Abstract
In sweet potato, rational nitrogen (N) assimilation and distribution are conducive to inhibiting vine overgrowth. Nitrate (NO3-) is the main N form absorbed by roots, and cultivar is an important factor affecting N utilization. Herein, a hydroponic experiment was conducted that included four NO3- concentrations of 0 (N0), 4 (N1), 8 (N2) and 16 (N3) mmol L-1 with two cultivars of Jishu26 (J26, N-sensitive) and Xushu32 (X32, N-tolerant). For J26, with increasing NO3- concentrations, the root length and root surface area significantly decreased. However, no significant differences were observed in these parameters for X32. Higher NO3- concentrations upregulated the expression levels of the genes that encode nitrate reductase (NR2), nitrite reductase (NiR2) and nitrate transporter (NRT1.1) in roots for both cultivars. The trends in the activities of NR and NiR were subject to regulation of NR2 and NiR2 transcription, respectively. For both cultivars, N2 increased the N accumulated in leaves, growth points and roots. For J26, N3 further increased the N accumulation in these organs. Under higher NO3- nutrition, compared with X32, J26 exhibited higher expression levels of the NiR2, NR2 and NRT1.1 genes, a higher influx NO3- rate in roots, and higher activities of NR and NiR in leaves and roots. Conclusively, the regulated effects of NO3- supplies on root growth and NO3- utilization were more significant for J26. Under high NO3- conditions, J26 exhibited higher capacities of NO3- absorption and distributed more N in leaves and in growth points, which may contribute to higher growth potential in shoots and more easily cause vine overgrowth.
Similar content being viewed by others
Introduction
The planting area and production of sweet potato in China rank first worldwide1. Nitrogen (N) is one of the essential nutrient elements in plant growth. In sweet potatoes, N plays crucial roles in dry matter accumulation, N absorption, and storage root formation and expansion2,3,4. Associations between N levels and the N uptake, yield, and quality of sweet potato have been reported5,6. Villagarcia et al.7 showed that sweet potato presents genotypic differences in N uptake and assimilation. Long vine cultivars with rapid growth of aboveground parts exhibited higher N metabolism levels in leaves8. Kelm et al.9 showed that the translocation efficiency of dry matter and N to storage roots is lower in cultivars with weak growth potential than in those with strong growth potential. Compared with early-maturity cultivars, cultivars with longer growth periods have higher N recovery efficiency and N physiological efficiency10. The N-tolerant cultivars showed higher root yields than N-sensitive cultivars under high N conditions11. Thus, cultivar is an important factor affecting yield and N absorption and assimilation. However, N utilization in sweet potato cultivars with different N tolerances has rarely been reported.
As the main inorganic N, nitrate (NO3-) affects crop yield and photosynthetic physiology and has a significant impact on plant root growth and nutrient absorption12,13,14. After absorbing NO3- in plants, one part is assimilated in roots, and the other part is transported to the aboveground parts through the xylem to participate in the assimilation reaction. Nitrate reductase (NR) is an NO3- inducible enzyme, which is the first step of its participation in assimilation; that is, NO3- is reduced to NO2- under the action of NR. The generated NO2- is reduced to ammonia under the action of nitrite reductase (NiR)15,16. Numerous genes, such as nitrate transporters (NRT), NR and NiR, are involved in NO3- sensing and signaling networks17,18. In sweet potatoes, NRT and NR genes are involved in N metabolism and are regulated by N concentrations19. Root morphology is closely related to N absorption. NO3- can affect root growth and its absorption rate20. Previous researchers have reported the relationships between root growth and N absorption, such as the root growth response of different cultivars to N levels and the impact of N management on root morphology21,22. However, there are few reports on the effects of NO3- supplies on early plant growth and N absorption in sweet potato cultivars with different N tolerances. The relationship between root morphology and NO3- absorption in sweet potato seedlings also needs to be studied.
In actual sweet potato production in northern China, due to unreasonable N application, vine overgrowth easily occurs even in hilly regions, which results in yield reduction11. The rational N distribution between aboveground parts and roots is conducive to source-sink coordination and inhibition of vine overgrowth in sweet potato23. NO3- is the main form of N uptake by crops, and its distribution between aboveground and underground parts in crops depends on the cultivar, external NO3- concentration, and root growth24,25. However, in sweet potatoes, the information on the physiological and molecular mechanisms involved in NO3- absorption and assimilation of cultivars with different N tolerances is limited. In previous studies, we found that the N accumulation in the N-sensitive Jishu 26 (J26) was higher than that in the N-tolerant cultivar Xushu 32 (X32) under similar N conditions supplied by urea. High N nutrition led to a greater increase in N accumulation in the fibrous roots of J264. In the present study, the seedlings of these two cultivars were cultured in modified Hoagland nutrient solution containing four NO3- concentrations. The following scientific hypotheses are proposed: (i) The N accumulation of the two cultivars under different nitrate supplies is related to their capacities for NO3- absorption and assimilation. (ii) Enzyme activities and the expression of genes such as NR, NiR and NRT1.1 in roots and leaves may vary between the two cultivars, thus affecting their influx rates of NO3- and NO3- assimilation. (iii) Root length and root surface area may also affect the influx rate of NO3-. Thus, in the present study, the responses of root growth, NO3- absorption and N distribution and the transcript levels of genes involved in NO3- assimilation were assessed. The results helped elucidate the differential mechanism of NO3- utilization in different cultivars and provide a theoretical reference for the mechanism of vine overgrowth in sweet potato.
