Abstract
Iliac vein compression syndrome (IVCS) is a clinical condition defined as obstruction of the iliac vein caused by chronic compression imposed by various causes. Currently, the clinical role of computed tomography venography (CTV) in the diagnosis of IVCS is unclear. Accurately diagnosing IVCS using CTV may enhance the understanding of the pathological anatomy of iliac veins, which may lead to better treatment outcomes, especially for recalcitrant venous leg ulcers (VLU). We aimed to investigate diagnostic criteria, contributing clinical factors, and stenting for IVCS with VLU in this study. CTV, digital subtraction angiography (DSV), and Doppler ultrasound (DUS) data were obtained from the medical and imaging records of 62 patients. Additionally, contributing factors and stenting for IVCS were analysed. Patients (100%) had clinical, aetiological, anatomic, or pathological C6 disease. CTV reduced the procedure time and contrast medium dose and provided more information than DSV. Risk factors for IVCS with VLU included female sex (P = 0.036) and advanced age (P = 0.014). The rate of ulcer healing was lower in the IVCS group without stent implantation (P = 0.020). Significant improvements were noted in venous clinical severity scores (P < 0.001) and chronic venous insufficiency questionnaire-20 scores (P < 0.001) after stenting for IVCS with C6 ulcers. CTV provides a more accurate diagnosis than DUS and DSV and allows detection of possible causes of IVCS. Female sex and advanced age were potential contributing factors for IVCS. Satisfactory outcomes were observed with stenting in the treatment of IVCS with C6 ulcers.
Similar content being viewed by others
Introduction
Iliac vein compression syndrome (IVCS), also referred to as May‒Thurner syndrome or Cockett syndrome, is a clinical disease defined as obstruction of the iliac vein caused by chronic compression imposed by an iliac artery or other various extraluminal causes, such as lumbar degenerative osteophytes, hyperlordosis, neoplasms or retroperitoneal fibrosis1,2,3,4. Clinically, IVCS can be characterized by leg swelling, pain, varicose veins, and venous leg ulcers (VLU), and shows a higher prevalence among women than among men5,6. Doppler ultrasound (DUS), computed tomography venography (CTV), digital subtraction venography (DSV), intravascular ultrasound (IVUS), and magnetic resonance venography (MRV) are used to diagnose IVCS. DUS has disadvantages in terms of accessing the iliac veins and inferior vena cava from within the abdomen, such as operator-dependent subjectivity, difficulty in obese patients due to inadequate penetration depth, vessel motion during breathing, and bowel gas and colour bleeding with movement of the bowel4,7. DSV is currently limited to patients who are difficult to diagnose or for whom an intervention is planned. IVUS has been established as the gold standard for diagnosing and treating IVCS8,9. Despite the advantages of IVUS, it is invasive and inappropriate for screening purposes. Thus, CTV may be a powerful alternative method for accurately evaluating iliac vein lesions and detecting external pressure sources on the iliac vein10,11, especially when IVUS and MRV expertise are not available in our health care system. At present, Stenting for IVCS results in improved quality of life, prevention of recurrent deep venous thrombosis, and healing of VLU12,13. Here, we aimed to investigate the diagnostic applicability of CTV and outcomes of stenting for IVCS with VLU in local Chinese patients.
Materials and methods
Patients
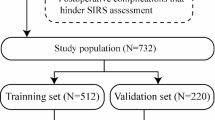
The records 82 consecutive of patients with unilateral lower extremity VLU (C6 according to CEAP classification14) between June 2019 and October 2022 in the Department of Vascular Surgery at the First Hospital of Hebei Medical University were retrospectively reviewed. A flow diagram of the study is shown in Fig. 1. All the patients underwent CTV, DSV, and DUS. Patient characteristics are shown in Table 1. This study was approved by the Ethics Review Board of the First Hospital of Hebei Medical University (20,160,690) and conformed to the Declaration of Helsinki guidelines. The inclusion and exclusion criteria for the study are listed in Table S1.
DUS
Using an ultrasonic Doppler instrument (M5, Mindray Medical International, ShenZhen, China), reflux investigation in the superficial and deep venous system was performed while the patient was standing and obstructed in the supine position. High-resolution ultrasound of veins was performed by a sonographer using a 2D Gateway Series with a 3–6 MHz linear-way transducer. Imaging revealed both legs from the ankle to the diaphragm. Colour flow imaging was used to assess luminal stenosis or occlusion. The colour scale and colour gain were adjusted to ensure that the flow was visible in the lumen without saturating the surrounding tissues. The ultrasonographic examinations were performed by a single ultrasonologist who was a specialist in vascular ultrasound, had 9 years of clinical experience and was blinded to all other imaging results.
