Abstract
Fish, being highly sensitive to changes in the physico-chemical parameters of water, are good indicators of contamination. Teesta, a prominent northern West Bengal River system, is increasingly contaminated due to anthropogenic activities. This study aims to determine agricultural pesticide contamination and its genotoxic impact on the resident fish, Pethia conchonius, as an experimental organism. Sample water analysis from three riverine sites I, II & III, showed the presence of the insecticides imidacloprid (IMI), chlorpyrifos (CPF), bifenethrin (BF), cypermethrin (CP), difenthiuron, acetamiprid (AC) in the sites II and III only with adjoining agricultural lands. Comet assay revealed a significantly lower % Head DNA (~ 1.2 times), higher %Tail DNA (~ 16 times), and %Tail length (~ 3.1 times) in the gills of Pethia conchonius from sites II and III. About 4 and 10 times increase of micronuclei and other nuclear abnormalities were also noted in the erythrocytes of the fish from sites II and III than I, which was not contaminated. The antioxidant enzymes SOD, CAT, and GST activity and MDA levels were significantly higher (p < 0.05) in the liver samples from sites II and III, while AChE activity was significantly decreased (p < 0.001) in the brain tissues. Moreover, the sod, cat, and gpx expression in the hepatic cells were significantly upregulated compared to the β actin mRNA indicating increased oxidative stress. Increased genomic damage, antioxidant enzyme activity, higher MDA levels, decreased AChE activity in the brain, and the upregulation of hepatic genes strongly suggested the genotoxic effects of the detected insecticides in combination with other contaminants.
Similar content being viewed by others
Introduction
India, a predominantly agricultural country, faces a rapid proliferation of arthropod pests due to its tropical climate1. This necessitates using various pesticides to control pests and increase crop production to meet the country’s economy and growing food demands. India’s most commonly used pesticide groups are organophosphates, neonicotinoids, and pyrethroids2,3.
In India, West Bengal is the 7th largest consumer of pesticides in the country1, and Maharashtra ranks first. West Bengal alone consumed 3630 and 3321 metric tonnes of pesticide in 2021–2022 and 2022–2023, respectively (Directorate of Plant Protection, Quarantine & Storage, the Government of India, 2023)4. Paddy, mustard, vegetables, fruits, and tea are the key crops in West Bengal5. Methylparathion (25.6%), imidacloprid (16.4%), dichlorvos (7.8%), and phorate (4.2%) are commonly used pesticides after alpha-cypermethrin (46%) in the state6.
Apart from controlling the target insect pests, the pesticides pose serious environmental and health issues due to their toxicity7. These compounds permeate food chains and accumulate in the highest trophic levels, potentially disrupting the ecosystem and causing damage to the non-target organisms, including humans, if applied inappropriately and without due consideration of recommendation8,9,10. Synthetic pesticides can inadvertently enter aquatic ecosystems, such as lakes, ponds, rivers, and oceans, through leaching from nearby areas, surface run-off due to rainfall, or irrigation conduits.
Fishes, the most common and diverse aquatic vertebrates, highly sensitive to even a slight physicochemical change in water parameters, have been effectively used as indicators of water contamination11. Studies have shown that insecticides, mostly from the groups of neonicotinoids, synthetic pyrethroid, organophosphates, organochlorines, and carbamates, can lead to genotoxic, physiological, neuro-behavioral, and biochemical stresses in fish12,13,14,15. Environmental pollution is known to generate oxidative stress, producing Reactive Oxygen Species (ROS), which can cause oxidative damage to the macromolecules, DNA, lipids, and proteins in different organisms, including fish16. Disruption of the balance of redox reactions in fish exposed to pesticides has been reported to cause oxidative damage17.
Pesticides have been detected in multiple rivers, namely, the Ganga and Hoogly in the Southern part of West Bengal and the rivers Deomoni, Changa, Manza, Tepu, and Karola in the Terai region of the state18,19,20,21. The mighty river Teesta, a significant waterway in the northern part of West Bengal, serves as a large drainage system for various urban and rural agricultural lands, cultivating tea, rice, jute, vegetables, fruits, and cash crops as monoculture19,22. Despite the extensive drainage of the river and the known use of pesticides on the plantations along the bank of Teesta, a study has yet to be conducted to assess the impact of pesticides on the non-target aquatic organisms of Teesta because of the risk of contamination of Teesta by surface run-off.
Therefore, a study has been undertaken to screen the presence of insecticide contamination in the river Teesta and the evaluation of their adverse effects on the non-target organism, Pethia conchonius using the parameters such as micronuclei, comet (Single-cell gel electrophoresis), antioxidant and cholinergic enzyme activity (SOD, CAT, GST, and AChE) and malonaldehyde (MDA) assays followed by pesticide-induced modulation of some antioxidant gene expression, to know the oxidative stress and DNA damage along with altered cholinergic neurotransmission.
Materials and methods
Selection of sampling sites and fish specimen
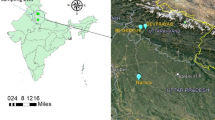
For the collection of adult fish specimens and water samples, three sites of the Teesta River were selected based on their proximity to the agricultural fields (Fig. 1; Table 1). An upstream riverine site (Site I) that has no agrarian fields along the river bank, and two downstream sites (Site I and Site II) were selected, which have vast stretches of agricultural fields in close proximity to the river Teesta, spanning two cities, Siliguri and Jalpaiguri, respectively in West Bengal. The resident fish Pethia conchonius (Rosy Barb) was selected as the experimental model due to its availability in the riverine sites, acceptable size for easy handling, and high local demand as food. As per Red Data Book, Pethia conchoniusis the least concerned. For the experiments, fish with mean length and weight of 6.20 ± 0.79 cm and 3.95 ± 0.65 g were considered as described by Dutta et al23.. The Pethia conchonius specimens collected from the riverine sites I, II, and III were designated as PcSV (fish specimens from site I at Sevok), PcGB (fish specimens from site II at Gajoladoba), and PcDM (fish specimens from site III near Domohoni), respectively. The sampling sites, designation of the fish population, and the geographical coordinates have been given in Fig. 1; Table 1. The entire study has no in vivo experiments.
