Abstract
Cardiovascular disease remains the leading cause of death worldwide, with air pollution’s impact on cardiovascular health being closely monitored. However, the specific effects of air pollution on the risk of hospital readmission for heart failure (HF) in patients with unstable angina (UA) have not been fully explored. We conducted a retrospective study involving 12,857 consecutive patients diagnosed with acute coronary syndrome (ACS) between January 2015 and March 2023. After rigorous screening, we included 8,737 patients with UA in the analysis. Furthermore, we used a Cox proportional hazards regression model to examine the relationship between air quality indicators and hospital readmission for HF in patients with UA. Additionally, a decision tree model identified air quality indicators levels that had the most significant impact on readmission for HF risk. After adjusting for confounding factors, we found that elevated levels of PM10 [hazard ratio (HR) = 1.003, 95% confidence interval (CI): 1.000-1.005, p = 0.04453] and CO (HR = 1.013, 95% CI: 1.005–1.021, p = 0.00216) were associated with an increased risk of hospital readmission for HF in UA patients. Specifically, patients exposed to PM10 levels above 112.5 µ g/m3 had a 1.61-fold higher risk of readmission for HF in UA patients. (HR = 1.609, 95% CI: 1.190–2.176, p = 0.00201), and those exposed to CO levels above 37.5 mg/m3 had a 2.70-fold higher risk of readmission for HF in UA patients. (HR = 2.681, 95% CI: 1.731–4.152, p < 0.00001). Higher concentrations of PM10 and CO significantly increased the risk of HF (HF) readmission in patients with UA after discharge, particularly when PM10 levels exceeded 112.5 ug/m3 and CO levels surpassed 37.5 ug/m3. Besides, female patients with UA, with fewer underlying diseases, were more susceptible to the adverse effects of PM10 and CO.
Similar content being viewed by others
Introduction
Cardiovascular disease remains the leading cause of death globally, imposing a significant burden on health systems worldwide1. In 2019, the Global Burden of Disease Study reported that approximately 4.58 million people died from cardiovascular disease, accounting for 42% of all deaths2. China, in particular, bears one of the highest cardiovascular diseases globally, making it a critical area of focus for research and intervention2.
Angina pectoris, often the initial sign of cardiovascular disease, indicates partial obstruction of the coronary artery and heightens the risk of myocardial infarction, heart failure (HF), and mortality3,4. Among its forms, unstable angina (UA) represents the most severe type, classified under acute coronary syndrome (ACS). Furthermore, UA is characterized by myocardial ischemia occurring at rest or with minimal exertion, without acute myocyte injury or necrosis5. Despite advances in treatment, patients with UA still face high rates of concurrent HF and rehospitalization, highlighting the need for targeted strategies to reduce these risks6.
While traditional risk factors contribute significantly to cardiovascular disease, increasing evidence highlights the severe impact of air pollution on global cardiovascular health. The World Health Organization (WHO) reported that in 2019, outdoor air pollution led to approximately 4.2 million excess deaths worldwide, with 37% of these deaths linked to stroke and cardiovascular disease7.
Several research studies have consistently demonstrated the harmful effects of air pollution on cardiovascular health. A 2014 prospective cohort study and meta-analysis from the ESCAPE project involving 11 European cohorts found that prolonged exposure to particulate matter increased the incidence of coronary events, even at concentrations below current European air quality limits8. Additionally, a study by Rus et al. revealed that short-term exposure to various air pollutants significantly heightened the risk of hospitalization for non-ST elevation ACS, particularly in patients with UA and those with hypertension9.
While some studies have examined the adverse effects of air pollutants on UA8,9, focusing primarily on morbidity and mortality, few have investigated how these pollutants influence the risk of HF readmission in UA patients. However, evaluating the risk of hospital readmission for HF provides a more sensitive measure of early damage caused by environmental exposure than mortality alone, offering opportunities for timely intervention to reduce death rates.
To address this gap, we conducted a retrospective analysis of 8,737 UA patients who were continuously hospitalized between January 2015 and March 2023. This study aimed to assess the impact of particulate matter with a diameter of ≤ 10 μm (PM10) and carbon monoxide (CO) on the risk of HF readmission in UA patients, providing crucial evidence in an area that has been largely overlooked.