CTV
During the procedure, direct CTV in this material was done with injection of a contrast agent in the foot with ascending acquisition of imaging to obtain detailed information. Patients were examined using a 64-detector CT scanner (GE Healthcare, Wisconsin, USA) with a tube voltage of 120 kV and current of 100 mAs. Images were reconstructed with a slice thickness of 1.25 mm and a reconstruction interval of 1 mm. A binocular high-pressure syringe (E-Z-EM, Westbury, New York, USA) was connected to a 22-gauge closed intravenous catheter system in the dorsal pedal vein bilaterally under ultrasound guidance. A bolus of 1.0 mL/kg of nonionic contrast material (350 mg/mL, Iohecol Injection, Yangtze River Pharmaceutical Group, TaiZhou, China) was administered with saline at a speed of 2 mL/s. CTV scanning performed under the Valsalva manoeuvre was delayed by 30 s after injection of iodinated contrast media. Scanning covered both legs from the ankle to the diaphragm. Computer software (CT Advantage Workstation 4.4; GE Healthcare) was used to create 3D reconstructions, including volume rendering and surface rendering. Two cardiovascular radiologists with 8 and 20 years of clinical experience were blinded to the patients’ symptoms, reviewed the films, and analysed the images.
DSV
DSV imaging was performed on C-arm fluoroscopy equipment (Philips Medical Systems, Netherlands). An ankle tourniquet was applied to the ankle, and a binocular high-pressure syringe was connected to the 24-gauge closed intravenous catheter system in the dorsalis pedis vein with ultrasound guidance to administer nonionic contrast material (350 mg/mL, Iohecol Injection, Yangtze River Pharmaceutical Group, TaiZhou, China) solution (mixed 1:1 with 40 mL of saline) within 1 min. The iliac vein lesions were imaged. To identify potential circulation abnormalities, patients were asked to perform a Valsalva manoeuvre to monitor venous compression. When pedal venograms suggested non-thrombotic iliac vein lesions, the femoral vein was accessed to detect the true extent of IVCS. Typical findings of compression include iliac vein stenosis or occlusion, reversal of flow within the ipsilateral internal iliac vein, tortuous venous collaterals crossing the pelvis, and an enlarged iliac or ascending lumbar vein. Two cardiovascular radiologists with 8 and 20 years of clinical experience were blinded to the patients’ symptoms, reviewed the films, and analysed the images.
Stent placement
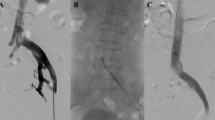
All procedures were performed under local anaesthesia (Fig. 2). The sheath (Terumo Corporation, Tokyo, Japan) was inserted into femoral vein of the patients using Seldinger technique, then an intravenous bolus of 100 UI/kg unfractionated heparin was administered. The diagnosis of IVCS was confirmed with DSV again. Guidewire traversal was accomplished, and lesion pre-dilatation was performed before stenting. Wallstents (Boston Scientific Corp.) with stent diameters ranging from 12 to 16 mm were placed across the obstructed segment. The stents were balloon dilated after deployment to achieve full expansion.
Diagnosis of IVCS on CTV images
The grade of iliac venous compression was measured by comparing the diameter of the iliac vein at the site of maximal compression divided by the average proximal and distal diameters of the uncompressed common iliac vein confined to cross-sectional imaging of the iliac vein on CTV scans. The cut-off value was set to 50%, which was considered clinically significant3. Images were randomly collected by two independent examiners who were blinded to study allocation. If a discrepancy in the initial categorization arose, a third examiner reviewed the images.