Screening and analysis of pesticides in the river water of selected sites
Water samples were collected from the selected sites of Teesta (Table 1) at an interval of 3 months in a year, during March-June, July-October, and November-February. Water samples were collected from a depth (10–30 cm) of the river in cleaned 1 L screw-capped high-density polyethylene narrow mouth bottles HDPE (Make: Tarson), immediately kept in ice to minimize degradation of pesticide residues, and stored at 4oC once brought to the laboratory. The water samples were analyzed by LC-MS/MS and GC-MS/MS using a mass detector at the Food Safety Referral Laboratory, ICAR-Indian Institute of Horticultural Research, Bangalore. The pesticide was extracted from the samples using the AOAC official method 990.06. The water sample was transferred into a 2 L separating funnel, and 100 g sodium chloride was added and mixed thoroughly. After 50 ml of dichloromethane was added to the separating funnel, the mixture was shaken vigorously. After separating the layers, the lower dichloromethane layer was transferred into a 500 mL round bottom flask through anhydrous sodium sulfate. This process was repeated twice and concentrated in a rotary vacuum evaporator (Buchi R-300) to dryness. The final volume was made up to 5 mL with either ethyl acetate or acetonitrile. The analysis was conducted by GC-MS/MS (TQ8040 Triple quadrupole, Shimadzu Corp., Japan) and LC-MS/MS (6460 Triple Quadrupole with 1290 Infinity HPLC, Santa Clara, CA, USA), following the injection sequence: solvent, matrix blank, calibration curve (CC) with a minimum of three levels (5 µgL[-1to 100 µgL[-1) and the test sample.
Fish collection and preparation of tissue sample
Fish specimens were collected from the three sites with the help of fishermen using cast nets laid out during the early hours of the day (4.00–6.00 AM). About 100–120 fish were randomly collected from each station (Site I, Site II & Site III). Fish were randomly and blindly sampled by one of the authors not involved in the experiments. A total of 300–350 specimens were used for the whole study. The gill, liver, and brain tissues were collected from the freshly captured fish specimens, PcSV, PcGB, and PcDM, from three sites (Table 1) by dissecting immediately near the sampling site, kept in a -20ºC mini-cooler in ice-cold 0.9% Phosphate buffer saline (PBS) (0.137 M NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4 pH 7.5) and were and stored at -20ºC after bringing to the laboratory. Tissues were homogenized in ice-cold PBS (pH 7.5) with a micro-pestle in a 1.5 ml microfuge tube and centrifuged at 10,000xg for 10 min at 4ºC. The supernatant was collected in a fresh autoclaved microfuge tube and stored at -20 ºC for further analysis. The current study has been authorized by the Institutional Animal Ethics Committee (under the Committee for Control and Supervision of Experiments on Animals, India) at the University of North Bengal vide reference No. IAEC/NBU/2022/30, dated 23.09.2022 (Proposal No. 12).
Protein estimation
Following the method of Lowry et al24.. , the protein was estimated from the liver homogenates of the freshly captured Pethia conchonius, PcSV, PcGB, and PcDM. Test samples were incubated in Reagent I (2% Na2CO3 in 0.1 N NaOH, 1% Sodium Potassium Tartrate, and 0.5% CuSO4) for 10 min, followed by incubation for 30 min after adding Reagent II (2 N Folin-Ciocalteau Reagent). A standard curve was plotted using BSA as standard. Protein was estimated by measuring the absorbance of the unknown concentration at 660 nm. The amount of protein in the homogenate was used to determine the glutathione S-transferase (GST), catalase (CAT), & superoxide dismutase (SOD) activities.
Genotoxicity studies
Comet assay
DNA damage in the gill tissue of the resident fish, Pethia conchonius, was determined using single-cell gel electrophoresis (SCGE) or comet assay following the method of Singh et al25.. with slight modifications, as described in Dutta et al26.. The EtBR-stained microgel slides were examined using a fluorescence microscope (Nikon Eclipse 200, Nikon, Japan) with a barrier filter of 590 nm and excitation filter of 510–560 nm connected to a CCD camera at 40x magnification.
Micronucleus (MN) assay
Micronuclei, notched and blebbed nuclei, were determined in the erythrocytes of the fish following the method of Beninca et al27. described in Dutta et al26.. The frequency of micronuclei (MN) and other nuclear abnormalities (NA) like notched (NO) and blebbed (BL) were calculated as:
Scoring criteria
Coding was done by one of the authors not involved in the experiments for both the Micronuclei and Comet Assays. Coded slides were scored blindly by the experimenters.
Micronucleus assay
A count of 9000 cells per specimen and 3000 cells per slide was scored for 3 different populations. Micronuclei (MNs) were distinguished based on smaller sizes than one-third of the primary nucleus, which had identical stain and intensity as the primary nucleus. Additionally, the presence of notched nuclei and blebbed nuclei was also assessed. Coding and blind scoring were conducted on all the slides.
Single-cell gel electrophoresis (comet assay)
Using an image analysis system (Casp lab 1.2.3 b2) attached to a fluorescent microscope (Nikon Eclipse E200, Nikon) with proper filters, 250 cells per concentration, with 50 cells per slide, were randomly scored. The comet software Casp lab 1.2.3 b2 was used to quantify DNA damage using % tail DNA, % head DNA, and tail length (L tail).
Biochemical analysis of Superoxide Dismutase (SOD), Catalase (CAT), Glutathione-S-Transferase (GST), Acetylcholinesterase (AChE) activities, and Malondialdehyde (MDA) level
SOD, CAT, GST, AChE activities, and MDA level were determined following the method of Nishikimi et al.28, Aebi29, Habig et al.30, Ellman et al.31, and Placer et al.32, respectively, in the liver or brain tissues of Pethia conchonius with minor modifications. The conditions and formulas of the assays are given in Table 2. The absorbance for each enzyme was determined spectrometrically (Shimadzu, UV 1900i) at 560, 240, 340, 412, and 532 nm, respectively. The results were expressed as U/mg protein or µmole/min/g tissue or µmole/min/mg tissue for enzymes and nmol MDA/g tissue for MDA.
