Abstract
To investigate the genetic relationship between end stage renal disease (ESRD) and human leukocyte antigen (HLA) alleles in the Guangxi Zhuang population. We performed polymerase chain reaction reversed sequence-specific oligonucleotide (PCR-rSSO) in 325 patients with ESRD and genotyped the HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 loci. The direct counting method was used to determine the frequencies of HLA alleles, and Arlequin software (version 3.5.2.2) was used for haplotypic frequency analyses to compare the included ESRD patients with 350 healthy donors from the Guangxi Zhuang population. In our study, 120 HLA alleles, 284 HLA-A-B-DRB1 haplotypes, and 332 HLA-A-C-B-DRB1-DQB1 haplotypes were detected. We found that only A*11:01-B*15:02-DRB1*12:02 had a positive association with ESRD (P = 0.001, Pc = 0.020, OR = 3.106, 95% CI = 1.497–6.446) after Bonferroni correction; thus, individuals with this haplotype may be susceptible to ESRD. A*11:01-B*15:02-DRB1*12:02 is a potentially valuable haplotype for evaluating the risk of ESRD in the Guangxi Zhuang population.
Similar content being viewed by others
Introduction
The most variable region on the 6th chromosome in the human genome, the human leukocyte antigen (HLA), plays important roles in resistance to infection, susceptibility to autoimmune disease, tumor resistance, and immune responses to allografts following organ donation1,2. In December 2023, the IMGT/HLA Database (version 3.54) reported that 38,008 HLA alleles had been found3. Racial and geographic restrictions govern the distribution of alleles and haplotypes at the HLA locus, and the distinction between racial and geographic restrictions is also significantly more pronounced at the allelic level than at the serologic level4,5.
End stage renal disease (ESRD) is the most severe stage of chronic kidney disease (CKD) and has become a significant global health problem6. A total of 10.8% of Chinese people have CKD, and the prevalence of ESRD is increasing each year7. Because it increases survival and improves patient quality of life, renal transplantation is acknowledged as the most successful therapeutic approach for ESRD8,9. HLA is a significant determinant of some autoimmune diseases, and it has been connected to the development of ESRD, along with genetics, race, age, and sex10. The allocation of kidneys for transplantation and the outcomes of kidney transplantation depend greatly on HLA matching11. Because the HLA-DRB1 alleles directly activate the recipient’s T helper lymphocytes, they should be given more weight in HLA matching12. Numerous studies have been conducted on the association between ESRD and HLA alleles. Some HLA alleles may increase susceptibility to ESRD, whereas other HLA alleles may provide protection13,14, indicating that specific susceptibility-associations alleles or variants may exist in different countries or races. Therefore, more evidence on the global distribution of susceptibility-associated HLA alleles and haplotypes can be obtained when genetic association research on different populations is reported. However, the majority of previously reported studies used low-resolution HLA typing.
Guangxi is a multiethnic province in China, and Guangxi Zhuang is the largest ethnic group, with a population of approximately 16 million. However, no studies have analyzed the relationships between ESRD and HLA polymorphisms in the Guangxi Zhuang population via high-resolution HLA typing. As a result, our findings provide new information on disease-associated factors in the Chinese population.
Subjects and methods
Subjects
This was a retrospective, case‒control study. We investigated the medical records of 5160 ESRD patients who were awaiting renal transplants at the transplant center of the Second Affiliated Hospital of Guangxi Medical University from 2014 to 2022. All research was performed in accordance with relevant guidelines and regulations. The 325 ESRD patients included in our study ranged in age from 15 to 65 years and included 188 men and 137 women. The patients were required to be of the Zhuang ethnicity and to have complete HLA typing data from all five loci to meet the inclusion criteria. We used the HLA typing results of 350 healthy volunteers from the Guangxi Branch of the Chinese Marrow Donor Program (CMDP) as controls. The donors were living at the time of the study and unrelated to the included patients. They included 176 men and 174 women between 18 and 48 years old who were of the Zhuang ethnicity and native to Guangxi15.
Ethical considerations
The Second Affiliated Hospital of Guangxi Medical University approved our study, and we obtained consent to collect original data. Therefore, the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University approved this study, and the need for informed consent was waived for (approval number: 2022-KY (0765)).