Definitions
VLU is defined as a full-thickness defect of the skin, most frequently in the ankle region, that fails to heal spontaneously and is sustained by chronic venous disease15. C6 was defined as an active VLU.. IVCS was defined as iliac vein compression > 50% on CTV scans (Fig. 3). CTV was used to determine which patients needed a stent. Current smokers were defined as those who reported smoking at the time of inclusion. Drinking was documented when a participant drank ≥ 2 drinks per day (10 g of pure alcohol per drink) for the past 6 months. According to Chinese criteria, obesity was defined as a BMI ≥ 28.0 kg/m2. All VLU patients were classified based on venous disease classification, including CEAP classification. Clinical severity was objectively documented at each time point using the venous clinical severity score (VCSS). Quality of life measures included the Chronic Venous Insufficiency Questionnaire-20 (CIVIQ-20)16 at baseline and after the operation. Reflux was defined as the presence of reverse flow in the femoral vein lasting > 1 s after distal augmentation and in the superficial venous system lasting > 0.5 s in the standing position17. Sixty-two patients with C6 disease (active VLU) were divided into Group A, Group B and Group C based on the presence or absence of IVCS and stenting (Table 4). Group A was defined as neither IVCS nor stenting. Group B was defined as with IVCS but no stenting. Group C was defined as with both IVCS and stenting. Resolved C6 disease was defined as complete epithelialization. Time to complete ulcer healing was calculated from the date of intervention to the last follow-up or healing of the active venous ulcer.
We deployed a 9F sheath and inserted the guide wire. A subtracted venogram showing the compressed left iliac vein and contralateral venous drainage via pelvic venous collaterals (A). A venogram after balloon inflation and stenting showing no stenosis or collaterals and a wall-stent placed sufficiently into the inferior vena cava to prevent migration (B, C, D).
Follow-up
After stenting, all patients received oral factor Xa inhibitors (rivaroxaban tablets, 20 mg/day, Bayer Health care Co., Ltd.) for six months. Patients were advised to attend visits every 2 weeks during follow-up time for clinical assessments consisting of an interview, a clinical examination questionnaire and DUS until complete healing of the ulcer. Ulcers were assessed based on healing conditions (completely or incompletely epithelialized). Clinical, VCSS, CIVIQ-20 and DUS outcomes were recorded. Patients who were lost to follow-up were censored on the last known date of contact.
Statistical analysis
Continuous variables with a normal distribution were compared by t tests, and those with a nonnormal distribution were compared by the Mann‒Whitney U or Wilcoxon signed-rank test. Normally distributed data are reported as the means and SDs and skewed data are presented as medians and interquartile ranges. Categorical variables are presented as counts and percentages, and the equality of variances was confirmed by Levene’s test. Qualitative parameters were described using frequency statistics and analysed using the Pearson chi-square test (χ2) and continuity correction (adjusted χ2). The consistency of the diagnostic tests was tested using kappa statistics. Kappa ≥ 0.75 was considered indicative of substantial agreement. Multivariate logistic regression analysis was conducted to identify potential risk factors for IVCS. The healing rate of leg venous ulcers was analysed using the Kaplan‒Meier method. The Wilcoxon signed-rank test was used to compare VCSS and CIVIQ-20 scores preprocedurally and during the follow-up period. Univariate and multivariate Cox regression models were used to evaluate independent factors affecting the healing of leg venous ulcers. Statistical significance was set at P < 0.05. Bonferroni correction was applied for multiple comparisons (α = 0.05/3 = 0.017). We considered an adjusted P < 0.017 to indicate statistical significance for comparisons among the three groups (A, B, and C). The data were analysed using SPSS software (version 26.0; SPSS Inc., IBM, Chicago, IL, USA).
Results
Comparison of CTV, DSV, and DUS for the diagnosis of IVCS
Among the 62 patients, 46 patients were diagnosed with IVCS by CTV, 38 patients were diagnosed with IVCS using DSV, and 17 patients were diagnosed with IVCS using DUS. Compared with those of DSV, the sensitivity and specificity of CTV were 100.0% and 66.7%, respectively (Youden index = 66.7%). The sensitivity and specificity of DUS were 42.1% and 95.8%, respectively (Youden index = 37.9%). Kappa values were 0.710 and 0.327, with concordance between DSV and CTV (P < 0.001) and between DSV and DUS (P < 0.001) (Table 2). The sensitivity of diagnosing iliac compression with CTV was better than that with DUS (χ2 = 34.008, P < 0.001). No significant difference in specificity was found between CTV and DUS (χ2 = 0.522, P = 0.470). The diagnosis of IVCS was confirmed by imaging in 46 patients according to the threshold definition. In addition, the duration of CTV (14.9 ± 1.2 min) was significantly shorter than those of DSV (20.5 ± 0.9 min, t = -29.671, P < 0.001) and DUS (22.4 ± 0.9 min, t = -37.469, P < 0.001) procedures. The dose of contrast medium administered (46.8 ± 5.1 min) for CTV was significantly lower than that administered for DSV (75.4 ± 2.6 min, t = -37.942, P < 0.001). Furthermore, CTV could accurately determine the causes of IVCS in 46 patients and provide a panoramic diagnosis of the collateral venous circulation in 16 patients.