Materials and methods
Experimental design
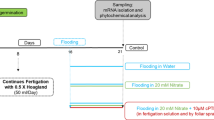
Hydroponic experiments were conducted in an artificial climate room at Shandong Academy of Agricultural Sciences, China (36°7′ N, 118°2′ E), in 2021. Two sweet potato cultivars, Jishu 26 (J26, N-sensitive) and Xushu 32 (X32, N-tolerant)4,11, were selected for hydroponic culture using Hoagland’s modified solution. Four NO3- concentrations were set for each cultivar: 0 (N0), 4 (N1), 8 (N2) and 16 (N3) mmol L-1. In the nutrient solutions with different NO3- concentrations, NO3- was supplied by Ca(NO3)2 and KNO3. CaCl2 or KCl was added to maintain consistent concentrations across the treatments19. The NO3- concentration preparation method is shown in Supplementary Table S1. The concentration of other nutrient elements was referred to a modified Hoagland’s nutrient solution with the following chemical composition: 1 mmol L-1 KH2PO4, 2 mmol L-1 MgSO4, 1 μmol L-1 H3BO3, 0.74 μmol L-1 MnSO4, 0.5 μmol L-1 ZnSO4, 0.25 μmol L-1 CuSO4, 0.1 μmol L-1 Na2MoO4·2H2O, 0.025 μmol L-1 KI, and 50 μmol L-1 Na-Fe-EDTA13,19. The culture conditions were a day/night temperature of 28/22 °C and 70% relative humidity with a light intensity of 300 μmol m-2 s-1 for 16 h. A completely randomized experimental design consisting of two factors was used in this study. The first factor was the two different cultivars, and the second factor was the four different NO3- concentrations. For each replicate of each treatment, twelve consistent seedlings (shoots with five to six functional leaves, approximately 20 cm in height) were selected and transplanted into a plastic container (50 cm × 36.5 cm × 14.5 cm) with 15 L of nutrient mixture. During the process of culture, the nutrient mixture was changed every 3 days, and the treatments were arranged completely at random, with four replicates.
Variable measurements
Seedlings were collected at 10 days after culture. The shoots and roots were divided, and the fresh weight (FW) was measured. In each replicate of each treatment, four seedlings were randomly collected and divided into leaves, petioles, stems, growth points (the part above the third expanded leaf from the shoot apex) and roots for dry matter and N content analysis. Another four seedlings were collected, and their third expanded leaves and roots were frozen in liquid N and stored at -80 °C for physiological index and gene expression analyses. The remaining seedlings were used for the flux measurements of NO3- and root scanning analysis.
Root growth characteristics
Root growth was evaluated by image analysis. The roots were floated on waterproof trays and scanned using a specialized EPSON V750 scanner (Seiko Epson Corp., Suwa, Nagano, Japan) at a resolution of 300 dpi. WinRHIZO Pro software (Regent Instruments Inc., Quebec, Canada) was utilized to analyse root images, which provided the total root length, root surface area, average root diameter and root volume26.
N content and N accumulation
The plant samples divided into different organs were oven-dried to a constant weight at 70 °C to measure their biomass. The total N content was determined with a Kjeldahl apparatus (Kjeltec 8100, FOSS, Hoganas, Sweden)27. The N accumulation amount of each organ was calculated by multiplying the element content by the dry weight of each organ. The N distribution ratio of each organ was calculated by dividing the N accumulation amount of each organ by the total N accumulation amount of all organs.
Nitrate content and activities of nitrate reductase (NR) and nitrite reductase (NiR)
Leaves and root samples of 0.1 g were added to 1 mL of distilled water in a centrifuge tube and boiled for 30 min to extract the nitrate. The samples were centrifuged at 12,000 × g for 15 min, and 0.1 mL of supernatant was mixed with 0.4 mL of 5% salicylic acid-sulfuric acid solution and incubated at room temperature for 30 min. The 8% sodium hydroxide solution was added, and nitrate was detected at 410 nm at room temperature.
NR and NiR activities were determined in accordance with the method described by Sun et al.12 and Yu et al.28. In brief, leaf and root samples were homogenized in a cold mortar and pestle with extraction buffer. The homogenate was centrifuged at 10,000 × g for 20 min at 4 °C. The reaction mixture for determining NR activity contained extract, 25 mM potassium phosphate buffer (pH 7.5), 10 mM KNO3, 0.2 mM NADH and 5 mM NaHCO3. Controls were generated by adding potassium phosphate buffer instead of NADH. The assays were conducted at 30 °C for 15 min. The reaction was terminated by adding 0.5 M zinc acetate, and excess NADH was oxidized by adding 0.15 mM phenazine methosulfate. The mixture was centrifuged at 10,000 × g for 5 min. The NO2- level was colorimetrically measured at 540 nm after the addition of 1% sulfanilamide in 2 M HCl and 0.02% N-(1-naphthyl)-ethylene-diammonium dichloride (NNEDD). The assay mixture for NiR activity contained extract, 25 mM potassium phosphate buffer (pH 7.5), 0.5 mM NaNO2, and 1 mM methylviologen. The reaction was initiated by adding 0.12 M Na2S2O4 and incubated at 25 °C for 60 min. The reaction was terminated by violently agitating the mixture in a vortex mixer. After 1 M zinc acetate was added, the mixture was centrifuged at 10,000 × g for 10 min. The NO2- level in the supernatant was determined at 540 nm after diazotation was performed using 1% sulfanylamide and 0.02% NNEDD.