Methods
Study population
In this retrospective single-center study, we analyzed 12,857 patients diagnosed with ACS who were treated in the heart center of xiangtan city central hospital from January 2015 to March 2023 (Fig. 1). We defined ACS according to the European Society of Cardiology’s 2023 guidelines for the Management of ACS5 and coded the diagnoses using the International Classification of Diseases Version 9 (ICD-9) code 428 or ICD-10 code I50.
We excluded patients based on the following criteria: (1) Those diagnosed with myocardial infarction patients. (2) Individuals under 18 years of age. (3) Pregnancy patients. (4) Patients with malignant tumors. (5) Patients with non-cardiac diseases with a life expectancy of less than six months, and (6) Cases with incomplete or missing critical data. After multiple screenings using these criteria, we included 8737 patients with UA in the final analysis.
Data collection
We retrieved and collected patients’ electronic medical records system, including demographic information, medical history, biochemical indicators at admission, and details of drug treatments.
Air quality indicators and pollutant data
We obtained air quality data from the National Urban Air Quality Real-time Release Platform (http://106.37.208.233:20035/) and the China Online Air Quality Monitoring Platform(https://www.aqistudy.cn/historydata/). These platforms provide daily average air quality results, based on hourly data published by the China Environmental Monitoring Station. The data cover all cities in China and include historical air pollution information from December 2013 onward, allowing for detailed temporal and spatial analysis.
We used air quality indicators and air pollutant data from UA patients on the day of initial hospitalization, and investigated their impact on readmission due to HF after discharge.
More importantly, this study includes the following air quality indicators: air quality index (AQI), particulate matter with an aerodynamic diameter of ≤ 10 μm (PM10; µg/m3), fine particulate matter (PM2.5; µg/m3), carbon monoxide (CO; mg/m3), sulfur dioxide (SO2; µg/m3), nitrogen dioxide (NO2; µg/m3), and the 8-hour moving average of ozone (O3-8 h; µg/m3).
Follow-up and outcome events
We followed all study participants until October 1, 2023. A team of five experienced cardiovascular physicians and ten nurses collected patient outcome data through on-site outpatient visits and telephone follow-ups. Next, we conducted clinical and laboratory evaluations10, focusing on outcomes such as readmission for HF, myocardial infarction, and stroke. Follow-up of all-cause mortality is ongoing.
The primary outcome of this study was readmission for HF. The median follow-up time for patients who experienced HF readmission was 1,496.15 ± 816.09 days, while for those without HF readmission, it was 1023.78 ± 750.77 days. Afterwards, we diagnosed HF based on the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic HF11 and coded these diagnoses using the International Classification of Diseases Version 9 (ICD-9) code 428 or ICD-10 code I50.
Statistical analysis
We compared normally distributed continuous variables using the T-test and expressed them as mean ± SD. For continuous variables that were not normally distributed, we used a non-parametric test and reported them as median (interquartile range). Categorical variables were presented as numbers (percentages) and compared using either Pearson’s Chi-square test or Fisher’s exact test. We calculated survival curves using the Kaplan-Meier method and compared them with the Log-rank test. To identify potential cutoffs for NO2 and PM10 associated with outcome events, we employed a classification tree for chi-square automated interaction detection (CHAID). We validated the relationship between these classifications and outcome events using multiple Cox regression models, adjusting for potential confounders such as age, sex, comorbidities, and blood biomarker levels. Subsequently, we calculated the hazard ratio (HR) and its 95% confidence interval (CI) to assess the risk for each group. The stratified analysis determined the relationship between these classifications and outcome events within each subgroup. We considered results significant when P < 0.05. Statistical analysis was performed using R (version 4.2.0) and IBM SPSS Statistics 26.0.0 (SPSS Inc., Chicago, IL, USA).
Ethics and informed consent
The Ethics Committee of Xiangtan Central Hospital (Xiangtan, China; Ethics Lot Number: 2023-02-001) approved the research protocol, and the study was conducted according to the principles outlined in the Declaration of Helsinki. As this was a retrospective study, we collected only clinical data, ensuring that sensitive or identifying information was removed, and treatment plans for patients were not altered. Consequently, the requirement for informed consent was waived.