Analysis of IVCS and clinical characteristics
Analysis of the relevance of categorical variables indicated that female sex (P = 0.036) and advanced age (P = 0.014) were significant factors for IVCS (Table 3). Clinical characteristics were analysed according to IVCS status.
Analysis of stenting for IVCS
Fifty-four patients with superficial vein reflux underwent high ligation and stripping of the great saphenous vein (37.0%, 20/54) or endovascular laser ablation (63.0%, 34/54). Incompetent perforating veins were surgically interrupted using endoscopic techniques (77.8%, 14/18), and foam sclerotherapy was performed using the Tessari method (22.2%, 4/18). During a follow-up period of 1 to 7 months, C6 healing was achieved in 56.5% (35/62) of the limbs at a median follow-up of 26 days (Figure S1). Among a total of 62 patients who were followed up in the three treatment groups, one patient was lost to follow-up due to a traffic accident. Incomplete ulcer healing was reported by 2 patients in Group B and 2 patients in Group C. Active venous ulcer healing times were significantly different among Group A, Group B and Group C (P = 0.020, Fig. 4, Table 4). The healing times of Groups A and C were both significantly shorter than that of Group B, which included patients with IVCS treated without iliac vein stent placement (χ2 = 5.872, P = 0.015 < 0.017; χ2 = 8.052, P = 0.005 < 0.017). No significant differences were found between Group A and Group C (χ2 = 0.096, P = 0.756 > 0.017). Ulcer healing time did not significantly differ between Groups A and C (P = 0.641, Figure S2). Univariate and multivariate Cox regression models showed that IVCS was the only risk factor for healing of active VLU (Table S2, S3). One month after stenting (6.0, 10.25), VCSSs significantly improved compared with those at baseline (13.75, 20.00; Z = -4.801, P < 0.001), and CIVIQ-20 scores also significantly improved after stenting (68.00, 85.00) compared with those at baseline (53.75, 62.25; Z = − 5.071, P < 0.001).
Discussion
In this study, we evaluated the important role of CTV in IVCS, as a mechanical problem. The outcomes may lead to better treatment strategies, especially for recalcitrant VLU, which often progress over time with advanced age, preexisting venous disease, and a large wound area18. The diagnosis of iliac vein obstruction largely depends on anatomical rather than haemodynamic criteria. Two main techniques are used for lower limb CTV: indirect and direct. Andreia et al.19 showed that direct CTV is valuable for defining dominant iliac vein inflow. Based on the present study, we suggest that CTV is more valuable than DSV or DUS for detecting IVCS among VLU patients. However, because of the smaller cross-sectional image of the iliac vein compared to that of IVUS in a previous study20, DUS is not considered a reliable diagnostic test for iliac vein stenosis.
Furthermore, the reduced procedure time and dose of contrast medium required compared with those of two-dimensional DSV, CTV, which has a lower specificity (Sp: 66.7%), helped detect IVCS that was not detectable using DSV and provided a panoramic assessment of collateral venous circulation and information about the causes of IVCS, such as arteries, neoplasms, and lumbar degenerative osteophytes. Magnetic resonance flow-sensitive sequences allow the prediction of the haemodynamic significance of compressive lesions by assessing the direction of venous flow21. However, MRV remains an expensive, time-consuming and scarcely available examination that requires a certain level of expertise that most centres have yet to reached. Furthermore, due to the lack of expertise, MRV for the diagnosis of IVCS cannot be performed in our hospital. For these reasons, CTV has been acknowledged as a useful modality for the confirmation of IVCS in our institution.