Flux measurements of NO3 -
The NO3- flow rate in roots was measured using a noninvasive microtest system (NMT 100 Series, USA) at Jiangsu Normal University, Jiangsu, China28. Under the microscope, the corresponding electrolyte (KON3) was filled into the microelectrode, and the liquid ion exchanger (LIX:XY-SJ-NO3-, Younger, USA) was filled into the tip of the electrode. The ion-selective microelectrode was corrected by KNO3 solutions with a certain concentration gradient. The root tips (3 cm long) from plants subjected to different treatments were collected and placed in a transparent dish. The root samples were fixed with sterile filter paper and resin in the dish. Ten millilitres of hydroponic solution was added, and the flux of NO3- was then measured. The ___location points of steady-state ion flow measurement were the meristem zone (300–600 μm from the root tip), elongation zone (1–3 mm from the root tip) and mature zone (10–15 mm from the root tip). Each root zone was measured for 5 min to obtain NO3- flow data, and the recording rate for the ion flux was one reading per 6 s. Positive values of the NO3- flow rate represented efflux, and negative values represented absorption. NO3- flux data were calculated with MageFlux1.
qRT–PCR assay
Total RNA was extracted using a total RNA extraction kit (Tiangen Biotech Co. Ltd. China) according to the manufacturer’s instructions. Reverse transcription was performed following the manufacturer's protocol (Takara Bio Inc. Japan). The expression levels of the target genes were determined by qRT–PCR using SYBR green fluorescent dye on a Bio–Rad CFX96 thermocycler (Bio–Rad, Hercules, CA, USA). The specific primers for the qRT-PCR assay were referred to Ren et al.27 and Yu et al.28. The expression levels of the genes (IbNR2, IbNiR2 and IbNRT1.1) were normalized to the level of constitutive IbActin expression, and three biological replicates were used to calculate relative gene expression levels by the 2−△△CT method29. The primers are listed in Supplementary Table S2.
Statistical analysis
Two-way ANOVA (cultivars and NO3- concentrations) and Duncan’s multiple range test were conducted using the statistical analysis software SPSS (version 17.0 for Windows, SPSS, Chicago, Illinois, USA).
Results
FW of shoots and roots
The effects of cultivar (C) and NO3- concentration (NC) on the FW of shoots and roots were significant (Table 1). Significant interactive effects of C × NC were detected only in the FW of the roots. With increasing NC to N3, for J26, the FW of shoots and roots first significantly increased but then decreased. However, for X32, there was no significant difference among N1, N2 and N3. Compared with X32, J26 presented significantly greater FW of roots under N2 and N3. These results suggested that the nitrate supply had more significant effects on the growth of J26 plants. However, the growth of X32 was insensitive to the nitrate supply. An excessive nitrate supply was not conducive to the growth of the roots of J26, and this cultivar presented greater growth vigour in its roots.
Root length, surface area, diameter and volume
The effects of C on the root surface area, diameter and volume were significant (Table 2). The effects of NC on root length, surface area and volume were significant. The interactive effects of C × NC on these four parameters were significant. With increasing NC to N3, the root length, surface area and volume of J26 significantly decreased. However, for X32, there was no significant difference in root length or surface area among N1, N2 and N3. Compared with X32, J26 presented a significantly greater root surface area, diameter and volume under N0 and N1. These data indicated that excessive nitrate application inhibited root growth, especially in J26. The regulatory effects of nitrate supply on the root growth of J26 were more significant than those on X32.
N content, N accumulation and distribution
For both cultivars, N accumulated mainly in the leaves, stems, and roots (Table 3). The effects of C on the N contents in leaves and roots and the N accumulation amounts in leaves, growth points and roots were all significant. The effects of NC on the N contents and N accumulation in all the divided organs were significant, whereas these regulatory effects on the N distribution ratio were not significant. With the exception of petioles, the interactive effects of C × NC on the N contents and N accumulation in other organs were significant. For both cultivars, the N content in all the divided organs increased with increasing NC to N2. N3 further significantly increased the N content in the leaves and roots of J26 and in the petioles of X32. The trends in the N accumulation amount were similar to those in the N content. Compared with X32, J26 presented significantly greater N contents and N accumulation amounts in leaves and roots under N1, N2 and N3. J26 also presented greater N accumulation at growth points under N1, N2 and N3. The results indicated that excessive nitrate increased the N contents and N accumulation amounts in the leaves and roots of J26 but did not further increase those of X32. J26 accumulated more N in the leaves, growth points and roots under relatively high nitrate levels.
