Abstract
There are considerable researches on risk factors for necrotizing enterocolitis (NEC), focusing primarily on the entire course before onset. However, fewer studies address risk factors within the brief period before NEC occurrence. The current study aims to retrospectively analyze the clinical data of NEC patients while focusing on relevant risk factors in the preceding week of NEC onset. Infants born between January 2019 and December 2021 at Suzhou Municipal Hospital and Suzhou University Children’s Hospital with a birth weight < 1500 g or a gestational age < 32 weeks were included. Around 54 NEC patients and 180 controls were recruited in the study. NEC patients satisfying the inclusion criteria formed the case group, while a 1:4 matching principle helped select the control group based on gestational age and birth weight. A statistically significant difference was observed between groups when red blood cell transfusions were compared the week before NEC onset (adjusted OR and 95% CI 2.16 (1.10, 4.24)). Broad-spectrum antibiotic usage before NEC occurrence was significantly lower in the NEC group than in the control group (adjusted OR and 95% CI 0.95 (0.91, 0.99)). A statistically significant difference was observed between groups while comparing patent ductus arteriosus (PDA) (adjusted OR and 95% CI 2.45 (1.23, 4.91)). The indication for packed red blood cell transfusion should be strictly controlled. Moreover, close monitoring of the patient’s condition for NEC occurrence should be conducted within one-week post-transfusion. Accurately identifying infections and using broad-spectrum antibiotics can reduce the incidence of NEC.
Similar content being viewed by others
Background
Necrotizing enterocolitis (NEC) among newborns is the most common life-threatening gastrointestinal emergency in the NICU. Mainly, this is prevalent in preterm infants, especially those with extremely low birth weight1. The survival rate of premature infants has significantly increased with improving perinatal medicine and neonatal critical care. However, NEC incidence in premature infants remains high. A survey in the United States revealed that 7% of infants with extremely low birth weight developed NEC2. A recent nationwide multicentric study in China indicated that NEC incidence in extremely preterm infants was 4.9%3. Currently, specific NEC treatment is lacking. Some studies have reported mortality rates of 20-30% in NEC patients4,5,6. Additionally, the postoperative mortality rate among extremely preterm infants with NEC undergoing surgical treatment could be upwards of 50%7. The early clinical manifestations of NEC in infants remain atypical, and diagnosis relies on abnormal findings on abdominal X-rays (e.g., pneumatosis intestinalis) and systemic microcirculatory disturbances during the progressive stage8,9. The disease has progressed to a certain degree of severity when meeting the clinical diagnosis standards. This severely affects the treatment success rate and the short- and long-term prognosis of surviving children.
The exact pathogenesis of NEC remains elusive. Despite the ongoing exploration of the pathophysiology of NEC and clinical risk factors, along with the continual optimization of management and treatment strategies, NEC incidence in premature infants has remained the same10,11,12,13. Current research on NEC risk factors has focused on the entire course leading up to NEC occurrence without deciphering the clinical characteristic changes during the short period preceding NEC onset. During this brief period, clinical features can provide crucial clues to the disease’s occurrence. Therefore, the current aim is to analyze relevant clinical information of infants the week before NEC diagnosis to identify risk factors predicting whether an infant can develop NEC. This study focused on infant clinical characteristics in the week leading up to NEC onset and analyzed associated factors to provide a reference for early diagnosis and intervention of NEC.
Materials and methods
Study population
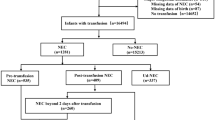
A retrospective clinical data analysis of infants with necrotizing enterocolitis admitted to the Neonatology Department of the Suzhou Municipal Hospital and Suzhou University Children’s Hospital between January 2019 and December 2021 was conducted. Inclusion criteria: (1) Premature infants with gestational age < 32 weeks or birth weight < 1500 g; (2) Satisfying the modified Bell staging criteria for NEC stages II and III. Exclusion criteria: (1) Genetic metabolic disorders, (2) Chromosomal disorders, and (3) Severe congenital malformations. The control group was matched according to the gestational age ± 3 days and birth weight ± 100 g of NEC cases in a 1:4 ratio during the same period.
The current study obtained approval from the Ethics Committee of Suzhou Municipal Hospital and Suzhou University Affiliated Children’s Hospital. Written informed consent has been obtained from the patient’s family, granting permission for the publication of their child’s clinical data. All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards.