Methods for the measurement of iliac vein stenosis on CT scans and confirmation of the significant stenosis rate used for treatment remain controversial. The diameter at the point of maximum compression divided by the average proximal and distal diameters of the uncompressed common iliac vein was measured. On routine CTV imaging, Raju et al.20 concluded that a diameter < 16 mm for the common iliac vein or < 14 mm for the external iliac vein appears to correlate with IVUS-calibre stenosis with good diagnostic metrics of low rates of false-positive and false-negative results. In the absence of haemodynamic information, most researchers believe that treating IVCS with minimal diameter stenosis > 50% is appropriate based on acceptable clinical outcomes in patients who are stented under this criterion3,22. Another study suggested that stent implantation should be performed when the stenosis rate of the iliac vein is greater than 70% and most blood flow occurs through the collateral vein into the contralateral iliac vein23. CIRSE advised that stenting of the iliac veins should be considered when the degree of stenosis is > 30% and venous collaterals are present24. In the present study, IVCS with venous cross-sectional stenosis > 50% with or without collateral veins were considered clinically significant and thus required treatment. Notably, however, not all patients with severe disease have collateral venous vessels25. In the present study, women had a higher incidence of IVCS than men, which may be due to the extra weight in the supine position during pregnancy and the additional strain on anatomic lesions after pregnancy26.
VLUs are open skin wounds on the lower leg that can last weeks, months, or even years23. No single operation is applicable to every type of VLU. In principle, superficial vein incompetence or perforating vein outflow should be corrected early. In this trial, we chose great saphenous vein high ligation and stripping or endovascular laser ablation procedures, which are the most commonly used techniques in many Chinese hospitals24. Therapy for perforating veins with endoscopic interruption and foam sclerotherapy eliminates the problem of extensive incisions without creating a major risk of infection. In recent years, Shi et al. reported that a common iliac vein diameter < 6.77 mm and a compression percentage > 42.9% were protective factors against pulmonary embolism27. However, iliac vein pathology has always been recognized as a risk factor for VLU28, and Raju et al.12 and Neglen et al.29,30 reported that iliac vein stenting is emerging as a safe and effective alternative to traditional open surgery to correct iliac vein obstruction with excellent long-term patency. Patients with CEAP classes of 3–6 and chronic venous outflow obstructions should be considered for stenting31. In our study, IVCS with C6 ulcers were treated with stenting to correct the severity of reflux and alleviate venous hypertension, which is consistent with previous studies32. The ulcer healing times of patients without IVCS or with IVCS treated with stent implantation were significantly shorter than those of patients with IVCS treated with other methods. This finding is consistent with our previous report33 and other studies26,34. Based on our findings, Stenting relieves the VLU symptoms associated with IVCS, and stent implantation may significantly reduce the VCSS and increase the CIVIQ-20 score, improving quality of life and increasing the rate of ulcer healing. Additionally, Li et al. reported that the short-term effects of stenting are beneficial for relieving IVCS and reducing blood stasis to improve clinical symptoms and signs. However, long-term effects increase the risk of thrombosis in the stent35.
Our study has several limitations. First, the power of the analysis is restricted by the nonrandomized design and selection bias due to the limited study population and single-centre study design. Second, compared with DSA and DUS, CTV requires more examination time and a higher radiation dose. Finally, VLU have a high recurrence rate, and a longer follow-up is needed to evaluate long-term symptom relief and reintervention rates after iliac vein stenting.
Conclusion
In our study, we found that the CTV could more accurately detect IVCS and identify the causes of compression. Advanced age and female sex are contributing factors for IVCS with VLU, and satisfactory outcomes including higher rate of ulcer healing, improved venous clinical severity scores and chronic venous insufficiency questionnaire-20 scores were observed with stenting in the treatment of IVCS with C6 ulcers.
Date availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Radaideh, Q., Patel, N. M. & Shammas, N. W. Iliac vein compression: Epidemiology, diagnosis and treatment. Vasc. Health Risk Manag. 9(15), 115–122. https://doi.org/10.2147/VHRM.S203349 (2019).
Lugo-Fagundo, C. et al. May-Thurner syndrome: MDCT findings and clinical correlates. Abdom. Radiol. (NY). 41(10), 2026–2030. https://doi.org/10.1007/s00261-016-0793-9 (2016).
Kim, J. H., Lee, S. K. & Kim, J. Y. Iliac vein compression syndrome by lumbar degenerative changes is associated with deep vein thrombosis after total knee arthroplasty. Arch. Orthop. Trauma Surg. https://doi.org/10.1007/s00402-023-04811-3 (2023).
Mousa, A. Y. & AbuRahma, A. F. May-Thurner syndrome: update and review. Ann. Vasc. Surg. 27(7), 984–995. https://doi.org/10.1016/j.avsg.2013.05.001 (2013).
