Abstract
The purpose of this study is to develop a nomogram model for early prediction of the severe mycoplasma pneumoniae pneumonia (SMPP) in Pediatric and Adult Patients. A retrospective analysis was conducted on patients with MPP, classifying them into SMPP and non-severe MPP (NSMPP) groups. A total of 550 patients (NSMPP 374 and SMPP 176) were enrolled in the study and allocated to training, validation cohorts. 278 patients (NSMPP 224 and SMPP 54) were retrospectively collected from two institutions and allocated to testing cohort. The risk factors for SMPP were identified using univariate analysis. For radiomic feature selection, Spearman’s correlation and the least absolute shrinkage and selection operator (LASSO) were utilized. Logistic regression was used to build different models, including clinical, imaging, radiomics, and integrated models (combining clinical, imaging, and radiomics features selected). The model’s discrimination was evaluated using a receiver operating characteristic curve, its calibration with a calibration curve, and the results were visualized using the Hosmer–Lemeshow goodness-of-fit test. Thirteen clinical features and fourteen imaging features were selected for constructing the clinical and imaging models. Simultaneously, a set of twenty-five radiomics features were utilized to build the radiomics model. The integrated model demonstrated good calibration and discrimination in the training cohorts (AUC, 0.922; 95% CI: 0.900, 0.942), validation cohorts (AUC, 0.879; 95% CI: 0.806, 0.920), and testing cohorts (AUC, 0.877; 95% CI: 0.836, 0.916). The discriminatory and predictive efficacy of the clinical model in testing cohorts increased further after clinical and radiological features were incorporated (AUC, 0.849 vs. 0.922, P = 0.002). The model demonstrated exemplary predictive efficacy for SMPP by leveraging a comprehensive set of inputs, encompassing clinical data, quantitative and qualitative radiological features, along with radiomics features. The integration of these three aspects in the predictive model further enhanced the performance of the clinical model, indicating the potential for extensive clinical applications.
Similar content being viewed by others
Introduction
Globally, the incidence of respiratory infections caused by Mycoplasma pneumoniae (MP) has increased significantly in recent years, with 10–40% of these infections progressing to Mycoplasma pneumoniae pneumonia (MPP)1,2. Children and adolescents of school age are the age group most frequently afflicted with pneumonia caused by MP, although this proportion fluctuates by age. However, this pathogen can also cause infections in adults and the elderly3. Recently, there have been an increasing number of reports on severe MPP (SMPP)4,5, presenting physicians with an immense challenge.
SMPP refers to the severe condition of MPP, wherein certain patients may potentially experience the development of complications such as pleural effusion, and atelectasis, rapidly progressing to respiratory failure or life-threatening extrapulmonary complications6,7. Children with SMPP may experience involvement of multiple systems and organs, including the nervous, digestive, blood, and mucous membrane skin systems, with potential long-term sequelae8,9. Chronic pneumonia, recurrent respiratory infections, and SMPP impose a significant suffering on both children and adult patients, therefore increasing the burden on healthcare and presenting significant challenges for clinical practitioners10,11.
Currently, common pathogen detection methods have numerous drawbacks, such as a long detection period, and false-positive and false-negative results12,13,14. The pathogenesis of SMPP is still unclear, and various factors make its treatment challenging. Given the persisting challenges associated with the detection of cytokines and other relevant indicators, there is a pressing need for a prompt and pragmatic approach that enables clinical practitioners to predict SMPP utilizing readily available clinical data. Computed tomography (CT) is widely used for the diagnosis and evaluation of pneumonia. The assessment of conventional CT features, however, is non-standardized and heavily dependent on the expertise of radiologists.
Furthermore, some researchers have used quantitative CT methods, such as the CT lesion percentage (CTLP), to assess the severity of lung injury in COVID-19 pneumonia15. This straightforward evaluation method can also be applied to non-severe MPP (NSMPP) and SMPP. In recent times, the utilization of artificial intelligence and big data-driven radiomics analysis has demonstrated significant advantages in determining the existence of disease types, predicting risks, and guiding of treatment16,17,18. Radiomics transforms medical images into high-dimensional images and uses high-throughput extraction for data analysis to explore effective data features. It can extract various information, such as texture features, that radiologists are unable to identify with the naked eye, thus aiding in the diagnosis and treatment of diseases16. At the same time, this technology is simple, fast, and may help in identifying and predicting problems between two different severity levels of MPP. It is also suitable for patients who are in critical condition and require accurate drug treatment immediately but are unable to obtain pathogen detection results in a timely manner.