Data collection
Completed demographic and clinical data were extracted from the medical records. These included the antepartum state of the mother (hypertension, chorioamnionitis, steroid, and MgSO4 use), neonatal gestational age, birth weight, Apgar score, feeding status from birth to NEC occurrence, feeding status in the preceding week of NEC onset, central venous catheter placement, invasive ventilation, vasopressors, ibuprofen, steroids, antibiotics, blood transfusions, and parenteral nutrition. Data were obtained for cases and controls from birth to the day before the documented onset of NEC. For instance, if the case developed NEC on the day life of 10, data before NEC for the case and its assigned controls were collected from birth up to the day of life 9. Moreover, data from one week before NEC were gathered from day of life 2 up to 9.
Statistical analysis
The statistical data analysis was conducted using R 4.2.3 statistical software. Normally distributed continuous data had been expressed as mean ± standard deviation (SD). Moreover, intergroup comparisons were performed with the t-test. Non-normally distributed continuous data were expressed as median (P25, P75). Furthermore, intergroup comparisons were performed with non-parametric tests. The categorical data were presented as n (%), and the chi-square or Fisher’s exact tests helped perform intergroup comparisons. A univariate analysis was performed by comparing variables between groups to analyze risk factors for NEC. Variables with statistically significant differences (P < 0.05) became potential risk factors and were included in a multivariate logistic regression model to characterize independent NEC risk factors.
Results
General characteristics
During the study period, 1334 premature infants with a birth weight < 1500 g or gestational age < 32 weeks were admitted to the Suzhou Municipal Hospital and Suzhou University Affiliated Children’s Hospital Neonatal Department. Twenty-seven cases with severe congenital malformations, chromosomal disorders, and genetic metabolic diseases were excluded. Ultimately, 54 infants (4.13%) with confirmed NEC (Bell staging ≥ II A) were enrolled in the study. A control group of 180 cases was included according to the gestational age and birth weight. The baseline characteristics of the case and control group infants are represented in Table 1. The NEC group infants had a mean gestational age of 29.6 weeks (± 2.0), a birth weight of 1175 g [IQR 1000–1372], and a median age at onset of 23.5 days [IQR 16.2–29.0] post-birth. The control group had a mean gestational age of 29.8 weeks (± 1.8) and a birth weight of 1250 g [IQR 1045–1400] without statistically significant difference (P > 0.05). No statistically significant differences (P > 0.05) could be observed between the groups based on maternal factors, including hypertension in pregnancy, chorioamnionitis, antenatal use of magnesium sulfate, and steroids. Moreover, there were no statistically significant differences between the groups amongst infant factors such as gender, mode of delivery, Apgar score, and small for gestational age.
Clinical procedures
The clinical procedures were compared between groups with single-factor logistic regression, as represented in Table 2.
Before NEC onset, no statistically significant difference was observed in the age of initiating feeding (2.0 days [IQR 1.0–7.2] vs. 2.0 days [IQR 1.0–3.2]), breastfeeding (4(7.5%) vs. 29(16.1%)), invasive mechanical ventilation (12(22.2%) vs. 46(25.6%)), inotropes (31(57.4%) vs. 95(52.8%)), ibuprofen (6(11.1%) vs. 16(8.9%)), steroids (5(9.3%) vs. 13(7.2%)), and packed blood red cells transfusion (31(57.4%) vs. 100(55.6%)) between the case and control groups. Moreover, no statistically significant difference between the two groups could be observed using probiotics, total parenteral nutrition, umbilical vein catheterization, and central venous catheterization (P > 0.05). A statistically significant difference was observed in the age at which the children attained full enteral nutrition (33.5 days [IQR 21.0-52.5] vs. 23.0 days [IQR 15.8–34.5]), correction of gestational age of FEF (34.6 weeks [IQR 32.9–36.7] vs. 33.4 weeks [IQR 32.3–34.9]), and days of enteral feeding (15.0 days [IQR 7.5–22.8] vs. 18.0 days [IQR 12.0–26.0]) (P < 0.05). Interestingly, a greater number of children in the control group (177 (98.3%)) were exposed to antibiotics than the case group (47 (87.0%)). The control group (10.0 days [IQR 5–18]) had a significantly longer duration of broad-spectrum antibiotic use than the case group (7.0 days [IQR 0–16]), with a statistically significant difference (P < 0.05).