Joh, M. & Desai, K. R. Treatment of Nonthrombotic Iliac Vein Lesions. Semin. Intervent Radiol. 38(2), 155–159. https://doi.org/10.1055/s-0041-1727101 (2021).
Poyyamoli, S. et al. May-Thurner syndrome. Cardiovasc. Diagn. Ther. 11(5), 1104–1111. https://doi.org/10.21037/cdt.2020.03.07 (2021).
Assi, I. Z. et al. An ultrasound imaging and computational fluid dynamics protocol to assess hemodynamics in iliac vein compression syndrome. J. Vasc. Surg. Venous Lymphat Disord. 11(5), 1023-1033.e5. https://doi.org/10.1016/j.jvsv.2023.05.017 (2023).
Esposito, A. et al. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur. J. Vasc. Endovasc. Surg. 60(1), 118–125. https://doi.org/10.1016/j.ejvs.2020.03.020 (2020).
Gagne, P. J. et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J. Vasc. Surg. Venous Lymphat Disord. 5(5), 678–687. https://doi.org/10.1016/j.jvsv.2017.04.007 (2017).
Jayaraj, A. & Raju, S. Three-dimensional computed tomography venogram enables accurate diagnosis and treatment of patients presenting with symptomatic chronic iliofemoral venous obstruction. J. Vasc. Surg. Venous Lymphat Disord. 9(1), 73-80.e1. https://doi.org/10.1016/j.jvsv.2020.07.012 (2021).
Rossi, F. H. et al. Comparison of computed tomography venography and intravascular ultrasound in screening and classification of iliac vein obstruction in patients with chronic venous disease. J. Vasc. Surg. Venous Lymphat Disord. 8(3), 413–422. https://doi.org/10.1016/j.jvsv.2019.09.015 (2020).
Raju, S. Best management options for chronic iliac vein stenosis and occlusion. J. Vasc. Surg. 57(4), 1163–1169. https://doi.org/10.1016/j.jvs.2012.11.084 (2013).
Bashar, K. et al. Endovascular versus medical treatment of venous compression syndrome of the iliac vein - a systematic review. Vasa. 50(1), 22–29. https://doi.org/10.1024/0301-1526/a000911 (2021).
Lurie, F. et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 8(3), 342–352. https://doi.org/10.1016/j.jvsv.2019.12.075 (2020).
O’Donnell, T. F. Jr. et al. Society for Vascular Surgery; American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. J. Vasc. Surg. https://doi.org/10.1016/j.jvs.2014.04.049 (2014).
Jayaraj, A., Powell, T. & Raju, S. Effect of body mass index on initial presentation and outcomes after stenting for quality of life-impairing chronic Iliofemoral venous obstruction. J. Vasc. Surg. Venous Lymphat Disord. 10(2), 325-333.e1. https://doi.org/10.1016/j.jvsv.2021.07.014 (2022).
Gloviczki, P. et al. The 2022 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities Part I. Duplex scanning and treatment of superficial truncal reflux: Endorsed by the society for vascular medicine and the international union of phlebology. J. Vasc. Surg. Venous Lymphat. Disord. https://doi.org/10.1016/j.jvsv.2022.09.004 (2023).
Raffetto, J. D., Ligi, D., Maniscalco, R., Khalil, R. A. & Mannello, F. Why venous leg ulcers have difficulty healing: overview on pathophysiology, clinical consequences, and treatment. J. Clin. Med. 10(1), 29. https://doi.org/10.3390/jcm10010029 (2020).
Coelho, A. & O’Sullivan, G. Usefulness of Direct Computed Tomography Venography in Predicting Inflow for Venous Reconstruction in Chronic Post-thrombotic Syndrome. Cardiovasc. Intervent Radiol. 42(5), 677–684. https://doi.org/10.1007/s00270-019-02161-5 (2019).
Raju, S. et al. Dimensional disparity between duplex and intravascular ultrasound in the assessment of iliac vein stenosis. Vasc. Med. 26(5), 549–555. https://doi.org/10.1177/1358863X211003663 (2021).
Costa, L. M. G. et al. Magnetic resonance imaging evaluation of left common iliac vein compression in patients with and without symptoms of venous disease. Circ. J. 84(5), 763–768. https://doi.org/10.1253/circj.CJ-19-0913 (2020).