Results
Baseline characteristics
We included a total of 8,737 patients with UA in the study. Of these, 445 patients (5.1%) were readmitted due to HF, while 8,292 patients (94.9%) were not. We statistically compared the demographic characteristics, medical history, biochemical indicators at admission, drug treatments, and air quality indices among the patients.
Analogously, patients readmitted for HF after discharge were older (68.65 ± 11.42 years vs. 66.97 ± 10.96 years, p = 0.002) and had a higher prevalence of atrial fibrillation (9.2% vs. 6.3%, p = 0.014) and renal insufficiency ( 16.2% vs. 8.6%, p < 0.001). Clinically, these patients had higher N-terminal pro-brain natriuretic peptide (NT-proBNP) levels (3,588.06 ± 7268.81 pg/ml vs. 731.27 ± 2,927.15 pg/ml, p < 0.001) and Troponin T (TNT) concentrations (0.06 ± 0.10 vs. 0.07 ± 0.10, p = 0.001). They also exhibited poorer left ventricular ejection fraction (56.66 ± 12.72 vs. 62.82 ± 8.71, p < 0.001), larger left atrium end-systolic diameter (37.36 ± 5.90 vs. 34.90 ± 4.84, p < 0.001), larger left ventricular end-diastolic diameter (51.28 ± 7.36 vs. 47.75 ± 5.31, p < 0.001), larger right atrium end-systolic diameter (36.16 ± 5.69 vs. 35.24 ± 3.99, p < 0.001), larger right ventricular end-diastolic diameter (31.80 ± 3.96 vs. 31.29 ± 3.39, p < 0.001). Regarding treatment, a higher proportion of these patients used spironolactone (13.48% vs. 7.57%, p < 0.001) but a lower proportion used sodium-dependent glucose transporter 2 inhibitors (2.70% vs. 5.41%, p < 0.001). Additionally, the air quality on the day at the initial admission of the patients with UA showed higher levels of PM10 (64.65 ± 40.78 vs. 60.37 ± 37.24, p = 0.019) and CO (6.28 ± 13.10 vs. 4.87 ± 9.82, p = 0.004) (Table 1).
We observed no statistical differences between the two groups in terms of sex, body mass index, valvular heart disease, hypertension, diabetes, chronic obstructive pulmonary disease, low-density lipoprotein levels, or the use of beta-blockers, angiotensin receptor-enkephalase inhibitors, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, PM2.5, PM10, SO2, CO, NO2, and O3-8 h (p all > 0.5).
Clinical outcomes
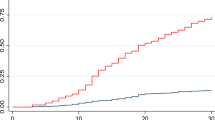
Table 2 presents a detailed comparison of air quality indicators related to the outcome events. Without adjusting for confounders (Table 2, Model I), PM10 (hazard ratio [HR] = 1.003, 95% confidence interval [CI] 1.000-1.005, p = 0.01888) and CO (HR = 1.012, 95% CI 1.004–1.020, p = 0.00394) increased the risk of HF readmission for HF in patients with UA. After adjusting for sex and age (Table 2, Model II), PM10 (HR = 1.003, 95% CI 1.000-1.005, p = 0.02726) and CO (HR = 1.011, 95% CI 1.003–1.019, p = 0.00527) continued to increase the risk of HF readmission. Besides, after adjusting for all confounders (Table 2, Model III), the risk of HF readmission due to PM10 exposure (HR = 1.001, 95% CI 1.000-1.006, p = 0.02023) and CO exposure (HR = 1.012, 95% CI 1.003–1.020, p = 0.00616) remained elevated. Figure 2 supports this finding, demonstrating that higher exposure to PM10 and CO correlates with an increased risk of HF readmission in patients with UA (Fig. 2).
Additionally, our study also found that all air quality indicators did not affect hospital mortality, initial HF, or stroke in UA patients (p > 0.05) (Supplementary Table 1).