During the week before the onset of NEC, there was a statistically significant difference between groups in terms of duration of enteral feeding (P < 0.05). The case group (26 (48.1%)) had a significantly higher proportion of children receiving red blood cell transfusions than the control group (51 (28.3%)), with a statistically significant difference (P < 0.05).
Complications and adverse outcomes
The NEC group had a significantly higher incidence of patent ductus arteriosus, late-onset sepsis, and death than the control group (P < 0.05). However, no statistically significant differences were observed between the groups in the incidence of neonatal respiratory distress syndrome, bronchopulmonary dysplasia, severe intracranial hemorrhage, periventricular leukomalacia, and retinopathy of prematurity (P > 0.05) (Table 3).
Multivariate analysis
The variables with statistically significant differences were incorporated from the univariate analysis into binary logistic regression analysis. The results indicated that patent ductus arteriosus had an odds ratio of 2.45, 95% confidence interval (1.23, 4.91), and red blood cell transfusion in the week before NEC had an odds ratio of 2.16, 95% confidence interval (1.10, 4.24), as independent risk factors for NEC. On the other hand, the duration of exposure to broad-spectrum antibiotics before NEC occurrence possessed an odds ratio of 0.95 and a 95% confidence interval (0.91, 0.99) (Table 4).
Disscussion
NEC incidence in this study population, comprising preterm infants with gestational age < 32 weeks or birth weight < 1500 g, was 4.13%, significantly lower than the internationally reported range of 5–7%2. This was lower than China’s national average incidence rate of 4.9%3. This was primarily attributed to improving our precise integrated treatment capabilities for preterm infants through precise nutritional management strategies and strict control over antibiotic and blood transfusion indications. Our research findings indicated that patent ductus arteriosus and the administration of suspended red blood cells the week before onset were linked with NEC. Our study observed that strict control over the indications for antibiotic use and adequate treatment duration may decrease NEC incidence, contrary to our previous understanding.
Preterm babies can develop anemia post-birth and require blood transfusions during hospitalization. Valieva et al.14 reported that 17% of extremely low birth weight infants developed NEC within seven days after receiving a blood transfusion (OR 2.37; 95% CI 0.12, 47.00). In contrast, Hay et al.15 reported that TANEC occurred within 48 h, with an odds ratio of 1.13 (95% CI 0.99, 1.29). Teišerskas and Wan-Huen16,17 observed that red blood cell transfusion within 48 h before NEC onset could be a risk factor for its occurrence. However, a recent meta-analysis showed that the unadjusted odds of developing TANEC were reduced in infants receiving red blood cell transfusions18. Sharma et al.19 identified no association between transfusion of red blood cells within 48 h or seven days before NEC onset. This study suggested no correlation between the transfusion of red blood cells throughout the NEC course and its occurrence. However, receiving red blood cell tranusions within one week before NEC onset enhanced NEC risk (adjusted OR 2.16, 95% CI 1.10, 4.24), consistent with the findings provided by Valieva et al. This could be associated with hemodynamic changes, blood viscosity alterations, and vascular reactivity changes caused by red blood cell transfusion, further triggering mesenteric ischemia-hypoxia necrosis. Simultaneously, blood transfusion-induced HLA antibody-mediated alloantigen-related immune response triggers activation, proliferation, and apoptosis of vascular endothelial cells, causing intestinal damage. However, a controversy involving whether red blood cell transfusion elevates NEC occurrence in premature infants still exists. Therefore, we should strictly control the indications for transfusion in clinical practice, closely monitor the clinical signs of infants within one week post-transfusion, and be vigilant for NEC development.