DNA extraction
We collected 2–5 ml of peripheral blood from ESRD patients using an EDTA anticoagulated blood collection tube. According to the manufacturer’s instructions, genomic DNA was extracted using a QIAamp blood kit (Qiagen, Hilden, Germany). The optical density at 260/280 nm was 1.65–1.9, and the DNA concentration was adjusted to between 30 and 100 ng/µl.
HLA genotyping
Samples from ESRD patients were subjecting to typing of the HLA-A, -B, -C, -DRB1, and -DQB1 loci via the PCR-rSSO method with LIFECODES HLA-SSO eRES TYPING KITS (LIFECODES, Waukesha, WI, USA) according to the manufacturer’s instructions. The product signals (One Lambda, Canoga Park, CA, USA) were detected with a Luminex-IS200 flow cytometer (Luminex Corporation, Austin, TX, USA), and HLA Fusion 3.0 software was used to perform HLA genotyping. The HLA genotyping results for the controls were derived from a previous study using sequence-based typing (SBT)15. The genotyping results of the two groups could be combined for analysis, although we used two different approaches, and the high-resolution standard for HLA typing was the same10.
Statistical analysis
The direct counting method was used to calculate the allele frequencies (AFs) of HLA-A, -B, -C, -DRB1, and -DQB1. The Arlequin software package (version no. 3.5.2.2) was used to estimate haplotypic frequencies with the expectation maximization (EM) algorithm16. The difference in the frequency of haplotypes between ESRD patients and controls was analyzed using SPSS version 19.0. Odds ratios (ORs) and 95% confidence intervals (CIs) were also computed with SPSS (version 19.0) and were used to express the degree to which disease was associated with a specific allele or haplotype. Bonferroni-corrected probability values (pc) were determined by multiplying individual p values by the number of comparisons made at the allele and haplotype levels. Results were considered statistically significant for p < 0.05.
Results
Association of HLA class I alleles (HLA-A, HLA-B, and HLA-C) with ESRD
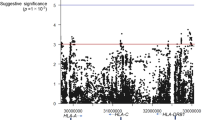
Twenty-one HLA-A alleles, 39 HLA-B alleles, and 19 HLA-C alleles were detected in the ESRD patients (Tables 1, 2 and 3). Before applying the Bonferroni correction, our analysis revealed that B*40:01 (P = 0.027) may act as a protective factor against ESRD. In contrast, A*11:01 (P = 0.031), B*15:02 (P = 0.007), B*13:02 (P = 0.031), C*08:01 (P = 0.012), and C*15:02 (P = 0.011) may be susceptibility markers for ESRD. We found that the significance of these associations was lost after applying the Bonferroni correction for the HLA-A, -B, and -C loci.
Association of HLA class II alleles (HLA-DRB1 and HLA-DQB1) with ESRD
Twenty-six HLA-DRB1 alleles and 15 HLA-DQB1 alleles were found in our study (Tables 4 and 5). Before applying the Bonferroni correction, we identified DRB1*14:54 (P = 0.005), DRB1*12:02 (P = 0.019), and DQB1*03:01 (P = 0.036) as potential susceptibility markers for ESRD. Concurrently, we found that DRB1*15:02 (P = 0.013) and DRB1*16:02 (P = 0.008) could serve as protective alleles against ESRD. After applying the Bonferroni correction, we found no association between HLA class II alleles (HLA-DRB1 and HLA-DQB1) and ESRD in the Guangxi Zhuang population.
Three-locus haplotype frequency in patients with ESRD and controls
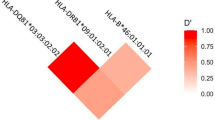
We identified 284 HLA-A-B-DRB1 haplotypes in patients with ESRD via statistical analysis. Table 6 displays the 20 most common three-locus haplotypes. The top 20 three-locus haplotypes account for 39.70% of all haplotypes, and twelve haplotypes with frequencies higher than 1% account for 32.67% of all haplotypes. The most prevalent haplotype among patients with ESRD and controls was A*33:03-B*58:01-DRB1*03:01 (ESRD, 6.12% vs. controls, 6.86%). After applying the Bonferroni correction, we found that only A*11:01-B*15:02-DRB1*12:02 was positively associated with ESRD (P = 0.001, Pc = 0.020, OR = 3.106, CI = 1.497–6.446), so this haplotype might be susceptible to ESRD.
