as in the previous study in which 100% of DSS was shown at the 5-year follow-up4.
Patients and methods
Study settings
The original study was a single-arm, multi-institutional prospective trial, and the results at the 5-year follow-up were already reported (JNETS 0601)4. The present study was another phase II trial to reveal the outcome of a 10-year follow-up with the same cohort as the original study. The study protocol was newly approved by the Institutional Review Board of the Faculty of Medicine, Yamagata University in 2020, followed by the approval by the Institutional Ethics Committees of all 12 participating institutions. The trial information was registered in the University Hospital Medical Information Network Clinical Trial Registration System, Japan (registration number: UMIN000046487; Date of registration: 27 December 2021). Details are available at https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000052889. Written informed consent was newly given by patients before the updated follow-up data were registered. For deceased patients and those whose follow-up had been terminated, the requirement for informed consent was waived by approval by the Institutional Review Board of the Faculty of Medicine, Yamagata University with the opt-out documents being posted.
Inclusion criteria
The inclusion criteria, surgical method, and definition of recurrence have been reported previously4. Briefly, the inclusion criteria of GGO lesions consisted of (1) adenocarcinoma or suspected adenocarcinoma; (2) MTD of 8–20 mm and GGO ratio of ≥ 80% (Fig. 1); (3) clinical T1N0M0 (UICC TNM classification, 6th edition); (4) FDG accumulation lower than that in the mediastinum on PET; (5) complete resection of the tumor possible with sublobar resection; (6) AIS on intraoperative pathological examination; (7) intraoperative cytological/ histological examination of the surgical margin negative. Regarding the procedures of the sublobar resections, wedge resection was basically performed, but segmentectomy was accepted when a sufficient margin (at least 10 mm) was not secured with wedge resection.
Follow-up, recurrence and pathological examination
The patients were followed-up at least once a year at the local hospital and underwent HR-CT examination every 1 or 2 years. Secondary primary lung cancer was defined according to the modification of the definition by Cortese et al.7; (i) the subsequent tumor had a different histological type from the original cancer, or (ii) the subsequent tumor had a similar histological type to the original cancer but was a carcinoma in situ or a cancer without any extra-thoracic metastases, and no carcinoma was found in the lymphatics in common with both cancers. Otherwise, the subsequent tumor was regarded as a recurrence of the original cancer.
Since this study began to enroll patients from 2006, the pathological classification of adenocarcinomas was in accordance with the WHO classification of 2004. Bronchioloalveolar carcinomas in the present study are equivalent to AISs, and adenocarcinomas with mixed subtype correspond to minimally invasive adenocarcinoma or invasive adenocarcinoma in the present 5th revised WHO classification, respectively2,8. The former was referred to AIS and the latter was expressed as adenocarcinomas with mixed subtype in this manuscript.
Endpoints
The primary endpoint of the present study was a 10-year DSS. The secondary endpoints were recurrence rates, the mode of recurrence and the OS.
Statistical analyses
Data were analyzed using JMP version 16.1.0 (SAS Institute Inc., Cary, NC, USA) and expressed as mean ± standard error of the mean. DSS and OS were estimated using the Kaplan–Meier method. The durations of DSS and OS were measured from the date of the surgery to the date of lung cancer death, the date of death due to any cause, or the last follow-up, respectively.
Results
Patient characteristics
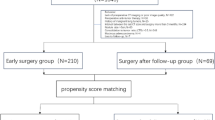
From November 2006 to April 2012, a total of 73 patients were prospectively enrolled from 13 institutions. The patient selection process is shown in Fig. 2. Among 73 patients enrolled, 53 patients were eventually judged eligible for inclusion in this study (Table 1). The mean age was 61.7 ± 10.8 years (range, 26–79 years); the mean tumor size was 14 ± 3.4 mm (8–9 mm in 4 patients, 10–14 mm in 26 patients, and 15–20 mm in 23 patients); the mean GGO ratio was 95.9 ± 7.2% (80–89% in 12 patients, 90–99% in 2 patients, and 100% in 39 patients). Wedge resection and segmentectomy were performed in 39 and 14 patients, respectively. Five open thoracotomies and 48 thoracoscopic surgeries were performed. Intraoperative evaluation of the cut margins was done by cytology and histology in 45 and 8 patients, respectively. The cut margin in 1 patient was positive for cancer cells. The patient underwent another wedge resection with a re-evaluation of the cut margin negative, and the patient was considered eligible. The final pathological diagnoses were AIS in 47 patients (88.7%) and adenocarcinomas with mixed subtype in 6 patients (11.3%), respectively. All the patients were staged as pT1N0M0-0, Stage I (UICC TNM classification, 6th edition). A completion lobectomy was not performed in any of the patients.