Antioxidant gene expression analysis
The liver tissues of the fish specimens, PcSV, PcGB, and PcDM of the three experimental sites, were homogenized separately in ice-cold PBS (pH 7.5) with a micro-pestle in a 1.5 ml microcentrifuge tube and centrifuged at 10,000x g at 4ºC for 10 min. The supernatant was transferred to a clean microcentrifuge tube and stored at -20 ºC for gene expression analysis. Expression of cat, sod, and gpx genes was quantified by the 2−ΔΔctmethod according to Livak and Schmittgen33 with minor modifications taking the housekeeping gene β actin as an internal control.
RNA extraction
Total RNA from each fish specimen was extracted following the manufacturer’s instructions (Invitrogen, Thermofisher Scientific) and Sambrook et al34.. The tissues were homogenized in TRIZOL™ reagent and centrifuged to remove debris, polysaccharides, and DNA. The RNA-containing phase was purified by chloroform/isopropyl alcohol treatment followed by 70% ethanol wash. The RNA pellet was air-dried at room temperature for 5–10 min. The RNA quality was checked in 1.5% Agarose gel and spectrophotometrically at 260/280 nm (UV 1900i, Shimadzu, Japan). The RNA samples were treated with DNase I (Thermo Fisher Scientific) to remove any genomic DNA contamination. Complementary DNA (cDNA) was synthesized using Go-Script Reverse Transcription Kit (Promega) from the purified RNA and used for gene expression analysis of cat, sod, and gpx by RT-PCR in Light Cycler 96 (Roche) using SYBR Green system (Sigma-Aldrich). The β-actin transcript was used as a standard to eliminate variation in mRNA expression. Each mRNA level was expressed as its ratio to β-actin mRNA. The primers used for the RT-PCR are given in Table 3. The PCR products were analyzed on 2% agarose gel stained with 0.5 µg/ml ethidium bromide. 100 bp DNA ladder (Promega) was used to calibrate the size. Gels were visualized under a UV-transilluminator (TFX-20 M, Life Technologies, India).
Statistical analysis
All the statistical analyses were carried out by using IBM SPSS Ver 27 software considering the significance level of p = 0.05 (Table 4). The data distributions for each group for all the dependent variables were primarily checked for normal distributions using the Shapiro-Wilk normality test and Levene’s test was performed to check the equality of variances. For Multiple comparisons among three different sites parametric test (ANOVA) was carried out for normally distributed data followed by post-hoc test (Duncun for homoscedastic/equal variances and Dunnett’s T3 for heteroscedastic/unequal variances data). Welch correction was also applied during ANOVA for the data having unequal variances. Non-parametric Kruskal Walli’s test was performed for non-normal data. For pair-wise comparison, an independent t-test was used for the same sample size and normal distributions, and for unequal sample size and non-normal distribution, a Mann-Whitney U test was conducted.
The entire study has been conducted in accordance with ARRIVE 2.0 guidelines.
Results
Analysis of water
The LC-MS/MS and GC-MS/MS analysis of water samples collected from three sites I, II, and III of river Teesta during pre-monsoon (March-June), monsoon (July-October), and post monsoon (November-February) revealed the presence of six insecticides, namely IMI, AC, CP, BF, CPF and Difenthiuron in the downstream site II (26.751499°N 88.597654°E). In contrast, four insecticides, IMI, CP, BF, and CPF, were detected in the downstream site III (6.556309°N, 88.763347°E) (Fig. 2). Pesticide residue in the water collected from Site I (26.881406 ºN, 88.474249 ºE) were found below detectable limit throughout the sampling seasons.
The concentration of IMI was the highest with 22.43, 0.06, and 7.43 µg L−1 during pre-monsoon, monsoon, and post-monsoon, respectively, followed by CPF, BF, CP, Difenthiuron, and AC in Site II, while IMI was recorded in the pre-monsoon (63.33 µg L−1) and post-monsoon (35.45 µg L−1) seasons only from site III (Fig. 2). CPF ranged from 0.00 to 17.62 µg L−1and 0.00–23.35 µg L−1 in sites II and III, respectively. CP, AC, and Difenthiuron in site II and BF in site III were detected during pre-monsoon only, but CP was detected at a low concentration in Site III compared to Site II during pre-monsoon and post-monsoon. IMI was the most prevalent insecticide in both the sites, followed by CPF and CP. Site III showed a relatively higher average insecticide concentration, but types of insecticides were more in Site II (Fig. 2).
Assessment of genotoxic & nuclear damages
Comet assay showed significantly lower % head DNA of 93.369 ± 1.074 and 85.562 ± 2.03 in the gill tissues of fish population PcGB and PcDM, respectively than PcSV (99.131 ± 0.254) (p < 0.05). On the other hand, the %tail DNA and tail length (LTail) values of 6.631 ± 1.074 & 16.000 ± 2.206 and 14.438 ± 2.031 & 19.863 ± 2.515 in PcGB and PcDM, respectively, were significantly higher compared to 0.869 ± 0.254 and 6.235 ± 0.787 in PcSV (p < 0.05). The % tail DNA was increased by approximately 8–16 times in PcGB and PcDM than in PcSV (Table 5; Fig. 3).
Micronucleus assay of whole blood (erythrocytes) in the fish population PcSV, PcGB, and PcDM from natural conditions in River Teesta revealed different degrees of nuclear damage. (Table 6; Fig. 4). Erythrocytes from PcGB and PcDM revealed a significantly higher frequency of MNi, NOs, and BLs than the PcSV (p < 0.05). The increase in MNi, NO, and BL was nearly 2 and 4 times, 5 and 10 times, and 4 and 7 times higher in PcGB and PcDM from sites II and III, respectively, compared to PcSV. The results showed that the nuclear damage was relatively higher in the contaminated downstream sites than in insecticide-free upstream sites.
Biochemical analysis
As the present study revealed the presence of neonicotinoids (IMI, AC), synthetic pyrethroids (CP, BF), and organophosphate insecticides (CPF) in the downstream sites II and III of Teesta, the AChE activity in the brain and SOD, CAT, GST and MDA activity in the liver tissue were analyzed.