Decision tree classification
We constructed a decision tree model (Fig. 3) to examine how different levels of PM10 and CO affect the risk of HF readmission in patients with UA after discharge. The initial split in the decision tree occurred at a PM10 concentration of 112.5 ug/m³. For patients with UA, the risk of HF readmission increased if PM10 exceeded this threshold. When PM10 was ≤ 112.5 µg/m3 and CO was ≤ 37.5 mg/m3, the risk of HF readmission remained low, indicating that CO’s impact on outcome events was minimal at this PM10 level. However, the risk for HF readmission significantly increased when CO levels exceeded 37.5 mg/m3, suggesting that higher CO concentrations may pose an additional health risk for patients with UA. Furthermore, we identified a new split point for PM10 at 251.0 µg/m3, revealing an unusual trend: when PM10 levels were extremely high (> 251.0 µg/m3), the proportion of patients with UA readmitted for HF decreased after discharge.
Group comparison after decision tree grouping
Table 3 shows how varying levels of PM10 and CO affect the risk of HF readmission in patients with UA after discharge, Without adjusting for any variables (Table 3, Model I), patients with UA faced a risk of HF readmission approximately 1.64 times higher when PM10 exceeded 112.5 ug/m3 (HR = 1.644, 95% CI: 1.226–2.205, p = 0.00089) compared to those with PM10 ≤ 112.5 µg/m3. Similarly, patients with CO levels > 37.5 mg/m3 had a 2.70-fold increase in the risk of HF readmission compared to those with CO ≤ 37.5 mg/m³ (HR = 2.698, 95% CI: 1.755–4.147, p < 0.00001). Figure 4 supports these findings, showing that elevated PM10 levels (> 112.5 ug/m3) and high CO levels (> 37.5 mg/m3) both increase the risk of HF readmission (Fig. 4A and B). After adjusting for sex and age (Table 3, Model II), the risk of HF readmission was about 1.62 times higher with PM10 levels > 112.5 ug/m3 (HR = 1.621, 95% CI: 1.209–2.175, p = 0.00127) and 2.60 times higher with CO levels > 37.5 mg/m³ (HR = 2.597, 95% CI: 1.688–3.996, p = 0.00001). Next, after adjusting for all confounders (Table 3, Model III), the risk of HF readmission increased approximately 1.69 times with PM10 > 112.5 ug/m3 (HR = 1.688, 95% CI: 1.240–2.298, p = 0.00089) and 2.43 times with CO > 37.5 mg/m3 (HR = 2.432, 95% CI: 1.539–3.844, p = 0.00014). These results confirm significant differences in the risk of HF readmission associated with varying levels of PM10 and CO, providing valuable insights for disease risk assessment and clinical decision-making.
Hierarchical analysis
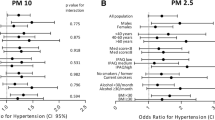
We analyzed how different characteristics affected the susceptibility of UA patients to PM10 and CO. The results in Table 4 reveal that patients aged 75 years or younger (HR = 1.00, 95% CI: 1.00-1.01, p = 0.0208), female patients (HR = 1.00, 95% CI: 1.00-1.01, p = 0.0245), those with a BMI under 28 (HR = 1.00, 95% CI: 1.00-1.01, p = 0.0151), and patients without a history of myocardial infarction (HR = 1.009, 95% CI: 1.003–1.014, p = 0.0017), atrial fibrillation ( HR = 1.00, 95% CI: 1.00-1.01, p = 0.0244), valvular heart disease (HR = 1.00, 95% CI: 1.00-1.01, p = 0.0095), hypertension (HR = 1.00, 95% CI: 1.00-1.01, p = 0.0099), chronic obstructive pulmonary disease (HR = 1.00, 95% CI: 1.00-1.01, p = 0.0103), and renal insufficiency (HR = 1.00, 95% CI: 1.00-1.01, p = 0.0088)were more sensitive to PM10. On top of that, patients with a history of diabetes (HR = 1.01, 95% CI: 1.00-1.01, p = 0.0085) also showed increased sensitivity to PM10.