The correlation between antibiotics and NEC occurrence is subject to debate. In a large multicentric cohort study of extremely low birth weight preterm infants undertaken by Li et al.20, after adjusting for other confounding factors, there was no increased incidence of NEC in the infant group who received early antibiotic treatment. Another study observed that antibiotic treatment in the first five days of life protected against NEC21. However, NEC occurrence risk increased by 7–20% for each additional day of antibiotic exposure within 7–14 days post-birth22,23. Studies on mouse and premature infant intestinal specimens depicted that premature infant intestines are prone to excessive inflammatory responses24,25. Limiting antibiotic use could delay bacterial colonization, giving the intestinal immune system more time to mature26. However, long-term antibiotic therapy may cause intestinal damage, such as dysbiosis27,28, reduced concentrations of short-chain fatty acids, intestinal tight junction barrier disruption, and elevated autophagy activation. The study results indicated that the timing of broad-spectrum antibiotic use protects against NEC, contradicting the findings of most literature. However, the trend of sepsis incidence before NEC within the case group was higher than in the control group. Moreover, broad-spectrum antibiotic usage was strictly regulated at the research center, demanding joint approval from the chief physician and clinical pharmacist. Therefore, adequate use of broad-spectrum antibiotics can effectively control infections, decrease inflammatory responses among children, and reduce the occurrence of sepsis and NEC.
The occurrence of NEC and PDA are closely associated. In infants with left-to-right shunting PDA, inadequate systemic perfusion can compromise intestinal blood supply, elevating NEC risk29. In a randomized controlled trial in 1989, NEC risk in PDA infants who underwent prophylactic surgical ligation was decreased (8% vs. 30%)30. In this study, the odds of NEC occurring in PDA infants were enhanced by 1.45 times. This could be associated with systemic blood flow redistribution caused by PDA. There is a sharp decline in blood supply to the gastrointestinal tract in infants with PDA, which provides blood supply to vital organs, including the brain and heart. In contrast, blood flow velocity in the superior mesenteric artery and abdominal aorta is significantly reduced. Moreover, hypoxia disrupts the balance between releasing oxygen free radicals (constricting factor) and synthesizing local tissue NO (diastolic factor). Due to their poor hemodynamic regulatory ability, premature infants could be prone to initiating the cascade reaction of hypoxanthine in the reperfusion phase post-hypoxia. This generates and releases many oxygen-free radicals, inducing organ damage31.
Unlike previous studies, this study attempted to identify risk factors predicting NEC in premature infants by focusing on relevant clinical information within a week preceding NEC onset. However, due to the study’s shorter duration and smaller sample size, the results may be biased.
Conclusion
The current study conducted a retrospective analysis of clinical data in premature infants to identify risk factors linked with NEC, focusing on clinical characteristics in the week preceding NEC onset. We identified associations between patent ductus arteriosus and packed red blood cell transfusion the week before NEC onset and occurrence. The current study observed that strict control of antibiotic indications and appropriate usage duration may decrease NEC occurrence, contrary to our previous comprehension. Therefore, it is necessary to control the indications for blood transfusions and antibiotic use in clinical practice and intervene promptly in PDA patients with hemodynamic changes. This would help reduce NEC incidence and improve the prognosis of surviving infants.
Data availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
References
Hull, M. A. et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J. Am. Coll. Surg.218(6), 1148–1155 (2014).
Holman, R. C. et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr. Perinat. Epidemiol.20(6), 498–506 (2006).
Cao, Y. et al. Assessment of neonatal Intensive Care Unit practices, morbidity, and mortality among very preterm infants in China. JAMA Netw. Open.4(8), e2118904 (2021).
Patel, R. M. et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med.372(4), 331–340 (2015).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med.364(3), 255–264 (2011).
Lin, P. W. & Stoll, B. J. Necrotising enterocolitis. Lancet368(9543), 1271–1283 (2006).
Fitzgibbons, S. C. et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg.44(6), 1072–1075 (2009).
Kliegman, R. M. & Walsh, M. C. Neonatal necrotizing enterocolitis: Pathogenesis, classification, and spectrum of illness. Curr. Probl. Pediatr.17(4), 213–288 (1987).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg.187(1), 1–7 (1978).
Pammi, M. & Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev.6(6), Cd007137 (2017).
Horbar, J. D. et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr.171(3), e164396 (2017).
AlFaleh, K. & Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev.2014(4), Cd005496 (2014).
Downard, C. D. et al. Treatment of necrotizing enterocolitis: An American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J. Pediatr. Surg.47(11), 2111–2122 (2012).
Valieva, O. A. et al. Effects of transfusions in extremely low birth weight infants: A retrospective study. J. Pediatr.155(3), 331–37.e1 (2009).