Five-locus haplotype frequency in patients with ESRD and controls
We found 332 HLA-A-C-B-DRB1-DQB1 haplotypes in patients with ESRD via statistical analysis. Table 7 displays the 20 most common five-locus haplotypes. The top 20 five-locus haplotypes accounted for 36.64% of all haplotypes, and the twelve haplotypes with frequencies higher than 1% account for 30.28% of all haplotypes. The most prevalent haplotype was A*33:03-C*03:02-B*58:01-DRB1*03:01-DQB1*02:01 in both ESRD patients and controls (ESRD, 6.15% vs. controls, 6.86%). After applying the Bonferroni correction, we found no association between ESRD and the five-locus haplotype of HLA in the Guangxi Zhuang population.
Discussion
HLA allele and haplotype distributions are restricted geographically and racially45, and the HLA matching is important in determining transplantation success17,18. According to many studies, there is a strong correlation between HLA alleles and ESRD10,13,14. China’s largest ethnic population is the Zhuang population from Guangxi. We conducted a high-resolution analysis of the HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 loci in patients with ESRD to identify the HLA polymorphisms associated with susceptibility to ESRD in the Guangxi Zhuang population. Our findings might be helpful for future research on genetic predispositions ESRD related to the HLA region.
We identified 21 HLA-A alleles, 39 HLA-B alleles, 19 HLA-C alleles, 26 HLA-DRB1 alleles, and 15 HLA-DQB1 alleles in our study. ESRD patients shared 16 alleles with frequencies greater than 10% with the controls: A*11:01, A*24:02, A*02:03, A*02:07, B*46:01, B*13:01, C*01:02, C*08:01, C*03:04, C*07:02, DRB1*15:01, DRB1*14:54, DRB1*16:02, DQB1*05:02, DQB1*03:01, and DQB1*06:01. The identification of these 16 alleles implies that matching donors for patients who carry these HLA alleles should be relatively easy to find, making kidney transplant allocation convenient and yielding better results. However, some susceptibility-associated alleles should receive more attention because they are significantly more common in ESRD patients than in controls before Bonferroni correction, which makes it more difficult to match patients with kidney transplants14.
In some studies, HLA alleles and ESRD were found to be significantly correlated, but most of these studies used low-resolution HLA typing. HLA-B*07, DQA1*06, and DQB1*03 may be susceptibility-associated alleles for ESRD in Vietnamese populations, while HLA-B*27 and DQB1*02 may be protective alleles19. HLA-A*11, HLA-A*34, HLA-A*69, HLA-B*41, HLA-B*50, HLA-DRB1*10, and HLA-DRB1*14 may be susceptibility-associated alleles for ESRD in Romanian populations, while HLA-DRB1*07, HLA-DRB1*08, and HLA-DRB1*13 may be protective alleles20. HLA-A*24 and HLA-B*35 may be protective alleles against ESRD in Indonesian populations21. HLA-B*50 may be a susceptibility-associated allele for ESRD in Pakistan, whereas HLA-B*40, DRB1*13, and DRB1*12 may be protective alleles22. HLA-DRB1*04 and DRB1*11 may be susceptibility-associated alleles for ESRD in the Henan Han population of China, whereas HLA-B*62 and DRB1*15 may be protective alleles23. HLA-B8 may be a susceptibility-associated allele for ESRD in Kuwaiti populations, and HLA-A*28 and HLA-DR*11 may be protective alleles24. HLA-A*02, -B*48, -B*52, and -B*55 were positively associated with ESRD in the Hunan Han population of China, whereas HLA-B*60 was negatively associated with ESRD25. HLA-A*24, B*54, B*55, B*60, and DRB1*04 may be susceptibility-associated alleles for ESRD in the Cantonese population in China26. HLA-B*15 and B*18 may be susceptibility-associated alleles for ESRD in Saudi Arabian populations, whereas HLA-A*26, B*39, and B*50 may be protective alleles27. DRB1*03 and DRB1*11 are markers for susceptibility to ESRD in Taiwanese populations, and HLA-DR8 might act as a protective factor28. HLA-DR17 is associated with ESRD due to MPGN in White and Black Americans29. HLA-A*11:01, A*31:01, B*15:01, B*55:02, B*39:05, DRB1*03:01, DRB1*04:03, DRB1*04:04, DRB1*04:05, DRB1*11:01, and DRB1*12:02 were found to be susceptibility-associated alleles in the Jiangsu Han population of China. In contrast, DRB1*15:01 was a protective allele10.