In contrast, patients older than 75 years (HR = 1.02, 95% CI: 1.00-1.03, p = 0.0135), female patients (HR = 1.02, 95% CI: 1.00-1.03, p = 0.0045), those with a BMI under 28 (HR = 1.00, 95% CI:1.00-1.01, p = 0.0151), and patients without a history of myocardial infarction (HR = 1.009, 95% CI: 1.003–1.014, p = 0.0017), atrial fibrillation (HR = 1.01, 95% CI: 1.00-1.02, p = 0.0061), valvular heart disease (HR = 1.01, 95% CI: 1.01–1.02, p = 0.0018), hypertension (HR = 1.02, 95% CI: 1.00-1.03, p = 0.0076), and renal insufficiency (HR = 1.01, 95% CI: 1.00-1.02, p = 0.0157)were more sensitive to CO. Additionally, patients with a history of diabetes (HR = 1.03, 95% CI: 1.01–1.04, p = 0.0002) were more sensitive to CO. Overall, the results show that female patients with fewer underlying diseases exhibited greater sensitivity to both PM10 and CO.
Discussion
This study reveals that exposure to PM10 and CO significantly heightens the risk of HF readmission in patients with UA, particularly when PM10 exceeds 112.5 µg/m3 and CO surpasses 37.5 mg/m3. We also identified that female patients with UA and fewer underlying diseases exhibit greater sensitivity to these pollutants. To the best of our knowledge, this research is the first clinical study to highlight how PM10 and CO exposure elevates the risk of HF readmission, defining hazard concentration thresholds and identifying characteristics of the most susceptible populations. This work therefore addresses a significant gap in the current literature.
Effect of PM10 and CO on cardiovascular disease
Numerous studies have investigated the harmful effects of air pollutants on cardiovascular diseases, similar to our findings. Remarkably, research has consistently shown that exposure to PM10 and CO adversely affects cardiovascular health, particularly coronary artery disease. Schwartz and Morris initially identified a link between PM10, CO, and cardiovascular disease hospitalization rates in Detroit, Michigan, USA in 199512. Later studies in Tucson, AZ, USA, and eight other US counties confirmed that PM10 and CO were associated with increased cardiovascular admissions13,14. A 2023 meta-analysis in China confirmed a significant impact of PM10 on HF incidence15. Furthermore, a meta-analysis conducted by Chen et al.16 which included studies from lower-income countries, corroborated these findings, reinforcing the impact of PM10 on HF.
Evidence increasingly highlights the pronounced effects of PM10 and CO on coronary artery disease. An Italian study demonstrated that PM10 exposure elevates the risk of ACS17. A large case-crossover study in China from 2013 to 2018 found that short-term exposure to PM10 significantly increased the risk of death from acute myocardial infarction18. Likewise, Cesaroni et al. showed that chronic exposure to PM10 raises the risk of acute coronary artery disease8. Additionally, a study from Spain established an association between CO exposure and increased cardiovascular admission risk19.
Further research has investigated the impact of PM10 and CO on UA. A 2023 systematic review and meta-analysis revealed that short-term exposure to PM10 and CO heightens the risk of angina20. Epidemiological data suggest that higher PM10 concentrations correlate with a higher incidence of UA among elderly patients17. Lin et al.21 found a significant link between CO exposure and emergency room visits for ischemic cardiovascular disease. A study conducted in Tehran from 1996 to 2001 reported a significant association between CO levels and daily angina admissions, with a 1.00934 increase in admissions for each unit rise in CO levels (95% CI: 1.00359–1.01512)22. While similar studies focus on the incidence, hospitalization, and mortality related to UA, our research uniquely explores the impact of PM10 and CO on outcome events, defining the hazard concentration thresholds and characteristics of the susceptible population. However, findings are not uniform; for example, Kleinman et al. reported an 18% reduction in angina episodes due to CO exposure23.