On the other hand, the final pathological diagnosis of 14 patients who were excluded from this study due to an intraoperative diagnosis of adenocarcinomas with mixed subtype was adenocarcinomas with mixed subtype in 12 patients, while that in 2 patients was AIS. Five patients underwent lobectomy and nine received wedge resection as a clinical practice based on the surgeon’s decision. Twelve patients were alive without recurrences during the 10-year follow-up period, and 2 patients died of other diseases (heart failure in one patient, and malignant lymphoma in the other patient).
Long-term outcomes after sublobar resection
Five patients were lost to follow-up during the period from 5 and 10 years, and 46 patients completed the follow-up for more than 10 years (assessment completion rate: 86.8%). Two deaths were recorded during the follow-up period. One patient died of bladder cancer 37 months after pulmonary resection. Another patient died of ileus 69 months after the surgery. No recurrence of lung cancer was found in these 2 patients. Another patient was diagnosed with bladder cancer 23 months and liver cancer 103 months after pulmonary resection but was alive at 121 months without recurrence of lung cancer. Four other patients suffered from another lung cancer, which was diagnosed as metachronous primary lung cancer according to the criteria of the protocol of this study. Three of them showed contralateral or ipsilateral solitary pulmonary adenocarcinoma, and these patients underwent pulmonary resection (2 patients underwent segmentectomy, 1 patient underwent wedge resection), again. The fourth patient had squamous cell carcinoma on the ipsilateral lung 76 months after the surgery. The patient underwent radiotherapy and was alive 162 months after the surgery in a second lung cancer-bearing state.
The median length of follow-up for all cases was 120 months (range, 37–162 months) and that for censored cases was also 120 months (range, 85–162 months). No recurrence was observed during the study period and the 10-year DSS was 100%. The 10-year OS was 96.2% (Fig. 3).
Discussion
The present study confirmed the validity of sublobar resection (wedge resection as the first choice) for GGO-dominant small lung adenocarcinoma with a 10-year follow-up. No recurrence was found in patients who underwent sublobar resection when they were selected with our strict criteria, which included MTD of 8–20 mm, GGO ratio of ≥ 80%, FDG accumulation lower than that in the mediastinum, AIS on intraoperative pathological examination, intraoperative cytological/histological examination of the surgical margin negative.
Recently, a couple of prospective multi-institutional studies have reported favorable 10-year outcomes with sublobar resection for GGO-dominant small-sized lung cancer9,10. The JCOG0804/WJOG4507L trial enrolled patients who had tumors with a MTD of 2 cm or less and with a consolidation tumor ratio (CTR) of 0.25 or less in the thin-section CT. They demonstrated that the 10-year relapse-free survival (RFS) and OS in 314 patients who underwent sublobar resections (258 wedge resections and 56 segmentectomies) were 98.6% and 98.5%, respectively. There was one local recurrence at the resection margin9. The other study from the National Cancer Center Hospital East and Kanagawa Cancer Center enrolled 100 patients who had lung adenocarcinoma with a MTD of ≤ 2 cm and tumor disappearance ratio (TDR) of ≥ 0.510. The TDR was defined as 1–the MTD measured in the mediastinal setting/ the MTD measured in the lung setting on HR-CT. Wedge resection was performed in 87 patients, segmentectomy in 9 patients and 4 cases were converted to lobectomy. The 10-year RFS and OS in 90 lung cancer patients who underwent sublobar resection were both 96.0%. Two patients experienced recurrence at resected ends > 5 years after wedge resection. The authors of these 2 studies concluded sublobar resections with their inclusion criteria to be acceptable, although the recurrence events at the resection margin must be taken seriously. They also emphasized that patients who undergo limited resection for radiologically non-invasive lung cancer should be followed up for > 5 years after surgery.