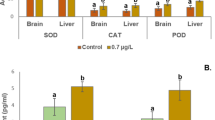
Analysis showed that AChE activities (in µmol/min/mg tissue) of 56.370 ± 0.581and 37.396 ± 0.410 in the brain tissue of PcGB and PcDM were significantly reduced compared to PcSV, 81.987 ± 1.363 (p < 0.001). (Table 6; Fig. 5). The results showed 1.40- and 1.6 times higher SOD activity, 37.630 ± 1.534 and 43.900 ± 3.548 in PcGB and PcDM, respectively, than the SOD activity 27.337 ± 1.441 of PcSV. The increase in SOD activity in site II was not significant. However, it was highly significant for site III (p < 0.05), possibly due to the rise in oxidative stress caused by the cumulative effect of pesticides found in the river water in these sites (Table 7; Fig. 5).
In contrast to the CAT activity of 0.186 ± 0.019 in PcSV, about 2- and 2.5 times higher activities of 0.393 ± 0.007 and 0.457 ± 0.022(p < 0.05) were recorded in PcGB and PcDM, respectively. (Table 6; Fig. 6). The hepatic GST activity showed significantly higher activity of 0.462 ± 0.014 and 0.341 ± 0.015 µM/min/g tissue in PcGB and PcDM, respectively, compared to the activity of 0.112 L ± 0.008 in PcSV (p < 0.05), which may be due to the rise in oxidative stress. (Table 6; Fig. 5). The increased MDA levels indicate higher lipid peroxidation (LPO) caused by oxidative damage. In comparison to the hepatic MDA level of 0.193 ± 0.013 in PcSV, the MDA levels of 0.344 ± 0.035 and 0.275 ± 0.015 in the downstream fish population PcGB and PcDM, respectively, were significantly higher (p < 0.05) (Table 7; Fig. 6).
Gene expression analysis
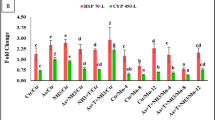
RT-PCR analysis revealed that the expression of hepatic catalase (cat), superoxide dismutase (sod), and glutathione peroxide (gpx) was significantly increased (p < 0.05) in PcGB and PcDM, compared to PcSV. In contrast to PcSV, the expression of cat, sod, and gpx was higher by 2.283 ± 0.367, 2.777 ± 1.040 and 4.580 ± 1.311 folds in PcGB and 4.550 ± 0.427, 4.230 ± 0.760 and 3.000 ± 0.760 folds in PcDM, respectively. PcGB from Site II showed relatively lower fold change than PcDM from Site III (Fig. 7).
Relative changes in the cat, sod, and gpx gene expression in the liver tissues of PcSV, PcGB, and PcDM from three sampling sites I, II, and III of river Teesta, respectively (Mean±SE). Different alphabets (lowercase) in superscript indicate values with distinct homogeneous subsets significantly differ at (p<0.05). An asterisk (*) in superscript denotes the p value for the pairwise comparisons between Site II and Site III with Site I. *** = p<0.001,** = p<0.01, * = p<0.05.
Discussion
The River Teesta, one of the significant waterways in North Bengal draining wide stretches of urban settlements and agricultural fields. Originates from Sikkim Himalaya, flows through different districts of West Bengal and ends in the mighty River Brahmaputra passing through Bangladesh. Human populations residing on the banks of Teesta depend on the river for food, agriculture and livelihood. While the insecticides are most important to control pests and increase the crop production in this region, its detrimental effects on non-target aquatic organisms like fish have not been critically investigated. The present study is the first of this kind to assess the extent of insecticide(s) induced genotoxic damages in the fish in natural riverine condition (river Teesta) using multidimensional approaches. A number of studies have shown that the pesticide contamination may induce impaired reproduction35, haematogical disorders36, respiratory problems37and behavioral alterations38in fish. Various respiratory issues39, carcinogenic effects40, neurotoxic effects41as well as reproductive disorders42 have been recorded in humans upon exposure to pesticides.
The wastewater effluent from households, industries, agriculture, aquaculture, animal husbandry, and hospital wastes produced due to anthropogenic activities constantly contaminating the aquatic bodies, such as rivers, lakes, ponds, and ultimately sea/ocean carrying various toxic substances8, which bioaccumulate in the resident organisms, including fish. These pollutants adversely affect the aquatic organisms and may disrupt the normal redox balance due to oxidative stress and overproduction of ROS via antioxidant enzyme-dependent biochemical pathways43,44,45. We have used a multidimensional approach with different parameters in the present investigation so that the biases of results may be ruled out to produce a robust and reliable data set with a high confidence level.
Pesticide contamination in Teesta
In the present study, six pesticides, IMI, AC (neonicotinoids), CP, BF (pyrethroids), CPF (organophosphate), and Difenthiuron (thiourea) were detected in the water samples collected from the downstream sites of river Teesta indicating heavy pesticide contamination in the areas where the river flows through, example like Gajoldoba (site II) and Domohoni (site III). Pesticides including IMI, AC, and difenthiuron were detected and quantified in LC-MS/MS; whereas CPF, CP and BF in GC-MS/MS. Contamination might have occurred due to surface run-off, spray drift, and/or air drift of the insecticides from the agricultural fields along the bank of Teesta, degrading the water quality in the downstream sites. This is also supported by the upstream site I (Sevok), which is devoid of adjoining agricultural fields and is free from pesticides. Higher pesticide contamination recorded during pre-monsoon and post-monsoon periods is due to the active spraying of pesticides on the crop plants in these areas than in monsoons due to the lack of active plantations and pesticide spraying. IMI is the most prevalent insecticide, followed by CPF in both the downstream sites, indicating its extensive use in agriculture. Pesticide contamination of other rivers like Ganga46, Brahmaputra47, Tapi48, Krishna49, Kaveri50, Ghaggar51, and Yamuna52in India and the rivers from the other parts of the world, including three rural rivers in Guangzhou, (China)53, Ebro River basin in Spain54, Kabul River in Pakistan55, Great Lakes in USA56, Ganges-Brahmaputra-Meghna estuary in Bangladesh57, and the river Nile (Africa)58 has been reported by different investigators.