Potential pathogenesis and pathways
Air pollutants entering the lungs cause oxidative damage, endothelial dysfunction, and systemic inflammation, which ultimately lead to vasoconstriction, increased cardiac afterload, and pulmonary artery disease, raising the risk of HF24,25. Research has shown that exposure to PM correlates with elevated levels of pro-inflammatory markers24, suggesting that air pollutants might heighten cardiovascular disease risk through inflammatory responses. Equally, evidence indicates that air pollutants can increase HF risk by affecting adrenaline, noradrenaline, and cortisol levels, and by causing oxidative deoxyribonucleic acid (DNA) damage26. Also, CO with a binding affinity for hemoglobin 250 times greater than that of oxygen, impairs effective oxygen delivery to body tissues, a critical issue for individuals with atherosclerotic disease or other cardiac conditions9. The impact of these pollutants varies based on pollutant type, exposure dose and duration, cardiovascular endpoints, and individual health status24.
Susceptible population
Our study found that female patients with UA and fewer underlying conditions were more sensitive to PM10 and CO. Previous epidemiological studies have shown that women are more sensitive to air pollution27. Basic research indicates that female mice are more susceptible to air pollution than males, likely due to the effects of gonadal hormones and increased inflammatory damage28. Some research suggests that women’s greater sensitivity may be related to structural and functional differences29. In like manner, women often engage in more household cleaning and kitchen work, leading to higher indoor air pollution exposure, which may interact with outdoor pollution and increase sensitivity. We hypothesize that individuals with fewer underlying diseases may be more sensitive to harmful stimuli due to their physiological and metabolic characteristics, resulting in more pronounced systemic reactions after exposure. However, other studies suggest that older adults (aged ≥ 65 years) and those with comorbid conditions, such as congestive HF, may be more vulnerable to air pollution30. As much of the current understanding of susceptibility remains speculative, further clinical and basic research is needed to provide stronger evidence.
Hazardous concentration threshold for PM10 and CO
On September 22, 2021, the WHO revised its air quality guidelines, recommending stricter standards based on the latest evidence and highlighting the urgent need to improve global air quality. China has established the Ambient Air Quality Standard (GB 3095 − 2012)31, which sets the 24-hour mean concentration limits for PM10 and CO at 150 ug/m3 and 4 mg/m3, respectively.
Our findings indicate that the risk threshold for HF readmission in patients with UA is when PM10 levels exceed 112.5 µg/m3 set by the Ambient Air Quality Standard. This suggests that the current secondary standard for PM10 is insufficient for reducing the risk of HF readmission in UA patients associated with PM10 exposure. Besides, we observed that at very high levels of PM10 (> 112.5 ug/m³), the risk of readmission decreased. This reduction might be due to patients taking extra precautions or undergoing health interventions not accounted for in the model during extreme air pollution events. We determined that the risk threshold concentration for CO is > 37.5 mg/m3, which is higher than the secondary criterion. This finding indicates that patients remain safe at the secondary standard CO level, but those with UA should pay particular attention when CO levels exceed 37.5 mg/m3.
Research strengths and potential impact
Our study aligns with global concerns about environmental pollution as a major public health issue. To our knowledge, no previous research has specifically addressed the impact of air pollutants on patients with UA, making our study a significant contribution to this field. We conducted a comprehensive analysis of several key environmental pollutants and identified PM10 and CO as particularly influential on UA patients. Thus, we established specific concentration thresholds for PM10 and CO that pose the greatest risk for HF readmission in these patients and pinpointed the characteristics of the most sensitive individuals. This research offers valuable insights into public health strategies and clinical practice.
Since PM10 and CO are prevalent in the air, the most effective way to mitigate the risk of exposure is through social action, with a particular emphasis on lowering PM10 levels. Government agencies can implement strategies to cut pollutant emissions and strengthen air quality standards based on the threshold of PM10 > 112.5 µg/m³. At the individual level, people can protect themselves from severe air pollution by wearing masks, using air purifiers, and staying indoors during high-pollution events. Patients with UA should avoid environments where PM10 exceeds 112.5 ug/m³. Taken together, increasing health education is crucial. This can involve raising public awareness through media, including pollution advice in hospital discharge instructions, and educating UA patients about avoiding dangerous PM10 and CO levels. Enhancing indoor and outdoor environments, particularly for women, can further reduce exposure. Hence, these measures will assist in optimizing environmental protection strategies, improve UA management, and reduce the economic and medical burden associated with UA readmissions.