The pre-operative inclusion criteria employed in above mentioned 2 prospective studies and ours were different one another. Although all studies enrolled patients with MTD ≤ 20 mm, the JCOG0804/WJOG4507L study included patients with CTR ≤ 0.25 based on the result of a prospective observational study, JCOG020111, which defined radiological non-invasive lung cancer as having an MTD ≤ 2 cm and a CTR ≤ 0.25. Using CTR alone to identify non-invasive lung adenocarcinoma indicated for wedge resection is a simple method suitable for widespread clinical application. The study conducted by the National Cancer Center Hospital East and Kanagawa Cancer Center defined radiologically less-invasive lung cancers as a TDR ≥ 0.5. TDR may also be a simple method to identify candidates for wedge resection with a low inter-observer divergence. This inclusion criterion was based on the background in which consolidation component in the mediastinal setting on HR-CT corresponded to non-lepidic components and correlated with the tumor malignancy10,11. On the other hand, our study incorporated “FDG accumulation lower than that in the mediastinum on PET” into the inclusion criteria, which was one of the unique points in our study. FDG accumulation on PET had been shown to be correlated well with the invasive ability of the tumor even in small-sized adenocarcinomas12,13, and therefore we considered that the combination of the GGO ratio and FDG accumulation may accurately identify good candidates for wedge resection. As for CTR, our study employed a GGO ratio of ≥ 80%, i.e. CTR ≤ 0.2, which was a little stricter than that in the JCOG0804/WJOG4507L.
Our study protocol also required intraoperative cytological/ histological assessments of the resected margins to be negative and the intraoperative pathological diagnosis to be AIS for patient inclusion. In our study, the cut margin in one patient was positive for cancer cells. The patient underwent additional wedge resection with a re-evaluation of the cut margin as negative, and the patient was included in the study and showed no recurrence during the follow-up period. The efficacy of the intraoperative cut-end evaluation in sublobar resections has been documented. Miyoshi et al.14 evaluated intraoperative lavage cytology of autostapling cartridges in 262 consecutive patients undergoing wedge or segmental resection for 311 lesions with primary lung cancer or pulmonary metastatic tumors, resulting in 22 (7%) positive cytology lesions found. They showed that recurrence at the margin developed in 2 of the 19 lesions which underwent additional resection (11%), while recurrence at the margin developed in 2 (67%) of the 3 lesions which did not undergo additional resection. However, when the subjects were restricted to the GGO-dominant peripheral small lung nodules, the incidence of cut-end recurrence was very low as shown in the JCOG0804/WJOG4507L trial10, in which the intraoperative cut margin assessment was required only for a macroscopically positive margin or a surgical margin less than 5 mm. Therefore, further studies would be needed to determine the feasibility and significance of the routine intraoperative cut-margin evaluation in sublobar resections for GGO-dominant peripheral small lung cancers.
The requirement of an intraoperative pathological examination was another unique characteristic of our study. Of 53 patients whose intraoperative pathological diagnosis was AIS, 6 patients (11.3%) had a final pathological diagnosis of adenocarcinomas with mixed subtype. The frozen-section accuracy was worse in our study compared with that of the previous single center study (98%) reported by Yoshida, et al.15, but no recurrence was found in our 6 patients without completion lobectomy during the 10-year follow up. In addition, two of the 14 patients who were excluded from this study due to an intraoperative diagnosis of adenocarcinomas with mixed subtype had a final pathological diagnosis of AIS. Further studies would be desirable to determine the reliability and necessity of intraoperative pathological examination for sublobar resection of GGO-dominant lung cancer.
Patients with GGO components sometimes experience synchronous or metachronous lung cancer. Among the patients enrolled in our study, four patients had secondary lung cancers during the follow-up period of 10 years. Three patients survived after the second sublobar resection. The fourth patient was diagnosed with squamous cell carcinoma with a transbronchial biopsy and was alive after the radiotherapy.
Whether GGO-dominant small lung adenocarcinoma should be resected is a matter of concern. Some of these tumors may grow in MTD and/ or CTR during a follow-up period, while some may remain with the same radiographic image for a long period. A multi-institutional, single-arm confirmatory trial to evaluate the efficacy and safety of watchful waiting for patients with radiologically non-invasive lung cancer (JCOG1906) began to enroll patients in 2020, in which patients having pulmonary nodules with a MTD ≤ 2 cm and a CTR ≤ 0.25 are to be registered16. The primary endpoint of this trial is 10-year survival.