Among different classes of pesticides detected in the present study, neonicotinoids (IMI, AC) and synthetic pyrethroids (CP and BF) are known for their acute neurotoxic effects on the target organisms. These are widely used classes of pesticides worldwide59. Organophosphates (CPF) are intensively used agro-pesticides due to their low mammalian toxicity, less persistence, and rapid biodegradability60. Ccaccanpa et al54.. assessed the effects of agricultural pesticides on non-target organisms like algae, daphnia, and fish using Risk Quotients (RQs) and Toxic Unit (TUs) Parameters for water and sediments. In the present study, the toxic effects of the pesticides contaminating river Teesta were assessed at cellular, DNA, enzymatic, and mRNA levels.
Assessment of genotoxic & nuclear damages
In the present study, the gill tissues of Pethia conchonius from different sites in the river Teesta were analyzed by comet assay. A significant decrease in % head DNA and a concomitant increase in % tail DNA and Ltail in downstream specimens (PcGB and PcDM) naturally exposed to the different insecticides suggested elevated nuclear DNA damage than upstream specimens (PcSV) (p< 0.05). Hussain et al61. reported significantly higher DNA fragmentation in Labeo rohitawhen exposed to polluted water of the Chenab River. Ali et al55.. have shown significantly higher DNA damage in the gill cells (24.10%) and lymphocytes (22.67%) of Channa punctatusexposed to sub-lethal concentrations of CPF for 5 days. Vierra et al62.. have reported increased DNA damage as indicated by a significant decrease in % head DNA in Prochilodus lineatus subjected to short-term in-situ exposure to streams from agricultural areas in Southern Brazil. Similarly, higher DNA damage (p = 0.018) was also recorded in response to the exposure to a sub-lethal concentration of IMI compared to the control, indicating the genotoxic nature of the pesticide. The above results are consistent with our findings showing higher DNA damage in the gill tissues of Pethia conchoniusfrom downstream sites (stations) of Teesta with multipesticide contamination. A load of pollutants in the aquatic ecosystem induces elevated micronuclei indicating cytotoxicity63,64 that may cause population decline. In the current study, significantly higher frequency of MNi, NOs, and BLs in the downstream populations PcGB and PcDM (p < 0.05) relative to the upstream PcSV suggested extensive genomic damage due to the collective effect of six insecticides detected in the riverine sites II and III. Our findings are in concurrence with the results of Hussain et al61,]. who reported single (50.00 ± 6.30) and double (14.40 ± 2.56) MN induction and the other nuclear abnormalities (150.00 ± 2.92) in Labeo rohitaharvested at the polluted experimental site of the Chenab River in Pakistan and showed 96% reduction in the fish population from the experimental site due to the high incidence of pollutants. Vierra et al62.. have also reported a higher frequency of nuclear abnormalities in Prochilodus lineatus exposed to agricultural polluted water. Induction of elevated MN in gill and kidney erythrocytes of Synodontis clarias and Tilapia niloticafrom five locations of the river Anambra due to environmental stress and pollution was noted more than the control group harvested from an ideal condition64. Our laboratory has also reported the genotoxic effects of IMI in Pethia conchoniusand showed a concentration and time-dependent increase in nuclear abnormalities26. Investigations have also indicated CPF-induced MN formations in a concentration and time-dependent response65,66. Several studies have also documented the genotoxic effects of polluted waters on the resident fish Astyanax lacustris, Hypostomus ancistroide, and Rhamdia quelen67,68, consistent with our findings.
Oxidative stress and biochemical alterations
Slight changes in the physicochemical parameters of water can cause stress in aquatic organisms, including fishes, in natural as well as laboratory conditions due to imbalance of ROS production and its elimination17,69. Oxidative stress triggered by ROS is balanced naturally by the upregulation of Phase I antioxidant enzymes, e.g., SOD or CAT, which are the first line of antioxidant stress management in fish, or by Phase II detoxifying enzymes, GST required to eliminate xenobiotics from the body[,35. However, if these pathways are inhibited or impaired, the oxidative stress leads to various physiological disturbances, including disruption of cellular integrity due to lipid peroxidation (LPO)70.
Cellular ROS converts polyunsaturated fatty acids (PUFAs) in fish tissues into hundreds of secondary compounds, aldehydes being the most harmful lipid peroxides, like Malonaldehyde (MDA)70,71. In the present study, 1.4 and 1.78-fold higher MDA levels in the livers of PcGB and PcDM, respectively compared to PcSV, suggested increased LPO that may be induced by the presence of different insecticides, like IMI, CP, and CPF in the downstream experimental sites II and III. Our findings are comparable with those of Vierra et al72.. , showing increased LPO levels in the liver of Prochilodus lineatusexposed to the polluted stream water from agricultural areas in Brazil. Soorya et al73.. demonstrated increased MDA levels in the liver, kidney, and brain of Anabas testudiniusexposed to sewage-polluted water for 7 days, with the highest damage in the kidney. Pyrethroids have been shown to elevate MDA levels in the gills, liver, and muscles of fish leading to different histopathological alterations like necrosis, vacuolization, and tissue layer detachment in the brain69. Neonicotinoids like IMI62or sulfoxaflor74 induce oxidative stress with the loss of membrane integrity in different freshwater fish exposed to sub-lethal concentrations for a short period.
Superoxide dismutase (SOD) initiates elimination of ROS by catalyzing the dismutation of O2 into H2O2, which is further reduced into H2O and O2 by CAT. An excess of H2O2 accumulation in the tissue may inhibit SOD activity, while an increase in O2may decrease the activity of CAT75. SOD and CAT activity increases with pesticide exposure as an adaptive response to oxidative stress56. However, with the increase of pesticide concentration and/or duration of exposure the SOD and CAT are unable to function due to inhibition and thus fail to eliminate ROS resulting in further oxidative stress56,76. Elevated hepatic SOD (10–15 times) and CAT (times increase) activity in PcGB and PcDM from polluted sites than PcSV of unpolluted upstream site I (Table 6) can be correlated with insecticide contamination in Teesta as revealed by water analysis. GST catalyzes the conjugation of reduced glutathione (GSH) and increases GST activity, increasing resistance to xenobiotic toxicity in fish77,78. The metabolic pathway associated with glutathione is the cells’ primary protective mechanism against oxidative stress. GST increases to detoxify organic compounds/pesticides and excrete them but it only works till a certain threshold of pesticide.