Some studies also reported no associations between specific HLA alleles and ESRD. One meta-analysis revealed no significant associations between the HLA-B*50, HLA-DQA1*3, B*40, DRB1*12, DRB1*13, and DQA1*6 alleles and ESRD30. HLA-B*51 was found to have no significant relationship with ESRD patients in Turkey31. The HLA and ESRD data presented above are contradictory to and inconsistent with those of our study. Vietnamese and Taiwanese individuals have different susceptibility-associated alleles but similar backgrounds, which may be related to differences in HLA polymorphisms across regions and races; more research on this topic is needed19,21,28.
According to one publication, HLA-C mismatch has a significant adverse effect on the outcomes of bone marrow transplants using unrelated donors32. Although no HLA-C alleles were associated with susceptibility to ESRD in the Guangxi Zhuang population in our study, further research should be conducted to determine whether the effect of HLA-C mismatch on survival in ESRD patients also has a significant negative clinical impact and may lead to poor prognoses. Haplotype polymorphisms are a component of HLA polymorphisms. The HLA haplotype and linkage disequilibrium are frequently used to search for donors and to elucidate the characteristics of the population being studied10,33. According to the findings of our study, the frequencies of most haplotypes did not differ significantly between ESRD patients and controls after Bonferroni correction, suggesting that should be relatively easy to find a donor to match a patient with those haplotypes23. A*11:01-B*15:02-DRB1*12:02 emerged as susceptibility-associated haplotypes for ESRD and showed strong and significant associations with disease. Donor selection on the basis of HLA haplotype mismatches was more critical than the number or type of HLA mismatches34. Specific HLA haplotypes may lead to an immune response to antigens, contributing to ESRD development10. For the long-term survival of renal transplantation patients, avoiding susceptibility-associated alleles and haplotypes in matched consanguineous donors is crucial35.
In our study, only A*11:01-B*15:02-DRB1*12:02 was positively associated with ESRD in the Guangxi Zhuang population after Bonferroni correction; thus, this haplotype may be associated with susceptibility to ESRD. We also found that the A*11:01-B*15:02-DRB1*12:02 haplotype is significant, while the single allele of these HLA alleles is not significant; linkage disequilibrium may lead to the above result. Our findings differ significantly from those reported for some populations, a typical outcome in genetic association studies. A few potential causes are differences in HLA allele distribution across geographical regions4,5, differences in pathogenic mechanisms and environmental triggers13, referral bias, the use of small populations with nonrepresentative sizes13,36, the use of different methods for HLA typing24, and differences in HLA allomorphs37. The previously common theories of linkage disequilibrium, receptor theory, and tumor immune escape mechanisms38 can only partially account for the relationship between HLA alleles and ESRD patients and do not fully elucidate the mechanisms involved; thus, further exploration is needed.
Our investigation was conducted without taking into account primary renal disease resulting in ESRD, as in some described studies21,22,23,24,,25,27, which is one limitation of our study. The other main limitations of this study were the small number of HLA loci and the small population of patients used; thus, further research with larger patient samples, more HLA loci, and every condition that can cause ESRD is needed. Our findings may increase the success rate of kidney transplantation, provide information on disease-associated factors in the Chinese population, and lead to future research on ESRD susceptibility, especially in the Guangxi Zhuang population. Further research should be performed to validate the findings of our study.
Conclusions
This is the first report of a high-resolution analysis of the HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 loci in patients with ESRD to identify the HLA polymorphisms associated with susceptibility to ESRD in the Guangxi Zhuang population. We found that only A*11:01-B*15:02-DRB1*12:02 had a positive association with ESRD in the Guangxi Zhuang population after Bonferroni correction (P = 0.001, Pc=0.020, OR = 3.106, 95% CI = 1.497–6.446), so this haplotype may be associated with susceptibility to ESRD. Our findings will increase the success rate of kidney transplantation, provide information on disease-associated factors in the Chinese population, and lead to future research on ESRD susceptibility, especially in the Guangxi Zhuang population.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Zhong, C., Cozen, W., Bolanos, R., Song, J. & Wang, S. S. The role of HLA variation in lymphoma aetiology and survival. J. Intern. Med.286(2), 154–180 (2019).