Study limitations
This study has several limitations. First, like many studies on air pollutants, we relied on data from online air quality platforms, using the mean 24-hour concentration of pollutants on the day of hospitalization. We did not directly measure individual real-time air pollutant levels or track individual behaviors such as staying indoors or using air purifiers, which may result in discrepancies between estimated and actual exposure. Second, despite adjusting for confounders, residual or unmeasured confounders may still affect the results. Third, there may be unrecorded cases of severe HF that did not result in hospitalization, potentially leading to an underestimation of hospitalizations. Fourth, this is a single center retrospective study based on China, the main limitation is the consistency and accuracy of data extraction, Although we asked the information department, the medical department, the medical department and the cardiovascular department to extract and organize the data, but there may still be population bias, therefore, the results may not be applicable to all people; thus, the results may not be applicable to all populations. Fifth. exposure to industrial work activities related air pollution may have an impact on the data and results. It is known that no large industrial-related air pollution events occurred during the study period, but it is not excluded that someone experienced industrial pollution events or arrived in industrial pollution areas, so the air pollution values we collected and the actual situation may be biased.Sixth, we conducted telephone follow-up for a small number of patients who cannot come to our hospital for field follow-up and who were readmitted for HF in other hospitals to obtain data on patient outcome events.Despite detailed inquiries with the patient and their families, there are limitations concerning data accuracy and authenticity.
To confirm the reliability of our findings, future research should include prospective multicenter studies. Additionally, further investigations should focus on exploring the biological mechanisms linking short-term exposure to air pollutants with the risk of readmission for HF in patients with UA.
Conclusion
Our study demonstrates that exposure to specific air pollutants, such as PM10 and CO, significantly raises the risk of HF readmission in patients with UA, particularly when PM10 concentrations exceed 112.5 ug/m³ and CO levels surpass 37.5 mg/m3. We also found that female patients with UA and fewer underlying diseases exhibit heightened sensitivity to these pollutants. As the first clinical study to address the impact of PM10 and CO on HF readmission risk in UA patients post-discharge, our research establishes critical risk concentration thresholds and identifies characteristics of vulnerable populations, offering a valuable foundation for developing targeted prevention and management strategies.
Data availability
The datasets generated and analyzed during the current study are not publicly available due the database owner is reluctant to make them public but are available from the corresponding author upon reasonable request.
References
Jiang, Y. et al. Ozone pollution and hospital admissions for cardiovascular events. Eur. Heart J.44 (18), 1622–1632 (2023).
GDB. (2019). https://vizhub.healthdata.org/gbd-compare
Murphy, N. F. et al. A population study of the long-term consequences of rose angina: 20-year follow-up of the Renfrew-Paisley study.Heart92 (12), 1739–1746 (2006).
Timmis, A. D., Feder, G. & Hemingway, H. Prognosis of stable angina pectoris: why we need larger population studies with higher endpoint resolution[J]. Heart93 (7), 786–791 (2007).
Byrne, R. A. et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur. Heart J.44 (38), 3720–3826 (2023).
Sinkovic, A., Marinsek, M. & Svensek, F. Women and men with unstable angina and/or non-ST-elevation myocardial infarction. Wien Klin. Wochenschr118 (Suppl 2), 52–57 (2006).
World Health Organization. Ambient (Outdoor) Air Pollution. Available online: (2021). https://www.who.int/news-room/fact_x0002_sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 30 November 2023)[J].
Cesaroni, G. et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE project. BMJ. 348, f7412 (2014).
Rus, A. A. et al. Impact of short-term exposure to Nitrogen Dioxide (NO(2)) and ozone (O(3)) on hospital admissions for Non-ST-Segment Elevation Acute Coronary Syndrome[J]. Toxics, 12(2). (2024).
Locuratolo, N. et al. [Follow-up of patients after an acute coronary event: the Apulia PONTE-SCA program. G Ital. Cardiol. (Rome)23 (1), 63–74 (2022).
McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J.42 (36), 3599–3726 (2021).
Schwartz, J. & Morris, R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am. J. Epidemiol.142 (1), 23–35 (1995).