We hypothesize that radiomics may reveal information not visible to the naked eye. Therefore, we included a cohort of 550 patients, including children and adults with MPP and SMPP, in this study, and validated the models using an entirely external validation dataset. According to the available literature, there is a scarcity of research addressing the precise differentiation between MPP and SMPP, as well as predicting the clinical severity of MPP through the application of radiomics. Thus, the purpose of this study is to explore the value of radiomics in distinguishing between MPP and SMPP and predicting associated risks. Additionally, we aim to investigate whether clinical prediction models can be enhanced by incorporating radiomic factors and quantitative and qualitative CT characteristics.
Methods
Patient population and groups
This study was approved by the Research Ethics Committee of the Affiliated Hospital of Hebei University and all methods are carried out in accordance with the relevant guidelines and regulations. Informed consent was waived by the Research Ethics Committee of the Affiliated Hospital of Hebei University. Clinical and imaging data of patients with MPP were retrospectively collected and analyzed from March 2022 to August 2023 at our medical institution. Two independent medical institutions in the same region provided the external validation data.
Inclusion criteria: (1) patients diagnosed with MPP with no age restriction; (2) CT images and clinical indicators obtained at the same time. Exclusion criteria: (1) patients with immunodeficiency diseases, chronic lung diseases, heart diseases, chronic glomerulonephritis, rheumatic diseases, malnutrition, diabetes, and other genetic metabolic diseases; (2) patients who were co-infected with other pathogens; (3) patients whose clinical records were incomplete; and (4) patients who had undergone various lung surgeries.
Patients with MPP were divided into two groups: NSMPP and SMPP. The diagnostic criteria19 for NSMPP were as follows: (1) symptoms and signs of community-acquired pneumonia (CAP), including fever, cough, and abnormal lung auscultation; (2) positive MP infection results, including MP immunoglobulin M (IgM) titers ≥ 1:160 or a fourfold rise in titers (with a 2-week interval), or positive results in MP polymerase chain reaction (PCR) in nasopharyngeal secretions.
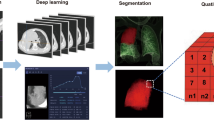
The diagnosis of SMPP was based on the guidelines recommended by the National Health Commission of China19, provided that any of the subsequent criteria were met: (1) persistent high fever (≥ 39 °C) for ≥ 5 days or fever for ≥ 7 days without a declining trend in peak temperature; (2) appearance of any of the following symptoms: wheezing, dyspnea, difficulty breathing, chest pain, or hemoptysis. Complicating plastic bronchitis, asthma attacks, pleural effusion, and pulmonary embolism are associated with severe lesions; (3) the development of extrapulmonary complications without reaching critical conditions; (4) the oxygen saturation during air inhalation at rest is ≤ 93%; (5) radiological findings that meet any of the following criteria: involvement of a single lung lobe ≥ 2/3, with a uniform and consistent high-density consolidation; two or more lung lobes showing high-density consolidation (regardless of the size of the affected area), possibly accompanied by a moderate to large amount of pleural effusion and features of localized bronchiolitis; diffuse involvement of a single lung or bilateral involvement of ≥ 4/5 lung lobes showing features of bronchiolitis, possibly with bronchiolitis and lung collapse; (6) clinical symptoms gradually deteriorate, as evidenced by imaging data indicating a progression of the lesion area by over 50% within 24–48 h; (7) a significant increase in one of the following: CRP, LDH, or D-dimer. The overall study workflow is illustrated in Fig. 1.