Kamiza, A. B., Kamiza, S. & Mathew, C. G. HLA-DRB1 alleles and cervical cancer: A meta-analysis of 36 case-control studies. Cancer Epidemiol.67, 101748 (2020).
Barker, D. J. et al. The IPD-IMGT/HLA database. Nucleic Acids Res.51(D1), D1053–D1060 (2023).
Zou, J., Shen, G., Qiang, W., Zhu, Y. Y. & Li, W. X. Study on the polymorphisms of HLA-ABCDQB1DRB1 alleles and haplotypes in Hubei Han population of China. Int. J. Immunogenet.48(1), 8–15 (2021).
Baek, I. C. et al. Allele and haplotype frequencies of human leukocyte antigen-A, -B, -C, -DRB1, -DRB3/4/5, -DQA1, -DQB1, -DPA1, and -DPB1 by next generation sequencing-based typing in Koreans in South Korea. PLoS ONE16(6), e0253619 (2021).
Holley, J. L. Advance care planning in CKD/ESRD: An evolving process. Clin. J. Am. Soc. Nephrol.7(6), 1033–1038 (2012).
Chen, F., Wang, M. & Jiang, Y. Prevalence of chronic kidney disease and metabolic related indicators in Mianzhu, Sichuan, China. Front. Public Health11, 1252110 (2024).
Vareldzis, R., Naljayan, M. & Reisin, E. The incidence and pathophysiology of the obesity paradox: Should peritoneal dialysis and kidney transplant be offered to patients with obesity and end stage renal disease?. Curr. Hypertens. Rep.20(10), 84 (2018).
Tepel, M. et al. Pretransplant characteristics of kidney transplant recipients that predict posttransplant outcome. Front. Immunol.13, 945288 (2022).
Pan, Q. et al. A single center study of protective and susceptible HLA alleles and haplotypes with end stage renal disease in China. Hum. Immunol.80(11), 943–947 (2019).
Fejzić, E. et al. HLA genotyping in patients with end stage renal disease waiting for cadaveric renal transplantation in Federation of Bosnia and Herzegovina. Open Access Maced. J. Med. Sci.5(1), 1–5 (2017).
Kosmoliaptsis, V. et al. Impact of donor mismatches at individual HLA-A, -B, -C, -DR, and -DQ loci on the development of HLA-specific antibodies in patients listed for repeat renal transplantation. Kidney Int.86(5), 1039–1048 (2014).
Shao, L. N. et al. Association between the polymorphism of HLA and ESRD in Dalian Han population located in North of China. Immunol. Investig.47(2), 212–219 (2018).
Hieu, H. T., Ha, N. T., Song, L. H. & Nghi, T. H. Association of human leukocyte antigen haplotypes with end stage renal disease in Vietnamese patients prior to first transplantation. Transplant. Proc.51(8), 2549–2554 (2019).
Pei, Y. F., Yu, M., Huang, H. N., Chen, J. R. & Li, H. C. Polymorphism of HLA-A, -B, -C, -DRB1 and -DQB1 allele and haplotype frequency at high resolution in Guangxi Zhuang population. Zhongguo Shi Yan Xue Ye Xue Za Zhi28(4), 1397–1405 (2020) (Chinese).
Excoffier, L. & Lischer, H. E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour.10(3), 564–567 (2010).
Kumru Sahin, G., Unterrainer, C. & Süsal, C. Critical evaluation of a possible role of HLA epitope matching in kidney transplantation. Transpl. Rev. (Orlando)34(2), 100533 (2020).
Alelign, T. et al. Kidney transplantation: The challenge of human leukocyte antigen and its therapeutic strategies. J. Immunol. Res.2018, 5986740 (2018).
Le Pham, N. M., Ong, T. P., Vuong, N. L., Van Tran, B. & Nguyen, T. T. H. HLA types and their association with end stage renal disease in Vietnamese patients: A cross-sectional study. Medicine (Baltimore)101(48), e31856 (2022).
Iancu Loga, L. I. et al. Association between human leukocyte antigen and end stage renal disease in patients from Transylvania, Romania. Int. J. Mol. Sci.24(17), 13383 (2023).