Melian, C. M. et al. Intravascular ultrasound in treating iliac vein compression with endovascular stenting: A necessary tool for optimal outcomes. Vasc. Endovascular Surg. 57(3), 299–305. https://doi.org/10.1177/15385744221145143 (2023).
Shi, C. et al. Compression bandages or stockings versus no compression for treating venous leg ulcers. Cochrane Database Syst. Rev https://doi.org/10.1002/14651858.CD013397.pub2 (2021).
Shao, C. et al. Single-stage treatment with iliac vein stenting and stripping of the great saphenous vein for patients with left iliac vein compression syndrome. Asian J. Surg. 45(1), 257–264. https://doi.org/10.1016/j.asjsur.2021.05.011 (2022).
Saleem, T. Hemodynamics of iliac venous compression syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 10(4), 978–979. https://doi.org/10.1016/j.jvsv.2021.10.017 (2022).
Rodrigues, L. D. S. et al. Association between deep vein thrombosis and stent patency in symptomatic iliac vein compression syndrome: Systematic review and meta-analysis. J. Vasc. Surg. Venous Lymphat. Disord. 9(1), 275–284. https://doi.org/10.1016/j.jvsv.2020.08.022 (2021).
Shi, Y. et al. Impact of common iliac vein compression on the incidence of pulmonary embolism in patients with acute deep vein thrombosis. Eur. J. Vasc. Endovasc. Surg. 65(6), 887–894. https://doi.org/10.1016/j.ejvs.2023.03.007 (2023).
George, R. et al. The effect of deep venous stenting on healing of lower limb venous ulcers. Eur. J. Vasc. Endovasc. Surg. 48(3), 330–336. https://doi.org/10.1016/j.ejvs.2014.04.031 (2014).
Neglén, P. et al. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J. Vasc. Surg. 46(5), 979–990. https://doi.org/10.1016/j.jvs.2007.06.046 (2007).
Neglén, P. Commentary on “The effect of deep venous stenting on healing of lower limb venous ulcers”. Eur J Vasc Endovasc Surg. 48(3), 337. https://doi.org/10.1016/j.ejvs.2014.05.016 (2014).
Mahnken, A. H. et al. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc. Intervent Radiol. 37(4), 889–897. https://doi.org/10.1007/s00270-014-0875-4 (2014).
Saleem, T. et al. Clinical tolerance of untreated reflux after iliac vein stent placement. J. Vasc. Surg. Venous. Lymphat. Disord. 11(2), 294-301.e2. https://doi.org/10.1016/j.jvsv.2022.09.009 (2023).
Liu, P. et al. Application of computed tomography venography in the diagnosis and severity assessment of iliac vein compression syndrome: A retrospective study. Medicine (Baltimore) https://doi.org/10.1097/MD.0000000000012002 (2018).
Cooke, P. V. et al. Patients with active venous leg ulcers at the time of iliac vein stenting require more reoperations. J. Vasc. Surg. Venous Lymphat. Disord. 10(6), 1304–1309. https://doi.org/10.1016/j.jvsv.2022.05.002 (2022).
Li, C. et al. Effect of stent treatment on Hemodynamics in iliac vein compression syndrome with collateral vein. Med. Eng. Phys. https://doi.org/10.1016/j.medengphy.2023.103983 (2023).
Funding
The Medical Science Research Project of the Key Research and Development Program of Hebei Province, China (No. 20377732D) and the Medical Science Research Project of Health and Family Planning Commission of Hebei Province, China (No: 20140360, 20201145).
Author information
Authors and Affiliations
Contributions
Peng Liu: writing—original draft and conceptualization;Feng Zhang: writing – review & editing;Zhao-peng He: data curation; Ya-ru Han: formal analysis and investigation;Bo-yu Wang: methodology and software;Hai-xia Song: supervision, validation and statistical analysis; Li-hua Zheng: critical revision of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Medical Ethics Committee of the First Hospital of Hebei Medical University, Shijiazhuang, Hebei, China. Informed consent was obtained from all participants or their legal guardians. The authors confirm that this study was conducted in accordance with the Declaration of Helsinki. All methods were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, F., Song, Hx., He, Zp. et al. Analysis of computed tomography venography for the diagnosis and endovascular treatment of iliac venous compression syndrome with venous leg ulcers: a retrospective study. Sci Rep 14, 22314 (2024). https://doi.org/10.1038/s41598-024-72425-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72425-9