Collection of clinical data and evaluation of CT radiological features
Data was collected retrospectively, comprising demographic information as well as clinical, laboratory, and imaging features. Based on previous research1,2,4 and the recommendations of clinical experts, a total of 25 clinical and laboratory features were investigated in this study. General patient characteristics included gender, age, type of fever (low-grade, mid-grade, or hyperpyrexia), and duration of fever. Laboratory biochemical examination indicators included white blood cell (WBC) count, neutrophil (NEUT) count, lymphocyte count, monocyte count, eosinophil count, basophil count, platelet (PLT) count, creatine kinase isoenzyme (CK-MB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), activated partial thromboplastin time (APTT), fibrinogen (FIB), procalcitonin, D-dimer, C-reactive protein (CRP), lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII, (PLT*ANC)/LYC).
All patients underwent chest CT examinations during their illness. Two radiologists (J.W.H with 6 years of experience and T.D.W with 8 years of experience, respectively), independently reviewed and analyzed all chest CT images. In instances where discrepancies were observed in the interpretation of imaging features, a third radiologist (L.Y.Z with 12 years of experience) was consulted to adjudicate and achieve consensus among the radiological team. Throughout this process, all readers were blinded to any patient-specific clinical information of significance. They assessed 18 CT imaging features, including pulmonary infarction, consolidation features, mixed ground-glass opacity, halo sign, cavity, nodule, ground-glass opacity (GGO), adjacent pleural thickening, pleural effusion, mediastinal lymph node enlargement, bronchial inflation sign, bronchial wall thickening, interlobular septal thickening, crazy-paving pattern, fibrous stripes, bilateral lung involvement, the number of lung lobes involved, and CT lesion percentage (CTLP). CTLP was defined as the lesion volume divided by the total lung volume20.
Image acquisition and lesion segmentation
Patients underwent chest CT scans using United-Imaging UCT968, Siemens SOMATOM Perspective, and Philips Brilince 64. All patients were scanned in the supine position and held their breath after deep inspiration, with breath-holding training conducted prior to each examination. The scanning range was from the costophrenic angle to the thoracic inlet. Specifications of the three scanners are shown in Supplementary Information 1 and Table S1.
The image segmentation, radiomics feature extraction, feature selection, and machine learning model building were established on the uAI Research Portal V1.1 (Shanghai United Imaging Intelligence, Co., Ltd.)21. The volume and density of the entire lung tissue and lesion areas within each lung lobe were segmented and calculated automatically based on a previously developed automatic segmentation algorithm22. The segmentation results were manually corrected by one radiologist (J.W.H with 6 years of experience) and confirmed by another radiologist (T.D.W with 8 years of experience). Additionally, they meticulously removed non-lesion areas or annotated lesions that were not initially identified. For segmentation results, interobserver reproducibility was assessed first using intraclass correlation coefficients. All physicians were blinded to the clinical information of their patients.
Radiomics feature extraction, selection
Firstly, pre-processing was conducted using resampling to adjust the x, y, and z spacing, achieving a spatial resolution of 1 mm × 1 mm × 1 mm. Subsequently, the Pyradiomics V3.023 tool integrated in uAI Research Portal V1.1 was utilized to automatically extract a total of 1,904 radiomic features from both the original images and the derived images. This was achieved by applying 15 filters, encompassing first-order static parameters (n = 378), morphological parameters (n = 14), gray-level co-occurrence matrix (GLCM) parameters (n = 441), gray-level run length matrix (GLRLM) parameters (n = 336), gray-level size zone matrix (GLSZM) parameters (n = 336), gray-level dependence matrix (GLDM) parameters (n = 294), and neighboring gray-tone difference matrix (NGTDM) parameters (n = 105). The details of the filters are described in Supplementary 2.
The detailed workflow for radiomics model development is depicted in Fig. 2. After normalization using Z-score, Spearman’s correlation we selected the least absolute shrinkage and selection operator (LASSO) based on its sparsity, stability, and interpretability24 to eliminate high correlation, reduce redundancy, The radiomics score (Rad score) for each patient was calculated through the linear combination of the selected features, weighted according to their respective coefficients in LASSO.
Development of predictive models
After conducting univariate analysis on all clinical and imaging features that were included, features with statistically significant differences were separately incorporated for predictive model development. We constructed a total of four models subsequent to Z-score normalization using logistic regression: the clinical model, the imaging model, the radiomics model, and the integrated model (clinical features + imaging features + radiomics). The predictive performance of all models was evaluated at an independent research center (external validation). The area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, and specificity were used to evaluate the performance of the four models. The calibration curves were utilized to assess the correlation between the predictive and actual outcomes, while the decision curve was used to calculate the net benefits of different threshold probabilities of the models.