Susianti, H. et al. Evaluation of human leukocyte antigen class I and class II in end stage renal disease occurrence in Indonesian transplantation patients. Int. J. Nephrol.2021, 4219822 (2021).
Noureen, N. et al. Revisiting the association between human leukocyte antigen and end stage renal disease. PLoS ONE15(9), e0238878 (2020).
Shang, W. et al. Comparison of HLA-A, -B and -DRB1 loci polymorphism between kidney transplants of uremia patients and healthy individuals in Central China. PLoS ONE11(10), e0165426 (2016).
Mosaad, Y. M. et al. Association between human leukocyte antigens (HLA-A, -B, and -DR) and end stage renal disease in Kuwaiti patients awaiting transplantation. Ren. Fail.36(8), 1317–1321 (2014).
Long, L. & Sun, Q. Association of end stage renal disease with HLA phenotypes and panel reactive antibodies in patients awaiting renal transplantation in Hunan Province. J. Clin. Lab. Anal.36(3), e24251 (2022).
Cao, Q. et al. HLA polymorphism and susceptibility to end stage renal disease in Cantonese patients awaiting kidney transplantation. PLoS ONE9(6), e90869 (2014).
Hamdi, N. M., Al-Hababi, F. H. & Eid, A. E. Correction: HLA Class I and Class II Associations with ESRD in Saudi Arabian Population. PLoS ONE12(12), e0190127 (2017).
Dai, C. S. et al. Association between human leucocyte antigen subtypes and risk of end stage renal disease in Taiwanese: A retrospective study. BMC Nephrol.16, 177 (2015).
Afolabi, H. et al. The association of class I and II human leukocyte antigen serotypes with end stage kidney disease due to membranoproliferative glomerulonephritis and dense deposit disease. Am. J. Kidney Dis.83(1), 79–89 (2024).
Noureen, N. & Zaidi, N. Association between human leukocyte antigen (HLA) and end stage renal disease (ESRD): A meta-analysis. Peer J.11, e14792 (2023).
Kavuzlu, M., Zengel, B. & Baştürk, B. HLA-B*51 frequency in transplant patients and donors. Exp. Clin. Transpl.22(Suppl 1), 265–269 (2024).
Woolfrey, A. et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol. Blood Marrow Transpl.17(6), 885–892 (2011).
Qi, J. et al. Relationship between high-resolution HLA-A,-B,-DRB1 alleles and haplotype polymorphisms with myeloid leukemia of Han People in North China. Zhongguo Shi Yan Xue Ye Xue Za Zhi26(1), 32–41 (2018) (Chinese).
Brifkani, Z. et al. The privilege of induction avoidance and calcineurin inhibitors withdrawal in 2 haplotype HLA matched white kidney transplantation. Transplant3(3), e133 (2017).
Miles, C. D., Schaubel, D. E., Liu, D., Port, F. K. & Rao, P. S. The role of donor-recipient relationship in long-term outcomes of living donor renal transplantation. Transplantation85(10), 1483–1488 (2008).
El-Gezawy, E. M. et al. Human leukocyte antigens as a risk factor for the primary diseases leading to end stage renal disease in Egyptian patients. Egypt. J. Immunol.18(2), 13–21 (2011).
Robson, K. J., Ooi, J. D., Holdsworth, S. R., Rossjohn, J. & Kitching, A. R. HLA and kidney disease: From associations to mechanisms. Nat. Rev. Nephrol.14(10), 636–655 (2018).
Lee, K. W., Yam, J. W. P. & Mao, X. Dendritic cell vaccines: A shift from conventional approach to new generations. Cells12(17), 2147 (2023).
Acknowledgements
The authors thank all the participants in the study.
Funding
This project was sponsored by the grants from the National Natural Science Foundation of China (No. 81670596).
Author information
Authors and Affiliations
Contributions
H.C.X. performed the experiments. Q.Y.H. analyzed the data. P.Y.F. and L.H.B. wrote the paper. S.X.Y. revised the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pei, Y., Li, H., Huang, C. et al. Associations between end stage renal disease and HLA polymorphisms in the Guangxi Zhuang population. Sci Rep 14, 21765 (2024). https://doi.org/10.1038/s41598-024-72688-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72688-2