We acknowledge several limitations in this study. Although this was a multi-institutional prospective study, the sample size was small. Five of 53 patients were lost to follow-up during the period from 5 to 10 years after surgery. The radiologic evaluation with CTR may vary among observers and CT scanning conditions.
Conclusion
GGO-dominant small-sized lung cancer can be cured by sublobar resection (wedge resection as the first choice) when patients are strictly selected by CTR on HR-CT, FDG-PET, negative intraoperative cytological/ histological assessments of the resected margins and the intraoperative pathological diagnosis of AIS by the frozen section.
Data availability
The data support the findings of this study are available on request from the corresponding author on reasonable request.
References
Kudo, Y. et al. Association between high-resolution computed tomography findings and the IASLC/ATS/ERS classification of small lung adenocarcinomas in Japanese patients. Lung Cancer 90, 47–54 (2015).
Noguchi, M. et al. Small adenocarcinoma of the lung Histologic characteristics and prognosis. Cancer 75, 2844–2852 (1995).
Suzuki, K. et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J. Thorac. Cardiovasc. Surg. 163, 289-301.e2 (2022).
Sagawa, M. et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur. J. Cardiothorac. Surg. 53, 849–856 (2018).
Yoshida, J. et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J. Thorac. Oncol. 5, 546–550 (2010).
Nakao, M. et al. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J. Thorac. Oncol. 7, 1563–1566 (2012).
Cortese, D. A. et al. Roentgenographically occult lung cancer. A ten-year experience. J. Thorac. Cardiovasc. Surg. 86, 373–380 (1983).
Travis, W. D. et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 6, 244–285 (2011).
Yoshino, I. et al. Long-term outcome of patients with peripheral ground-glass opacity-dominant lung cancer after sublobar resections. J. Thorac. Cardiovasc. Surg. 166, 1222-1231.e1 (2023).
Niimi, T. et al. Ten-year follow-up outcomes of limited resection trial for radiologically less-invasive lung cancer. Jpn. J. Clin. Oncol. https://doi.org/10.1093/jjco/hyad187 (2024).
Suzuki, K. et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J. Thorac. Oncol. 6, 751–756 (2011).
Yoshioka, M. & Ichiguchi, O. Selection of sublobar resection for c-stage IA non-small cell lung cancer based on a combination of structural imaging by CT and functional imaging by FDG PET. Ann. Thorac. Cardiovasc. Surg. 15, 82–88 (2009).
Kagimoto, A. et al. Patient selection of sublobar resection using visual evaluation of positron-emission tomography (PET) for early-stage lung adenocarcinoma. Ann. Surg. Oncol. 28, 2068–2075 (2021).
Miyoshi, T. et al. Stapling cartridge lavage cytology in limited resection for pulmonary malignant tumors: Assessment of cytological status of the surgical margin. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e1240 (2019).
Yoshida, J. et al. Limited resection trial for pulmonary ground-glass opacity nodules: Fifty-case experience. J. Thorac. Cardiovasc. Surg. 129, 991–996 (2005).
Miyoshi, T. et al. Prospective evaluation of watchful waiting for early-stage lung cancer with ground-glass opacity: A single-arm confirmatory multicenter study: Japan Clinical Oncology Group study JCOG1906 (Evergreen study). Jpn. J. Clin. Oncol. 51, 1330–1333 (2021).
Acknowledgements
We thank Mrs. Yuko Ito for managing the case report forms at the data center; Dr. Hiroyuki Oizumi, and Dr. Akira Sakurada for their assistance with the study protocol; and Dr. Jun Suzuki for reporting the follow-up patient data. We also thank Mr. Brent Bell for assistance in English language editing.
Funding
The authors received no funding for this study.
Author information
Authors and Affiliations
Contributions
H.K. and Y.O. wrote the main manuscript text and H.K. prepared Figs. 1, 2, 3. S.S. revised the manuscript for important intellectual content. H.S., H.U., J.A., S.M., T.H, H.D., M.E., N.S., M.A., and J.S., recruited patients and collected the data. M.S. and N.H. assisted designing the study protocol. All authors recruited patients, collected the data, and contributed to data interpretation, review and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kato, H., Shiono, S., Suzuki, H. et al. A prospective 10-year follow-up study after sublobar resection for ground-glass opacity-dominant lung cancer. Sci Rep 14, 21243 (2024). https://doi.org/10.1038/s41598-024-72248-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72248-8