NO3 - content and activities of NR and NiR in leaves
The effects of C and NC on the NO3- content and activities of NR and NiR in leaves were significant (Fig. 1). The significant interactive effects of C × NC existed only for the NO3- content. For both cultivars, the NO3- content in leaves significantly increased with increasing NC to N3. A similar trend was observed for the NR activity in the leaves of J26. However, N3 did not further increase these values for X32. N3 did not further increase NiR activity in J26, whereas no significant difference was detected among N1, N2 and N3 for X32. Compared with X32, J26 presented significantly greater NO3- content and NR activity in leaves under N1, N2 and N3 and greater NiR activity in leaves under N2 and N3. These data suggested that excessive nitrate could further increase the NR activity in leaves only for J26. J26 had a greater ability to assimilate and metabolize nitrate in its leaves.
Nitrate content (a) and NR (b) and NiR (c) activities in leaves under different N treatments. Means denoted by different letters are significantly different at P < 0.05 as determined by Duncan's multiple range test. N0, N1, N2 and N3 represent 0, 4, 8 and 16 mmol L-1, respectively. J26 and X32 represent cultivar Jishu 26 and Xushu32, respectively.
NO3 - content and activities of NR and NiR in roots
The effects of C on the NO3- content and NR activity in roots were significant (Fig. 2). The effects of NC and the interactive effects of C × NC on the NO3- content and activities of NR and NiR in roots were significant. For both cultivars, the NO3- content in the roots significantly increased with increasing NC to N3. A similar trend was observed for NiR activity in the roots of both cultivars. N3 further increased the NR activity in the roots of J26, whereas no significant difference was detected between N2 and N3 for X32. Compared with X32, J26 presented significantly greater NO3- contents under N1, N2 and N3 and greater NR activity in roots under N2 and N3. The results suggested that excessive nitrate could further increase the activities of NR and NiR in roots only for J26. The regulatory effects of NC on the activities of NR and NiR in roots were more significant for this cultivar, which presented a greater ability for nitrate assimilation and metabolism in roots.
Nitrate content (a) and NR (b) and NiR (c) activities in roots under different N treatments. Means denoted by different letters are significantly different at P < 0.05 as determined by Duncan's multiple range test. N0, N1, N2 and N3 represent 0, 4, 8 and 16 mmol L-1, respectively. J26 and X32 represent cultivar Jishu 26 and Xushu32, respectively.
Flux measurements of NO3 -
For both cultivars, the mean flux of NO3- exhibited an influx under the NO3--supplemented treatments in all the tested root regions (Fig. 3). The effects of C on the mean flux of NO3- in the meristem and mature zones were significant. The effects of NC and the interactive effects of C × NC on the mean flux of NO3- in all the tested root regions were significant. For both cultivars, the mean influx of NO3- in the meristem zone significantly increased with increasing NC from N1 to N3. A similar trend was observed in the elongation zone for J26. In the mature zone, the mean influx of NO3- was the highest under N2 for this cultivar. For X32, there was no significant difference in the mean influx of NO3- in the elongation and mature zones between N2 and N3. Compared with X32, J26 presented a significantly greater mean influx of NO3- in the meristem and mature zones under N1, N2 and N3. These data indicated that excessive nitrate could further increase the mean influx of NO3- in the meristem and elongation zones of J26 but did not further increase NO3- in the elongation and mature zones of X32. The roots of J26 presented a greater ability to absorb nitrate under the same NC.
The mean rate of NO3- flux at the meristem (300 ~ 600 μm from the tip), elongation (1 ~ 3 mm from the tip), and mature (10 ~ 15 mm from the tip) root zones. Means denoted by different letters are significantly different at P < 0.05 as determined by Duncan's multiple range test. N0, N1, N2 and N3 represent 0, 4, 8 and 16 mmol L-1, respectively. J26 and X32 represent cultivar Jishu 26 and Xushu32, respectively.
Gene expression of NiR2, NR2, and NRT1.1 in roots
The effects of C and NC and the interactive effects of C × NC on the gene expression of NiR2, NR2, and NRT1.1 in roots were significant (Fig. 4). With increasing NC to N3, the expression level of NR2 significantly increased for both cultivars. Similar trends were observed in the expression of NiR2 and NRT1.1. Compared with X32, J26 presented significantly greater expression levels of NR2 under N2 and N3 and greater expression levels of NiR2 and NRT1.1 under N1, N2 and N3. The results suggested that excessive nitrate could further increase the gene expression of NiR2, NR2, and NRT1.1 in the roots of both cultivars. The regulatory effects of NC on the expression of NiR2, NR2, and NRT1.1 in J26 were more significant than those in X32. J26 presented increased expression of genes related to nitrate assimilation under higher NC.
The expression levels of the genes involved in nitrogen metabolism in roots under different N treatments. Means denoted by different letters are significantly different at P < 0.05 as determined by Duncan's multiple range test. N0, N1, N2 and N3 represent 0, 4, 8 and 16 mmol L-1, respectively. J26 and X32 represent cultivar Jishu 26 and Xushu32, respectively.