Hay, S. et al. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Semin. Perinatol.41(1), 80–91 (2017).
Teišerskas, J., Bartašienė, R. & Tamelienė, R. Associations between red blood cell transfusions and necrotizing enterocolitis in very low birth weight infants: Ten-year data of a tertiary neonatal unit. Medicina (Kaunas)55(1) (2019).
Wan-Huen, P. et al. Packed red blood cell transfusion is an independent risk factor for necrotizing enterocolitis in premature infants. J. Perinatol.33(10), 786–790 (2013).
Rai, S. E., Sidhu, A. K. & Krishnan, R. J. Transfusion-associated necrotizing enterocolitis re-evaluated: A systematic review and meta-analysis. J. Perinat. Med.46(6), 665–676 (2018).
Sharma, R. et al. Packed red blood cell transfusion is not associated with increased risk of necrotizing enterocolitis in premature infants. J. Perinatol.34(11), 858–862 (2014).
Li, Y. et al. Early use of antibiotics is associated with a lower incidence of necrotizing enterocolitis in preterm, very low birth weight infants: The NEOMUNE-NeoNutriNet cohort study. J. Pediatr.227, 128-134e2 (2020).
Esmaeilizand, R. et al. Antibiotic exposure and development of necrotizing enterocolitis in very preterm neonates. Paediatr. Child. Health. 23 (4), e56–e61 (2018).
Abdel Ghany, E. A. & Ali, A. A. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann. Saudi Med.32 (5), 521–526 (2012).
Alexander, V. N., Northrup, V. & Bizzarro, M. J. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J. Pediatr.159(3), 392–397 (2011).
Yu, W. et al. SIGIRR mutation in human necrotizing enterocolitis (NEC) disrupts STAT3-dependent microRNA expression in neonatal gut. Cell. Mol. Gastroenterol. Hepatol.13(2), 425–440 (2022).
Claud, E. C. et al. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc. Natl. Acad. Sci. U S A. 101 (19), 7404–7408 (2004).
van Elburg, R. M. et al. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child. Fetal Neonatal Ed.88(1), F52–F55 (2003).
Gasparrini, A. J. et al. Antibiotic perturbation of the preterm infant gut microbiome and resistome. Gut Microbes7(5), 443–449 (2016).
Jiang, P. et al. Antibiotics increase gut metabolism and antioxidant proteins and decrease acute phase response and necrotizing enterocolitis in preterm neonates. PLoS ONE7(9), e44929 (2012).
Dollberg, S., Lusky, A. & Reichman, B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: A population-based study. J. Pediatr. Gastroenterol. Nutr.40(2), 184–188 (2005).
Cassady, G. et al. A randomized, controlled trial of very early prophylactic ligation of the ductus arteriosus in babies who weighed 1000 g or less at birth. N. Engl. J. Med.320(23), 1511–1516 (1989).
Samuels, N. et al. Risk factors for necrotizing enterocolitis in neonates: A systematic review of prognostic studies. BMC Pediatr.17(1), 105 (2017).
Acknowledgements
We thank all the children and their guardians who participated in this study.
Funding
This study was financially supported by the National Natural Science Foundation of China (82271741), Jiangsu Provincial Health and Family Planning Commission Medical Research Project (ZD2021013), SuZhou Health Talent Program (GSWS2022055), Soochow University Translational Platform Program (ML13101523), and “Suiyuan” Clinical Research Program (SY003). Recipient: Xueping Zhu. Gusu Talent Program (GSWS2021036). Recipient: Zongtai Feng.
Author information
Authors and Affiliations
Contributions
The authors L.L. and W.S. contributed to the study conception and design. Data collection was performed by Y.C. and Z.F. Analyses were conducted by Y.Y. Reviewed and revised manuscript by Z.Y. and X.Z. All authors commented on all versions of the manuscript and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The current study obtained approval from the Ethics Committee of Suzhou Municipal Hospital and Suzhou University Affiliated Children’s Hospital. Written informed consent has been obtained from the patient’s family, granting permission for the publication of their child’s clinical data. All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, L., Sun, W., Cai, Y. et al. The clinical characteristics and risk factors analysis within one week before the onset of necrotizing enterocolitis. Sci Rep 14, 22380 (2024). https://doi.org/10.1038/s41598-024-73212-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73212-2