Statistical analysis
Descriptive statistics were calculated using the mean ± standard deviation (\(\:\stackrel{-}{X}\) ± s) for quantitative data. T-tests or Mann-Whitney U tests were employed for comparing groups, depending on the distribution of the data. The count data were represented as numbers (percentages) (n[%]), and chi-squared tests were used for group comparisons. Univariate analysis was utilized for variable selection. The performances of the four models were assessed by area under the receiver operating characteristic curve (AUC), specificity, and sensitivity. The optimal cut-off points to predict the SMPP were determined by Youden’s index. The DeLong test was used for pairwise comparisons among the four models. Statistical significance was defined as P < 0.05. All statistical analyses were performed using SPSS version 22.0. Graphs were created using R software.
Results
Patient characteristics
A total of 550 patients were included in the study (Fig. 1). However, 35 patients were excluded, encompassing 9 with underlying diseases, 22 with co-infections with other pathogens, and 4 with incomplete clinical records. The final cohort consisted of 374 patients with non-severe mycoplasma pneumonia (NSMPP) and 176 patients with severe mycoplasma pneumonia (SMPP). A total of 440 patients were assigned to the training set, while 110 patients were designated for internal validation. These two groups were selected at random in an 8:2 ratio. A total of 278 patients were included from two independent centers in the external validation cohort (224 patients had NSMPP and 54 had SMPP, Fig. 1).
There were no statistically significant differences in age and gender between the NSMPP and SMPP groups in the training set and validation set, as indicated in Table 1. Type of fever, WBC, NEUT, CK-MB, LDH, APTT, FIB, D-dimer, CRP, NLR, PLR, and SII exhibited statistically significant differences between the NSMPP and SMPP groups in the training set (all P < 0.05), as shown in Table 1. In the training set, there were no statistically significant differences in PLT between the NSMPP and SMPP groups (P = 0.13). There were statistically significant differences in gender and age across the training set, validation set, and testing set (P = 0.014, P < 0.001). Additionally, in all three groups, there were statistically significant differences in factors such as type of fever, CK-MB, LDH, FIB, D-dimer, NLR, and PLR (all P < 0.05).
In terms of CT imaging features (Table 2), lobar atelectasis, consolidation pattern, adjacent pleura thickening, pleural effusion, mediastinal enlargement of lymph nodes, air bronchogram sign, interlobular septal thickening, reticular pattern, fiber cords, and average lesion density displayed significant statistical differences between the NSMPP and SMPP groups (all P < 0.05) in the training set. There were no statistically significant differences observed in the consolidation mixed GGO, number of lobes involved, or CTLP between the NSMPP and SMPP groups in the training set. There were statistically significant differences between the NSMPP and SMPP groups in the training set, validation set, and testing set for consolidation pattern, consolidation mixed GGO, adjacent pleura thickening, pleural effusion, mediastinal enlargement of lymph nodes, air bronchogram sign, fiber cords, and average lesion density (all P < 0.05). In all three sets, however, there was no statistically significant difference between the NSMPP and SMPP groups for lobar atelectasis and reticular pattern (P = 0.83, P = 0.26).
Evaluation of models and comparison of predictive model performance
After conducting a univariate analysis, 13 clinical features and 13 imaging features were selected. Furthermore, CTLP was included in the imaging model due to its potential correlation with the severity of pulmonary lesions, as suggested by prior research15. The selected features and their respective coefficients in the clinical model and imaging model are listed in Table S2. Following the detection of 1904 imaging features using the Pearson correlation coefficient, it was determined that 1,486 imaging features were found to be correlated with SMPP (all P < 0.05, r > -0.321). Subsequently, a total of 25 features were chosen by LASSO, including 3 first-order features, 4 GLDM, 4 GLRLM, 3 wavelet-based features, 8 GLSZM features, 4 NGTDM features, and 1 GLCM features, and RadScore was conducted. The details of the radiomics feature selection process in LASSO are shown in Fig. 2.