Discussion
High NO3- supply could impair the FW of roots and shoots in plants30. However, in wheat, a high NO3- supply revealed no significant effects on the dry weight (DW) of roots in cultivar BTS but decreased these in cultivar GE18. Similar results of FW of shoots were observed in sweet potato seedlings, and the FW of roots increased initially and then decreased for most of the tested cultivars19. In the present study, there were differences in plant growth between the two cultivars with different N tolerances in response to nitrate supply. The growth of the roots and shoots of J26 was significantly regulated by the NO3- supply, whereas that of X32 was relatively stable, indicating that the regulatory effects of the nitrate supply on the growth of J26 plants were more variable. For sweet potato seedlings, Yao et al.19 reported that the total N content in roots and shoots was increased by high N concentrations. In the present study, for both cultivars, increasing NO3- concentrations to 8 mM increased the N contents and N accumulation amounts in the leaves and roots. For J26, 16 mM NO3- further increased the N contents in leaves and roots and the N accumulation amounts in the organs of leaves, growth points and roots. However, these parameters showed no significant increase for X32. Although higher NO3- concentrations caused a decrease in the FW of roots for J26 or showed no significant increase for X32, higher N accumulation amounts in roots were obtained for both cultivars (Table 3), suggesting that higher N content was the reason for the higher N accumulation. Compared with X32, J26 may have a higher N assimilation capacity, which resulted in higher N contents in leaves and roots, and more N was accumulated in these organs. Moreover, the higher N accumulation amount at the growth points of J26 suggested that high N accumulation in this metabolic centre may increase the source-sink distance and promote shoot growth8.
High NO3- supplies could inhibit root growth25. According to Adavi and Sathee18, high NO3- supply reduced the total root length but showed no significant effects on the root surface area for wheat cultivar BTS. However, it decreased the root surface area for cultivar GE. In the present study, increasing NO3- concentrations decreased the total root length and surface area of the N-sensitive cultivar J26. However, for X32, the NO3- supply had no significant effect on these two parameters. Thus, the regulatory effects of nitrate supply on root length and surface area were more significant for J26 than for X32. The NO3- supply had significant effects on the NO3- influx in roots, and the regulatory effects were related to NO3- concentrations in nutrient solution and plant species31,32. In the present study, for J26, the highest mean influx of NO3- in the meristem and elongation zones was both obtained under 16 mM NO3- while that in the mature zone was obtained under 12 mM NO3-. For X32, 16 mM NO3- further increased the mean influx of NO3- merely in the meristem zone. Moreover, compared with X32, J26 presented a significantly greater mean influx of NO3- in the meristem and mature regions. Thus, the relatively high NO3- content in the roots and leaves of J26 was related to the relatively high mean influx of NO3- in the roots. The N-sensitive cultivar J26 presented a high capacity for nitrate uptake, especially under relatively high nitrate levels. Crop plants may expand their absorption area by maintaining a higher total root length and surface area, aiming to absorb a larger amount of N nutrients under low-N conditions32. With sufficient N supply, the increased N uptake was achieved mainly by the increased root density and total root length, rather than by the N uptake rate33,34. In the present study, high NO3- concentrations decreased the total root length and surface area for J26 and showed insignificant effects on these two parameters for X32. However, a higher NO3- influx rate was obtained under high NO3- concentrations. Thus, the higher NO3- influx rate in roots for these two sweet potato cultivars was not associated with the total root length and surface area. The assimilation of N genes and upregulation of NRT may contribute to a stronger N uptake capacity for plant N accumulation. In apple seedlings, a higher NO3- influx rate in roots was associated with higher NR activities and upregulated transcription of MdNRT1.1 in roots35. For the sweet potato seedlings in this study, the high NO3- concentrations upregulated the expression levels of NRT1.1, NR2 and NiR2 in roots and increased the activities of NR and NiR in roots (Figs. 2), indicating a higher N uptake capacity36, which was the main reason for the higher NO3- influx rate in roots. Similarly, compared with X32, J26 exhibited higher N uptake capacity in roots and higher levels of leaf N metabolism, especially under higher NO3- concentrations, thus leading to the accumulation of more N in roots and shoots (Table 1). Thus, for the two cultivars, the relatively high NO3- influx rate was related mainly to the upregulated expression of NRT1.1, NR2 and NiR2 and the relatively high activities of NR and NiR but not to the parameters of root length and surface area.