Schwartz, J. Air pollution and hospital admissions for cardiovascular disease in Tucson. Epidemiology8 (4), 371–377 (1997).
Schwartz, J. Air pollution and hospital admissions for heart disease in eight U.S. counties. Epidemiology10 (1), 17–22 (1999).
Zhang, D. et al. Air pollution exposure and heart failure: a systematic review and meta-analysis. Sci. Total Environ.872, 162191 (2023).
Jia, Y. et al. Effect of Air pollution on heart failure: systematic review and meta-analysis. Environ. Health Perspect.131 (7), 76001 (2023).
Kuzma, L. et al. Effect of air pollution on the number of hospital admissions for acute coronary syndrome in elderly patients[J]. Pol. Arch. Intern. Med.130 (1), 38–46 (2020).
Liu, Y. et al. Short-term exposure to Ambient Air Pollution and Mortality from myocardial Infarction[J]. J. Am. Coll. Cardiol.77 (3), 271–281 (2021).
Ballester, F., Tenias, J. M. & Perez-Hoyos, S. Air pollution and emergency hospital admissions for cardiovascular diseases in Valencia, Spain. J. Epidemiol. Community Health55 (1), 57–65 (2001).
Yang, M. et al. A systematic review and meta-analysis of air pollution and angina pectoris attacks: identification of hazardous pollutant, short-term effect, and vulnerable population. Environ. Sci. Pollut Res. Int30 (12), 32246–32254 (2023).
Lin, C. A. et al. Association between air pollution and ischemic cardiovascular emergency room visits. Environ. Res.92 (1), 57–63 (2003).
Hosseinpoor, A. R. et al. Air pollution and hospitalization due to angina pectoris in Tehran, Iran: a time-series study. Environ. Res.99 (1), 126–131 (2005).
Kleinman, M. T. et al. Urban angina in the mountains: effects of carbon monoxide and mild hypoxemia on subjects with chronic stable angina. Arch. Environ. Health53 (6), 388–397 (1998).
Brook, R. D. et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American heart association. Circulation121 (21), 2331–2378 (2010).
Konduracka, E. & Rostoff, P. Links between chronic exposure to outdoor air pollution and cardiovascular diseases: a review. Environ. Chem. Lett.20 (5), 2971–2988 (2022).
Clougherty, J. E. Invited perspective: temporality and recursive dynamics in stress-pollution interactions. Environ. Health Perspect.130 (12), 121302 (2022).
Clougherty, J. E. A growing role for gender analysis in air pollution epidemiology. Environ. Health Perspect.118 (2), 167–176 (2010).
Durrani, F. et al. Gonadal hormones and oxidative stress interaction differentially affects survival of male and female mice after lung Klebsiella pneumoniae infection[J]. Exp. Lung Res.38 (4), 165–172 (2012).
Redfield, M. M. et al. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 112 (15), 2254–2262 (2005).
Pope, C. A. et al. Short-term exposure to fine particulate matter air pollution is preferentially associated with the risk of ST-segment elevation acute coronary events. J. Am. Heart Assoc., 2015,4(12).
Ambient air quality standard (GB3095-2012.). https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/dqhjbh/dqhjzlbz/201203/t20120302_224165.htm
Funding
This study is supported by Scientific Bureau of Xiangtan City (SF-ZDJH20231037), Xiangtan City, Hunan Province, China and Xiangtan Medical Association (2023-xtyx-35), Xiangtan City, Hunan Province, China.
Author information
Authors and Affiliations
Contributions
L.Z. and Z.L. established the hypothesis, performed the statistical analysis, wrote the manuscript; data collection and participated follow-up. L.Z., Z.L., J.Z., M.W.: initiated the study hypothesis, edited the manuscript.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Xiangtan Central Hospital (Xiangtan, China, No.2023-02-001) and conformed to the principles outlined in the Declaration of Helsinki.The need for informed consent was waived by the ethics committee Review Board of Xiangtan Central Hospital, because of the retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Liu, Z., Zhou, X. et al. Long-term impact of air pollution on heart failure readmission in unstable angina patients. Sci Rep 14, 22132 (2024). https://doi.org/10.1038/s41598-024-73495-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73495-5