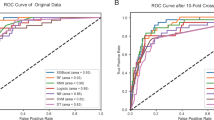
The diagnostic performance of each model is presented in Table 3, whereas the ROC curve analysis results and the calibration curve are depicted in Fig. 3. In the training set, the intergraded model achieved an AUC of 0.922 (95% CI: 0.900; 0.942), sensitivity of 0.853, and specificity of 0.879. The intergraded model of the internal validation set yielded an AUC of 0.869 (95% CI: 0.806; 0.920), sensitivity of 0.793, and specificity of 0.800. For the external validation set, the integrated model obtained an AUC of 0.877 (95% CI: 0.836; 0.916), sensitivity of 0.802, and specificity of 0.907. The Delong test indicated that in the external validation set, the integrated model outperformed the clinical model in predicting SMPP (P = 0.002). Although there were no statistically significant differences in predictive performance compared to the imaging model and the radiomics model, the integrated model still achieved the highest area under the curve (AUC = 0.877) in the external validation set. The predictive performance of the three independent models did not exhibit statistical differences in the external validation set (Clinical Model vs. Imaging Model, P = 0.479; Clinical Model vs. Radiomics Model, P = 0.884; Imaging Model vs. Radiomics Model, P = 0.613).
Discussion
Statistical analyses were performed on clinical data, quantitative and qualitative radiological features, and radiomic features of patients with NSMPP and SMPP. Thirteen clinical features, fourteen radiological features, and twenty-five radiomic features were ultimately included in the model-building process. In the training set, the integrated model achieved AUCs of 0.922 (95% CI: 0.900; 0.942); in the internal validation set, 0.869 (95% CI: 0.806; 0.920), and in the external validation set, 0.877 (95% CI: 0.836; 0.916). Sensitivity and specificity varied across sets: 0.853 and 0.879 for the training set, 0.793 and 0.800 for the internal validation set, and 0.802 and 0.907 for the external validation set. The comprehensive inclusion of clinical, radiological, and radiomic features in the models highlights the multidimensional nature of the diagnostic process for NSMPP and SMPP. These findings were validated using independent datasets from other institutions. The enhanced performance of the integrated model underscores the value of combining these diverse data sources for a more accurate prediction of SMPP.
Gender and age were found significantly different between the two groups in all cohorts (P = 0.014, P < 0.001). The potential bias in external validation data, sourced from both a children’s hospital and a comprehensive hospital in the same region, may be attributed to the higher tendency of children seeking medical care at children’s hospitals.
The complex pathogenesis of SMPP remains unclear; however, it frequently arises from a combination of factors that are closely related to both the direct pathogenic mechanisms of MP and the dysregulation of the immune response of the host. Several links between innate and adaptive immunity are disrupted following an infection with MP, leading to excessive inflammation in both the lungs and the entire body25. When these inflammatory responses are triggered, cytokines and chemokines are released, which initiates a which starts a chain reaction that makes inflammation worse and results in elevated levels of various inflammatory markers26. The findings of our research showed that the SMPP group exhibited higher degrees of fever, CK-MB, LDH, FIB, D-dimer, NLR, and PLR than the NSMPP group (all P < 0.05) in the training set, the internal validation set, and the external validation set. This suggests that the SMPP group experienced a more pronounced systemic inflammatory response. This is consistent with the results of prior studies that identified LDH-D-dimer as a risk factor for SMPP2,27,28,29,30,31.
The results of our study revealed a higher proportion of segmental and wedge-shaped patterns in the pulmonary consolidation features of patients with SMPP, indicating a larger extent of lung consolidation (all P < 0.05). As a consequence, the average lesion density was significantly elevated (all P < 0.001), and the likelihood of pleural effusion was heightened (all P < 0.001). This phenomenon may be attributed to MP infection, where the organism adheres to respiratory epithelial cells, induces the expression of respiratory epithelial adhesion proteins, significantly increases airway mucus secretion, and leads to the formation of bronchial mucous plugs. Plastic bronchitis may facilitate the detection of pulmonary symptoms, including reduced breath sounds and radiological indications of lung collapse or segmental consolidation11,30,32. In the SMPP group, the progression of pulmonary lesions and pleural effusion may further contribute to prolonged fever and hospitalization11,28.