NRT1.1 is a well-known NRT gene involved in the uptake and translocation of NO3-. When NO3- is sufficient in the environment, NRT1.1 displays robust nitrate transport activity to meet the N demand37. In cucumber, a high NO3- supply upregulated NRT1.1 gene expression in roots38. In sweet potato seedlings, the coding genes for NRT were regulated by N concentration, and the expression of the NRT1.1 gene in roots was upregulated under higher NO3- concentrations19. In the present study, with increasing NO3- levels, the expression of the NRT1.1 gene was upregulated for both cultivars. J26 exhibited higher expression levels of this gene, i.e., NO3- induced higher expression levels of NRT1.1, which was conducive to a higher NO3- influx rate and higher NO3- content in roots for this cultivar34. Moreover, the range of increase in the NRT1.1 gene for J26 was greater than that for X32, suggesting that the regulatory effect of NO3- supplies on the expression of NRT1.1 was more significant for J26. This may be one reason that the NO3- absorption of J26 was sensitive to the NO3- supply. The activities of N-assimilating enzymes play a significant role in maintaining growth and development39. An appropriate amount of N increased the activities of NR and NiR in the organs of leaves and roots40,41. In the present study, for J26, the activities of NR and NiR in roots and leaves were significantly increased when increasing NO3- concentrations to 16 mM. However, for X32, this concentration merely increased the root NiR activity but did not further increase the NR activity in roots and leaves. NO3- supplies did not affect the leaf NiR activity for this cultivar. The changes in the enzyme activities of J26 were relatively more variable than those of X32. Compared with X32, J26 presented greater NR and NiR activities in roots and leaves under higher NO3- concentrations, suggesting that J26 presented greater NO3- assimilation capacity and greater demand for N. Enzyme activity is subject to regulation at the level of gene expression42. According to Liao et al.41, for citrus cultivar ‘Huangguogan’, the trends in the activities of NR and NiR in roots were consistent with those of HgNR and HgNiR transcription, respectively. In sweet potato, the expression level of the NR gene in leaves was upregulated by high N conditions, and the leaf NR activity was increased43. In the present study, for both cultivars, the expression levels of NR2 and NiR2 in roots were upregulated by NO3- concentrations. The trends in the activities of NR and NiR were subject to regulation of NR2 and NiR2 transcription, respectively. Moreover, the range of increase in the NR2 and NiR2 genes for J26 was greater than that for X32. Combined with the expression data of the NRT1.1 gene, the regulatory effects of NO3- supply on the expression of these genes in J26 were more significant, accordingly, the changes in NO3- transportation and in enzyme activities related to NO3- assimilation were more variable, which may explain why the N availability of this cultivar was sensitive to NO3- supply. Although NO3- induced NR protein and activity, it is important to note that NR transcripts were present in the roots and leaves of plants grown in NO3--free medium44. This phenomenon also existed in the present study and was related to the NO3- already contained in the sweet potato seedlings.
As the external NO3- concentration increases, NO3- assimilation into amino acids in plants is increasingly achieved, which become the main sites of NR activity45. Since the NR activity is much higher than that of NiR, there is almost no accumulation of NO2- in plants, so NR is the rate-limiting enzyme. The NO3- contents were enhanced both by increasing NR activity and upregulating NRT genes27. In the present study, NO3- supplies induced higher NR activity in roots and leaves and upregulated the expression level of NRT1.1 in roots, which promoted more N absorption in roots and N assimilation in leaves for both cultivars. For J26, 16 mM NO3- further increased the NR activities in roots and leaves and the NO3- influx rate in roots. However, for X32, this concentration exhibited no significant increase in these parameters. J26 maintained higher N absorption and assimilation capacity under higher NO3- supplies. On the one hand, this was related to the higher N absorption and assimilation capacity for this cultivar, as indicated by upregulated expression of NRT1.1 and higher NR activity, on the other hand, this may be related to the higher N distribution in leaves and at growth points (Table 1), which may transmit feedback signals from long-distance NO3- transport and contribute to higher NO3- influx in roots34. Plants with higher growth rates may require more N metabolites, and growth can be a driving force for the N metabolism of plants46. Higher root growth can lead to greater N demand47. The N accumulation amount of J26 was higher than that of X32 (Table 1), suggesting that more N was needed for plant growth of J26 and that the N demand for this cultivar was higher, as indicated by the higher FW of roots (Table 1) and higher source-sink average distance observed in our previous research11.
Conclusion
For J26, NO3- supplies exhibited obvious regulatory effects on root length, root surface area, and NO3- absorption and assimilation. 16 mM NO3- still yielded significant increases in the NR activities in leaves and roots and the NO3- influx rate in roots for this cultivar. The N accumulation was also increased in leaves, growth points and roots. However, for X32, NO3- supplies showed a more moderate influence. Under higher NO3- nutrition, compared with X32, J26 exhibited higher expression levels of the NR2, NiR2, and NRT1.1 genes and promoted a higher net influx of NO3- in roots. The higher NO3- influx rate was mainly associated with the upregulated expression of NRT1.1, NR2 and NiR2 and higher activities of NR and NiR. Based on statistical analysis (C, NC, C × NC), J26 is a cultivar to have higher N availability and this contributes biomass production, especially below ground. The key differentially expressed genes related to N utilization in the roots of the two cultivars need further exploration under different NO3- concentrations.
Data availability
All data generated and/or analyzed during the current study are included in this article.
References
Food and Agriculture Organization of the United Nations. Statistics database of food and agriculture organization of the United Nations-Food and agriculture data-Production-Crops. http://www.fao.org/statistics/en/.FAOSTAT. (accessed 14 January 2020).
Villordon, A. Q. & Clark, C. A. Variation in virus symptom development and root architecture attributes at the onset of storage root initiation in ‘Beauregard’ sweetpotato plants grown with or without nitrogen. PLoS ONE 9, e107384 (2014).
Taranet, P., Harper, S., Kirchhof, G., Fujinuma, R. & Menzies, N. Growth and yield response of glasshouse- and field-grown sweetpotato to nitrogen supply. Nutr. Cycl. Agroecos. 108, 309–321 (2017).