Compared to PcSV, increased hepatic GST activity in PcGB and PcDM also suggested elevated environmental stress which is in agreement with the study of Shah and Parveen79, who reported significantly higher SOD, CAT, and GST activities in the freshwater fish Rita rita and Cyprinus carpiofrom the river Ganges at downstream sites, Naora Valley and Rishikesh polluted by agricultural pesticides compared to the relatively less polluted upstream site (Devprayag). Clasen et al80.. in a study reported increased GST activity in the liver, gill, and brain of C. carpioreared in the mixed fish and rice culture syste showing the potential of agricultural pesticides to cause oxidative stress. Mohanty and Samanta56 analyzed the tissue-specific response of the oxidative biomarkers and metal accumulation in fish from river systems like Mahanadi. Lipid peroxidation and protein carbonylation were reported to be increased gradually in the fish tissues collected from experimental sites along the course of the river in the Choudwar-Jagatpur industrial area of Cuttack city experimental sites (two sites downstream of Choudwar-Jagatpur industrial area) comparison to the upstream reference site (upstream of Cuttack industrial region) in Mahanadi. The significant inhibitory effect of CP, a pyrethroid, on acetylcholinesterase and catalase was reported in freshwater fish, Oreochromis niloticusleading to oxidative stress81. On the other hand, the hepatic antioxidant enzyme (SOD and CAT) and detoxifying enzyme (GST) activities were significantly induced in a time and dose-dependent response in goldfish exposed to agricultural pesticide 2,4-dichlorophenol (2,4-DCP)35. An increase in hepatic SOD and a concomitant decrease in CAT activity were shown in P. lineatusexposed to IMI, triggering oxidative stress as evidenced by the elevated lipid peroxidation in the tissues62.
Pyrethroids and neonicotinoids are known to act as an agonist of ACH causing continuous stimulation of nACHRs resulting in neurotoxicity59. Neonicotinoids act as direct agonists while pyrethroids like Chlorpyrifos can act by inhibiting the acetylcholinesterase enzyme affecting its structure. It inactivates an active hydrolyzable serine group important for enzymatic activity82. Neonicotinoids, pyrethroids, and organophosphates act on target organisms83as well as non-target organisms of various trophic levels including fishes and planktons in aquatic systems triggering neurotoxic effects12, including morphological, histological, and biochemical alterations in fish tissues84. AChE is an effective biomarker of neurotoxicity as it has also been correlated with behavioral changes due to its inhibition23,72,73,85. Our findings of 2.1- and 1.45-fold reduction of AChE activity in the brain tissues of PcGB and PcDM, than PcSV from river Teesta suggesting neurotoxic effect of the insecticides on the Central nervous system (CNS) are in strong agreement with the findings of Vierra et al65.. who reported a significant reduction of AChE activity in the brain and muscle tissues of the fishes exposed to waters from the natural systems polluted with agricultural pesticides. Inhibition of AChE in and subsequent neurotoxicity in the brain of Pethia conchoniuscould be due to agonist activity of IMI and CP as suggested by Boelsterli et al75.. and Tomizawa et al59.. Recently, our laboratory reported the inhibitory effect of sub-lethal concentrations of IMI on AChE activity in Pethia conchoniuswith concomitant behavioral alterations, including brain tissue damages23. Similar results were also shown in the freshwater teleost, O. mossambicus86. Combining neonicotinoids, viz., IMI with thiamethoxam87and IMI with nitenpyram88have identical effects. Neurotoxic effects of synthetic pyrethroid, like CP, also inhibited AChE activities and caused mild to severe behavioral changes in freshwater teleost and rainbow trouts89,90.
Antioxidant gene expression analysis
SOD, CAT, and GPX enzymes are responsible for the elimination of ROS generated in tissues by redox reactions that prevent oxidative damage in tissues. Superoxide radicals, hydrogen peroxide, and organic hydroperoxides are metabolized by SOD, CAT, and GPX, respectively91. In the present study, the heightened expression of sod, cat, and gpx genes in hepatic tissues is comparable with the increased hepatic antioxidant enzymes as a result of oxidative stress in contaminated experimental sites of the river Teesta (sites II and III) in comparison to site I. This could be due to the combined effect of insecticides detected in the Teesta River (sites II and III). An increase of 2.2, 2.7, and 4.5 folds (PcGB) and 4.5, 4.2, and 3.0 folds (PcDM) of sod, cat, and gpx gene expression, respectively than PcSV is comparable with the MN, comet, and enzyme assay data.
Agus et al92.. have shown a 1.1- and 1.6-fold increase in sod and gpx transcripts in Cyprinus carpioexposed to di-n-butyl phthalate (DBP) for 96 h. Veldhoen et al93.. reported a significant increase in the hepatic catexpression in females compared to males of migratory Pacific salmon in the Fraser River (Columbia), which is known to be contaminated with pesticides. Jin et al94.. have reported upregulation of Cu/Zn–sod, Mn–sod, cat, and gpx in the liver of zebrafish exposed to various concentrations of atrazine (ATZ) for 14 days.
In the current study, different insecticides in the experimental sites II and III of river Teesta might have synergistic effects on the cellular, physiological, and nuclear components of Pethia conchonius than uncontaminated site I leading to severe genotoxicity as supported by the multidimensional study. It is also imperative to comment that pollutants, like heavy metals (not analyzed in this investigation) may cause genotoxic impacts on the organisms as also reported in Salmo trutta from three rivers, Stribekken, Naustebekken, and Rugla, contaminated with heavy metals in Norway, that showed a significant increase (p < 0.05) of sod, cat, and gpxmRNA transcripts in the metal-exposed trouts compared to the specimens from an uncontaminated site95.