The utilization of radiomics holds significant potential in the extraction of clinically relevant information for the enhancement of the accuracy of clinical differential diagnoses. Notably, current literature lacks instances where radiomics has been applied to risk stratification for predicting severe mycoplasma pneumoniae pneumonia (SMPP). We extracted 1,904 candidate radiomics features from CT images as part of this investigation; 25 potential predictors were then selected after feature selection. The selected radiomics features were identified as shape and texture features, encapsulating intrinsic data on the distribution of pixel intensity and textural morphology. These are details that are not readily apparent to radiologists33. As an example, the “Short Run Low Gray Level Emphasis” in GLRLM class extracted from the image filtered by wavelet-LHL signifies intensity and textural characteristics within high-intensity CT voxels of the lesion. This feature is one of the three radiomic features that exhibit the most robust correlation with SMPP. Another feature, “Size Zone Non-Uniformity Normalized” in GLSZM class measures the variability of size zone volumes throughout the image, with a lower value indicating more homogeneity among zone size volumes in the image. The relationship between the maximum and minimum principal components within the shape of the ROI is denoted by the “Flatness” property of the SHAPE class. These parameters effectively capture microstructural alterations in the infected lung region, serving as pivotal markers for distinguishing between NSMPP and SMPP.
We compared our study with previous research34 that utilized a combination of clinical and imaging features to predict refractory MP pneumonia (RMPP), as there has been a limited focus in scientific literature on SMPP prediction. In the absence of an external validation cohort, the AUCs in the training cohort were 0.881 (95% CI: 0.843; 0.918) and 0.777 (95% CI: 0.661; 0.893) in the validation cohort. In contrast, our intergraded model achieved AUCs of 0.922 (95% CI: 0.900; 0.942) in the training cohort, 0.869 (95% CI: 0.806; 0.920) in the internal validation cohort, and 0.877 (95% CI: 0.836; 0.916) in the external validation cohort. Overall, our model demonstrated better diagnostic and predictive performance. The integrated model demonstrated superior predictive performance for SMPP in the external validation set when compared to the clinical model (P = 0.002). Additionally, the inclusion of easily accessible clinical and biological data may enhance the feasibility of our model in future applications. Notably, radiomics models alone achieved almost identical predictive performance to clinical and imaging models in our study (Clinical Model VS Radiomics Model, P = 0.884; Imaging Model VS Radiomics Model, P = 0.613). This also highlights the equally significant role of radiomics alone in predicting SMPP compared to clinical and imaging features.
There are certain limitations to this study that necessitate acknowledgment. The exclusion of cases from outside Hebei Province in the study may impact the stability and generalizability of the predictive model. However, our attempt to incorporate a multicenter research approach involving internal and external validation sets and subjective and objective CT assessments assures the validity of the conclusions drawn. Furthermore, the retrospective nature of this study may introduce inherent biases during the identification and recruitment of participants. Future investigations should aim for broader representation across diverse regional populations and incorporate case studies involving image-pathology correlation.
Conclusion
In conclusion, outstanding performance in predicting SMPP was achieved by leveraging clinical data, quantitative and qualitative radiological features, and radiomics models that were developed and validated across training, validation, and testing cohorts. The amalgamation of these three components into an integrated predictive model further enhances the predictive capabilities of the clinical model, indicating its potential for extensive applications in clinical practice.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due the hospital’s policies or confidentiality agreements but are available from the corresponding author on reasonable request.
References
Kutty, P. K. et al. Mycoplasma pneumoniae among children hospitalized with community-acquired Pneumonia. Clin. Infect. Dis.68, 5–12. https://doi.org/10.1093/cid/ciy419 (2019).
Choi, Y. J., Jeon, J. H. & Oh, J. W. Critical combination of initial markers for predicting refractory Mycoplasma pneumoniae pneumonia in children: a case control study. Respir Res.20, 193. https://doi.org/10.1186/s12931-019-1152-5 (2019).
Gadsby, N. J. et al. Increased reports of Mycoplasma pneumoniae from laboratories in Scotland in 2010 and 2011 - impact of the epidemic in infants. Euro. Surveill17 (2012).
Yan, C. et al. Molecular and clinical characteristics of severe Mycoplasma pneumoniae pneumonia in children. Pediatr. Pulmonol.54, 1012–1021. https://doi.org/10.1002/ppul.24327 (2019).