Duan, W. X. et al. Nitrogen utilization characteristics and early storage root development in nitrogen-tolerant and nitrogen-susceptible sweet potato. Physiol. Plantarum. 173, 1090–1104 (2021).
Phillips, S. B., Warren, J. G. & Mullins, G. L. Nitrogen rate and application timing affect “Beauregard” sweetpotato yield and quality. Hortscience. 40, 214–217 (2005).
Fernandes, A. M., Assunção, N. S., Ribeiro, N. P., Gazola, B. & Silva, R. M. Nutrient uptake and removal by sweet potato fertilized with green manure and nitrogen on sandy soil. Rev. Bras. Cienc. Solo. 44, e0190127 (2020).
Villagarcia, M. R., Collins, W. W. & Raper, C. D. Nitrate uptake and nitrogen use efficiency of two sweetpotato genotypes during early stages of storage root formation. J. Am. Soc. Hortic. Sci. 123, 814–820 (1998).
Duan, W. X. et al. Comparative study on carbon-nitrogen metabolism and endogenous hormone contents in normal and overgrown sweetpotato. S. Afr. J. Bot. 115, 199–207 (2018).
Kelm, M., Brück, H., Hermann, M. & Sattelmacher, B. The effect of low nitrogen supply on yield and water-use efficiency of sweet potato (Ipomoea batatas L.). In: Horst, WJ. et al. (Eds.), Plant Nutrition. Developments in Plant and Soil Sciences, Springer, Dordrecht, pp: 402–403 (2001).
Ankumah, R. O., Khan, V., Mwamba, K. & Kpomblekou-A, K. The influence of source and timing of nitrogen fertilizers on yield and nitrogen use efficiency of four sweet potato cultivars. Agr. Ecosyst. Environ. 100, 201–207 (2003).
Duan, W. X. et al. Differences between nitrogen-tolerant and nitrogen-susceptible sweetpotato cultivars in photosynthate distribution and transport under different nitrogen conditions. PLoS ONE 13, e0194570 (2018).
Sun, H. W., Feng, F., Liu, J. & Zhao, Q. Z. Nitric oxide affects rice root growth by regulating auxin transport under nitrate supply. Front. Plant Sci. 9, 659 (2018).
Wang, P. et al. Increased biomass accumulation in maize grown in mixed nitrogen supply is mediated by auxin synthesis. J. Exp. Bot. 70, 1859–1873 (2019).
Ma, H. P. et al. Heterologous expression of nitrate assimilation related-protein DsNAR2.1/NRT3.1 affects uptake of nitrate and ammonium in nitrogen-starved Arabidopsis. Int. J. Mol. Sci. 21, 4027 (2020).
Orsel, M., Filleur, S., Fraisier, V. & Daniel-Vedele, F. Nitrate transport in plants: Which gene and which control?. J. Exp. Bot. 53, 825–833 (2002).
Davenport, S., Lay, P. L. & Sanchez-Tamburrrino, J. P. Nitrate metabolism in tobacco leaves overexpressing Arabidopsis nitrite reductase. Plant Physiol. Bioch. 97, 96–107 (2015).
Takushi, H. & Hitoshi, S. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 10, 2501–2512 (2017).
Adavi, S. B. & Sathee, L. Elevated CO2 alters tissue balance of nitrogen metabolism and downregulates nitrogen assimilation and signalling gene expression in wheat seedlings receiving high nitrate supply. Protoplasma 258, 219–233 (2021).
Yao, Z. F., Wang, Z. Y., Fang, B. P., Chen, J. Y. & Yang, Y. L. Involvement of nitrogen in storage root growth and related gene expression in sweet potato (Ipomoea batatas). Plant Biol. 22, 376–385 (2020).
Mcinenly, L. E., Merrill, E. H., Cahill, J. F. & Juma, N. G. Festuca campestris alters root morphology and growth in response to simulated grazing and nitrogen form. Funct. Ecol. 24, 283–292 (2010).
Rasmussen, I. S., Dresbll, D. B. & Thorup-Kristensen, K. Winter wheat cultivars and nitrogen (N) fertilization effects on root growth, N uptake efficiency and N use efficiency. Eur. J. Agron. 68, 38–49 (2015).
Meng, L. et al. Differential responses of root growth to nutrition with different ammonium/nitrate ratios involve auxin distribution in two tobacco cultivars. J. Integr. Agr. 18, 2703–2715 (2020).
Du, X. B., Xi, M. & Kong, L. C. Split application of reduced nitrogen rate improves nitrogen uptake and use efficiency in sweetpotato. Sci. Rep. 9, 14058 (2019).
Darnell, R. L., Stutte, G. W. & Sager, J. C. Nitrite concentration effects on NO3- uptake and reduction, growth, and fruit yield in strawberry. J. Am. Soc. Hortic. Sci. 126, 560–563 (2001).
Wang, X. L., Tao, Y. Y., Sheng, H. J. & Feng, K. Effects of nitrate supply on morphology development and nitrate uptake kinetics of wheat roots. J. Tritic. Crops. 30, 129–134 (2010).
Chen, X. G. et al. Responses of root physiological characteristics and yield of sweet potato to humic acid urea fertilizer. Plos ONE 12, e0189715 (2017).