Conclusion
In the present study, an integrated biomarker approach has revealed the induction of oxidative stress and genotoxicity in the non-target fish, Pethia conchonius due to agricultural insecticide contamination. DNA damage as evidenced by the increased frequency of Comet parameters, micronuclei, and the other nuclear abnormalities (notched and blebbed nuclei), changes (induction) of SOD, CAT, and GST activities, and MDA levels in the liver of fish from the downstream polluted sites II and III of river Teesta indicated heightened ROS generation and oxidative stress. This can induce an increased frequency of DNA damage. Reduced AChE activity in the brains of Pethia conchonius from the contaminated downstream sites and concomitant upregulation of hepatic sod, cat, and gpx genes confirmed the overall oxidative stress caused by the synergistic effect of agricultural pesticides present in the river Teesta. Moreover, the implications of these findings can be extended beyond Pethia conchonius to broader prospects of environmental health and human welfare. Pesticide contamination in the river Teesta poses significant environmental risks, affecting aquatic organisms, i.e. fish health and survival and potentially affecting human populations reliant on river resources for water and food. Appropriate management solutions are essential to reduce these hazardous pesticides. Regulation on pesticide use, enforcement of pollution control measures, and periodic water quality monitoring are essential steps toward safeguarding aquatic ecosystems. Additionally, public awareness campaigns and education on sustainable agricultural practices can promote judicious pesticide usage to reduce the adverse impacts on the environment. Further, our results open up directions for future research.
Data availability
The datasets created and analyzed during the study are accessible from the corresponding author upon an adequate request.
Change history
04 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-90548-5
References
Subash, S. P., Chand, P., Pavithra, S., Balaji, S. J. & Pal, S. Pesticide use in Indian agriculture: trends, market structure and policy issues (2017).
Nayak, P. & Solanki, H. Pesticides and Indian agriculture—a review. Int. J. Res. Granthaalayah9(5), 250–63. https://doi.org/10.29121/granthaalayah.v9.i5.2021.3930 (2021).
Statistical Database. Directorate of Plant Protection, Quarantine & Storage. GOI. (2021). http://ppqs.gov.in/statistical-database
Statistical Database. Directorate of Plant Protection, Quarantine & Storage. GOI. (2023). http://ppqs.gov.in/statistical-database
Khatun, H. & Jamal, A. Uses of Chemical pesticides in Agricultural fields in West Bengal and their effects on Non-target Species-A Review study. IJISRT. 8 (6), 64–69 (2021).
Banerjee, I., Tripathi, S. K., Roy, A. S. & Sengupta, P. Pesticide use pattern among farmers in a rural district of West Bengal, India. J. Nat. Sci. Biol. Med.5 (2), 313. https://doi.org/10.4103/0976-9668.136173 (2014).
Gibbons, D., Morrissey, C. & Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Poll. Res.22, 103–118. https://doi.org/10.1007/s11356-014-3180-5 (2015).
Rana, A., Sharma, N. & Arya, V. Heavy uses of pesticides in India: a quantitative analysis. Indian J. Ecol.49 (3), 994–1004. https://doi.org/10.55362/IJE/2022/3627 (2022).
Amenyogbe, E., Huang, J. S., Chen, G. & Wang, Z. An overview of the pesticides’ impacts on fishes and humans. Int. J. Aquat. Biol.9 (1), 55–65. https://doi.org/10.22034/ijab.v9i1.972 (2021).
Mahboob, S., Al-Ghanim, K.A., Sultana, S., Al-Balawi, H.A., Sultana, T., Al-Misned, F. & Ahmed, Z. A study on acute toxicity of triazophos, profenofos, carbofuran and carbaryl pesticides on Cirrhinus mrigala. Pak. J. Zool. 47(2), 461-466 (2015).
Hedayati, A. Fish biomarkers, suitable tools for water quality monitoring. IJVAR. 1 (3), 63–69 (2018).
Merga, L. B. & Van den Brink, P. J. Ecological effects of imidacloprid on a tropical freshwater ecosystem and subsequent recovery dynamics. Sci. Total Environ.784, 147–167. https://doi.org/10.1016/j.scitotenv.2021.147167 (2021).
Inyang, I. R. & Korogbegha, T. F. Haematological and biochemical responses of Heterobranchus bidorsalis to imidacloprid. Annals Ecol. Environ. Sci.2 (2), 41–46 (2018).
Altun, Ö. S., Arslan, H. & S., & Imidacloprid exposure cause the histopathological changes, activation of TNF-α, iNOS, 8-OHdG biomarkers, and alteration of caspase 3, iNOS, CYP1A, MT1 gene expression levels in common carp (Cyprinus carpio L). Toxicol. Rep.5, 125–133 (2018).
Topal, A. et al. Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: Assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere. 175, 186–191. https://doi.org/10.1016/j.chemosphere.2017.02.047 (2017).
Rajak, P. et al. Agricultural pesticides–friends or foes to biosphere?. J. Hazard. Mater. Adv.10, 100264. https://doi.org/10.1016/j.hazadv.2023.100264 (2023).
Lushchak, V. I., Matviishyn, T. M., Husak, V. V., Storey, J. M. & Storey, K. B. Pesticide toxicity: a mechanistic approach. EXCLI J. 17, 1101. https://doi.org/10.17179/excli2018-1710 (2018).
Aktar, M. W. et al. Impact assessment of pesticide residues in fish of Ganga river around Kolkata in West Bengal. Environ. Monit. Assess. 157, 97–104. https://doi.org/10.1007/s10661-008-0518-9 (2009).
Mandal, N. K. The Trend of Agricultural Development in West Bengal: a District Level Study. RAY: Int. J. Multidisciplinary Stud. 3, 67–87 (2018).
Singh, S. et al. Analyses of pesticide residues in water, sediment and fish tissue from river Deomoni flowing through the tea gardens of Terai Region of West Bengal, India. Int. J. Fish. Aquat. Stud. 3 (2), 17–23 (2015).
Das, A. Comparative study of pollution status of two main rivers: Karola and Tista of Jalpaiguri, West Bengal. India J. Chem. Pharm. Res. 9 (7), 76–81 (2017).
Goyal, M. K. & Goswami, U. P. Teesta river and its ecosystem. The Indian rivers: Scientific and socio-economic aspects. 537 – 51. https://doi.org/10.1007/978-981-10-2984-4_37 (2018).