Liu, J. et al. Mycoplasma pneumoniae pneumonia associated thrombosis at Beijing Children’s hospital. BMC Infect. Dis.20https://doi.org/10.1186/s12879-020-4774-9 (2020).
Wang, X. et al. Necrotizing pneumonia caused by refractory Mycoplasma pneumonia pneumonia in children. World J. Pediatr.14, 344–349. https://doi.org/10.1007/s12519-018-0162-6 (2018).
San Martin, I., Zarikian, S. E., Herranz, M. & Moreno-Galarraga, L. Necrotizing pneumonia due to Mycoplasma in children: an uncommon presentation of a common disease. Adv. Respir Med.https://doi.org/10.5603/ARM.a2018.0049 (2018).
Narita, M. Classification of Extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front. Microbiol.7, 23. https://doi.org/10.3389/fmicb.2016.00023 (2016).
Meyer Sauteur, P. M. et al. Frequency and clinical presentation of Mucocutaneous Disease due to Mycoplasma pneumoniae infection in Children with Community-Acquired Pneumonia. JAMA Dermatol.156, 144–150. https://doi.org/10.1001/jamadermatol.2019.3602 (2020).
Kassisse, E., García, H., Prada, L., Salazar, I. & Kassisse, J. Prevalence of Mycoplasma pneumoniae infection in pediatric patients with acute asthma exacerbation. Arch. Argent. Pediatr.116, 179–185. https://doi.org/10.5546/aap.2018.eng.179 (2018).
Waites, K. B., Xiao, L., Liu, Y., Balish, M. F. & Atkinson, T. P. Mycoplasma pneumoniae from the respiratory tract and Beyond. Clin. Microbiol. Rev.30, 747–809. https://doi.org/10.1128/cmr.00114-16 (2017).
Totten, A. H. et al. Allergic airway sensitization impairs antibacterial IgG antibody responses during bacterial respiratory tract infections. J. Allergy Clin. Immunol.143, 1183–1197e1187. https://doi.org/10.1016/j.jaci.2018.07.021 (2019).
Bénet, T. et al. Microorganisms Associated with Pneumonia in Children < 5 years of age in developing and emerging countries: the GABRIEL Pneumonia Multicenter, prospective, case-control study. Clin. Infect. Dis.65, 604–612. https://doi.org/10.1093/cid/cix378 (2017).
Wang, L. et al. A comparison study between GeXP-based multiplex-PCR and serology assay for Mycoplasma pneumoniae detection in children with community acquired pneumonia. BMC Infect. Dis.17, 518. https://doi.org/10.1186/s12879-017-2614-3 (2017).
Shen, C. et al. Quantitative computed tomography analysis for stratifying the severity of Coronavirus Disease 2019. J. Pharm. Anal.10, 123–129. https://doi.org/10.1016/j.jpha.2020.03.004 (2020).
Rizzo, S. et al. Radiomics: the facts and the challenges of image analysis. Eur. Radiol. Exp.2, 36. https://doi.org/10.1186/s41747-018-0068-z (2018).
Li, G. et al. An MRI radiomics approach to predict survival and tumour-infiltrating macrophages in gliomas. Brain145, 1151–1161. https://doi.org/10.1093/brain/awab340 (2022).
Chen, Q. et al. Radiomics in precision medicine for gastric cancer: opportunities and challenges. Eur. Radiol.32, 5852–5868. https://doi.org/10.1007/s00330-022-08704-8 (2022).
China, N. H. C. o. t. P. s. R. o. guidelines for the diagnosis and treatment of Mycoplasma pneumoniae pneumonia in children (2023 version). Ration. drug use Explor. China20, 16–24 (2023).
Pan, F. et al. Time Course of Lung Changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19). Radiology295, 715–721. https://doi.org/10.1148/radiol.2020200370 (2020).
Wu, J. et al. uRP: an integrated research platform for one-stop analysis of medical images. Front. Radiol.3, 1153784. https://doi.org/10.3389/fradi.2023.1153784 (2023).