Ren, Z. T. et al. Overexpression of IbSnRK1 enhances nitrogen uptake and carbon assimilation in transgenic sweetpotato. J. Integr. Agr. 17, 296–305 (2018).
Yu, Y. C. et al. NaCl-induced changes of ion homeostasis and nitrogen metabolism in two sweet potato (Ipomoea batatas L.) cultivars exhibit different salt tolerance at adventitious root stage. Environ. Exp. Bot. 129, 23–36 (2016).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008).
Liang, Y. L., Zheng, P., Li, S., Li, K. Z. & Xu, H. N. Nitrate reductase-dependent NO production is involved in H2S-induced nitrate stress tolerance in tomato via activation of antioxidant enzymes. Sci. Hortic. 229, 207–214 (2018).
Wang, J. Y., Wang, H. Q., Liang, X. D. & Liu, J. H. Response of root morphology and N absorption to nitrate nitrogen supply in hydroponic oats. J. Plant Nutr. Ferti. 22, 1049–1055 (2016).
Lv, X. M. et al. Low-nitrogen stress stimulates lateral root initiation and nitrogen assimilation in wheat: roles of phytohormone signaling. J. Plant Growth Regul. 40, 436–450 (2021).
Yan, H. F. et al. Root morphological and proteomic responses to growth restriction in maize plants supplied with sufficient N. J. Plant Physiol. 168, 1067–1075 (2011).
Huang, S. J. et al. A transcription factor, OsMADS57, regulates long-distance nitrate transport and root elongation. Plant Physiol. 180, 882–895 (2019).
Xing, Y. et al. Role of calcium as a possible regulator of growth and nitrate nitrogen metabolism in apple dwarf root stock seedlings. Sci. Hortic. 276, 109740 (2021).
Gao, R. H. et al. Enhancement of root architecture and nitrate transporter gene expression improves plant growth and nitrogen uptake under long-term low-nitrogen stress in barley (Hordeum vulgare L.) seedlings. Plant Growth Regul. 95, 343–353 (2021).
Su, H. et al. Abscisic acid signaling negatively regulates nitrate uptake via phosphorylation of NRT1.1 by SnRK2s in Arabidopsis. J. Integr. Plant Biol. 63, 597–610 (2021).
Lan, G. L., Jiao, C. J., Wang, G. Q., Sun, Y. H. & Sun, Y. Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Sci. Hortic. 260, 108790 (2020).
Singh, M., Singh, V. P. & Prasad, S. M. Nitrogen modifies NaCl toxicity in eggplant seedlings: assessment of chlorophyll a fluorescence, antioxidative response and proline metabolism. Biocat. Agr. Biotech. 7, 76–86 (2016).
Zhang, Z. M., Wan, S. B., Dai, L. X., Ning, T. Y. & Song, W. W. Effects of nitrogen application rates on nitrogen metabolism and related enzyme activities of two different peanut cultivars. Sci. Agr. Sin. 44, 280–290 (2011).
Liao, L. et al. Effect of nitrogen supply on nitrogen metabolism in the citrus cultivar ‘Huangguogan’. PLoS ONE 14, e0213874 (2019).
Ren, B. Z., Dong, S. T., Zhao, B., Liu, P. & Zhang, J. W. Responses of nitrogen metabolism, uptake and translocation of maize to waterlogging at different growth stages. Front. Plant Sci. 8, 1216 (2017).
Xia, H. Q. et al. Drought-induced responses of nitrogen metabolism in Ipomoea batatas. Plants 9, 1341 (2020).
Carilloa, P., Mastrolonardoa, G., Naccaa, F. & Fuggia, A. Nitrate reductase in durum wheat seedlings as affected by nitrate nutrition and salinity. Funct. Plant Biol. 32, 209–219 (2005).
Andrews, M. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ. 9, 511–519 (2010).
Lawlor, D. W. Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J. Exp. Bot. 53, 773–787 (2002).
Luo, J. et al. Net fluxes of ammonium and nitrate in association with H+ fluxes in fine roots of Populus popularis. Planta. 237, 919–931 (2013).
Funding
The research program was sponsored by the Natural Science Foundation of Shandong Province (ZR2021MC092), the China Agriculture Research System of MOF and MARA (CARS-10-GW09), the Tubers and Root Crops Innovation Team of Modern Agricultural Technology System in Shandong Province, China (SDAIT-16–09) and the Key Research and Development Program of Shandong Province, China (2023TZXD001).
Author information
Authors and Affiliations
Contributions
Wenxue Duan, Haiyan Zhang and Liming Zhang conceived and designed the study. Wenxue Duan, and Shasha Wang performed the experiments. Wenxue Duan and Beitao Xie analyzed the data. Wenxue Duan wrote the paper. Liming Zhang reviewed and edited the manuscript. The final version was read and approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Experimental research and field studies on plants, including the collection of plant material, was carried out in accordance with relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Duan, W., Wang, S., Zhang, H. et al. Plant growth and nitrate absorption and assimilation of two sweet potato cultivars with different N tolerances in response to nitrate supply. Sci Rep 14, 21286 (2024). https://doi.org/10.1038/s41598-024-72422-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72422-y