Shan, F. et al. Abnormal lung quantification in chest CT images of COVID-19 patients with deep learning and its application to severity prediction. Med. Phys.48, 1633–1645. https://doi.org/10.1002/mp.14609 (2021).
van Griethuysen, J. J. M. et al. Computational Radiomics System to Decode the Radiographic phenotype. Cancer Res.77, e104–e107. https://doi.org/10.1158/0008-5472.Can-17-0339 (2017).
Jiang, L., Greenwood, C. M. T., Yao, W. & Li, L. Bayesian Hyper-LASSO classification for feature selection with application to Endometrial Cancer RNA-seq data. Sci. Rep.10, 9747. https://doi.org/10.1038/s41598-020-66466-z (2020).
Guo, L., Liu, F., Lu, M. P., Zheng, Q. & Chen, Z. M. Increased T cell activation in BALF from children with Mycoplasma pneumoniae pneumonia. Pediatr. Pulmonol.50, 814–819. https://doi.org/10.1002/ppul.23095 (2015).
Lee, Y. C. et al. Altered chemokine profile in Refractory Mycoplasma pneumoniae pneumonia infected children. J. Microbiol. Immunol. Infect.54, 673–679. https://doi.org/10.1016/j.jmii.2020.03.030 (2021).
Chen, P. et al. The relationships between LncRNA NNT-AS1, CRP, PCT and their interactions and the refractory mycoplasma pneumoniae pneumonia in children. Sci. Rep.11, 2059. https://doi.org/10.1038/s41598-021-81853-w (2021).
Bi, Y. et al. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci. Rep.11, 6595. https://doi.org/10.1038/s41598-021-86086-5 (2021).
Li, G. et al. High co-expression of TNF-α and CARDS toxin is a good predictor for refractory Mycoplasma pneumoniae pneumonia. Mol. Med.25, 38. https://doi.org/10.1186/s10020-019-0105-2 (2019).
Gong, H., Sun, B., Chen, Y. & Chen, H. The risk factors of children acquiring refractory mycoplasma pneumoniae pneumonia: a meta-analysis. Med. (Baltim).100, e24894. https://doi.org/10.1097/md.0000000000024894 (2021).
Fang, C., Mao, Y., Jiang, M. & Yin, W. Pediatric critical illness score, clinical characteristics and Comprehensive Treatment of children with severe Mycoplasma Pneumoniae Pneumonia. Front. Surg.9, 897550. https://doi.org/10.3389/fsurg.2022.897550 (2022).
Gao, L. W. et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol. Infect.147, e192. https://doi.org/10.1017/s0950268819000839 (2019).
Song, L. et al. Clinical, conventional CT and Radiomic feature-based machine learning models for Predicting ALK Rearrangement Status in Lung Adenocarcinoma patients. Front. Oncol.10. https://doi.org/10.3389/fonc.2020.00369 (2020).
Shen, F. et al. Development of a Nomogram for Predicting Refractory Mycoplasma pneumoniae Pneumonia in Children. Front. Pediatr.10, 813614. https://doi.org/10.3389/fped.2022.813614 (2022).
Funding
This work was supported by Youth Research Fund Project (2023QA06).
Author information
Authors and Affiliations
Contributions
Writing-original draft: Li-yong Zhuo, Jia-wei Hao and Zi-jun Song. They contributed to the work equally and should be regarded as co-first authors.Data collection: Huan Meng, Tian-Da Wang, Lu-Lu Yang, Zi-Mei Yang, Wei-Yang, Li-Li ZangData analysis: Jia-Mei Ma, Dan-ShenVisualization of results: Jing-Jing Cui Wen-Jing ChenWriting-review: Xiao-ping YIN, Jia-ning.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhuo, LY., Hao, JW., Song, ZJ. et al. Predicting the severity of mycoplasma pneumoniae pneumonia in pediatric and adult patients: a multicenter study. Sci Rep 14, 22978 (2024). https://doi.org/10.1038/s41598-024-74251-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74251-5
Keywords
This article is cited by
-
Predicting and interpreting key features of refractory Mycoplasma pneumoniae pneumonia using multiple machine learning methods
Scientific Reports (2025)
-
A comparison of diagnostic and therapeutic approaches for Mycoplasma pneumoniae pneumonia in children and adults, during the post-COVID-19 pandemic era
European Journal of Clinical Microbiology & Infectious Diseases (2025)