Dutta, D., Ray, A., Bhattacharya, E., Ghosh, B. & Bahadur, M. Neurotoxic effects of Imidacloprid on Pethia conchonius (Rosy Barb), a common freshwater fish of India. Toxicol. Int. 31, 43–54. https://doi.org/10.18311/ti/2024/31i1/35473 (2024).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193 (1), 265–275. https://doi.org/10.1016/S0021-9258(19)52451-6 (1951).
Singh, N. P., McCoy, M. T., Tice, R. R. & Schneider, E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cel l Res. 175 (1). https://doi.org/10.1016/0014-4827(88)90265-0 (1988). 184 – 91.
Dutta, D., Ray, A., Ghosh, B. & Bahadur, M. Assessment of imidacloprid induced genotoxicity in Pethia conchonius (rosy barb), a common freshwater fish of India. Drug Chem. Toxicol. 47 (1), 101–114. https://doi.org/10.1080/01480545.2023.2222931 (2024).
Benincá, C. Biomonitoring of the Camacho-Jaguaruna (SC) and Santa Marta-Laguna (SC) estuarine lagoons, using Geophagus brasiliensis (Cichlidae) (Doctoral dissertation, Master’s Dissertation, University of Paraná, Paraná, Brazil) (2011).
Nishikimi, M., Rao, N. A. & Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46 (2), 849–854 (1972).
Aebi, H. Catalase. In: Methods of Enzymatic Analysis, (ed Bergmeyer, H.U.) 673-680 (Academic Press Inc., New York, 1974). https://doi.org/10.1016/b978-0-12-091302-2.50032-3.
Habig, W. H., Pabst, M. J., Jakoby, W. B. & Glutathione, S. -transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249 (22), 7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8 (1974).
Ellman, G. L., Courtney, K. D., Andres, V. Jr. & Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7 (2), 88–95. https://doi.org/10.1016/0006-2952(61)90145-9 (1961).
Placer, Z. A., Cushman, L. L. & Johnson, B. C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 16 (2), 359–364. https://doi.org/10.1016/0003-2697(66)90167-9 (1966).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods. 25 (4), 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, New York, 1989).
Yang, C., Lim, W. & Song, G. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 234, 10875–10878. https://doi.org/10.1016/j.cbpc.2020.108758 (2020).
Srivastava, P., Singh, A. & Pandey, A. K. Pesticides toxicity in fishes: biochemical, physiological and genotoxic aspects. Biochem. Cell. Arch. 16 (2), 199–218 (2016).
Mishra, A. & Devi, Y. Histopathological alterations in the brain (optic tectum) of the fresh water teleost Channa punctatus in response to acute and subchronic exposure to the pesticide Chlorpyrifos. Acta Histochem. 116 (1), 176–181 (2014).
Crosby, E. B., Bailey, J. M., Oliveri, A. N. & Levin, E. D. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol Teratol. 49, 81–90 (2015).
Mattila, T. et al. Scoping review—the association between asthma and environmental chemicals. Int. J. Environ. Res. Pub Health. 18 (3), 1323 (2021).
Calaf, G. M. Curcumin, Oxidative Stress, and Breast Cancer. In Cancer: Oxidative Stress and Dietary Antioxidants 159-169 (Elsevier Inc., 2016). https://doi.org/10.1016/B978-0-12-405205-5.00015-5.
Ganie, S. Y., Javaid, D., Hajam, Y. A. & Reshi, M. S. Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: a critical appraisal. Toxicol. 472, 153181 (2022).
Oliva, S. U. et al. Impairment of adult male reproductive function in rats exposed to ethanol since puberty. Reproduct Toxicol. 22 (4), 599–605 (2006).
Zhang, J. F. et al. Responses of the antioxidant defenses of the Goldfish Carassius auratus, exposed to 2, 4-dichlorophenol. Environ. Toxicol. Pharmacol19(1), 185–90. https://doi.org/10.1016/j.etap.2004.07.001 (2005).
Suchiang, P. A review on toxicity of pesticides in catfishes: reproductive, haematological and biochemical aspects. Annu. Res. Rev. Biol. 36 (9), 47–59 (2021).
Rani, S. N. Effect of Pesticide Application on aquatic environments and Fish Diversity in Indian Scenario. Int. J. Emerg. Technol. Innovative Res.7(2), 614–621 (2020) http://www.jetir.org/papers/JETIR2002497.pdf.
Shah, Z. U. & Parveen, S. Distribution and risk assessment of pesticide residues in sediment samples from river Ganga, India. PLoS One. 18 (2), e0279993. https://doi.org/10.1371/journal.pone.0279993 (2023).
Chakraborty, P. et al. Polychlorinated biphenyls and organochlorine pesticides in River Brahmaputra from the outer Himalayan Range and River Hooghly emptying into the Bay of Bengal: occurrence, sources and ecotoxicological risk assessment. Environ. Pollut. 219, 998–1006. https://doi.org/10.1016/j.envpol.2016.06.067 (2016).
Hashmi, T. A., Qureshi, R., Tipre, D. & Menon, S. Investigation of pesticide residues in water, sediments and fish samples from Tapi River, India as a case study and its forensic significance. Environ. Forensics. 21 (1), 1–0 (2020).
Patil, N. N., Selvaraj, K. K., Krishnamoorthy, V., Elaiyaraja, A. & Ramaswamy, B. R. Organochlorine pesticide contamination in the Kaveri (Cauvery) river, India: a review on distribution profile, status, and trends. In Water Challenges and Solutions on a Global Scale 115–28 (ACS Publications, 2015). https://doi.org/10.1021/bk-2015-1206.ch007.
Gupta, H. et al. Freshwater discharge from the large and coastal peninsular rivers of India: a reassessment for sustainable water management. Environ. Sci. Pol. Res. 1–8. https://doi.org/10.1007/s11356-021-16811-0 (2022).
Kaushik, A., Sharma, H. R., Jain, S., Dawra, J. & Kaushik, C. P. Pesticide pollution of river Ghaggar in Haryana, India. Environ. Monit. Assess. 160, 61–69. https://doi.org/10.1007/s10661-008-0657-z (2010).
Water Quality Status of Yamuna River (1999-2005), Assessment and Development of River Ba-sin Series: ADSORBS/41/2006-07, Central Pollution Control Board, Delhi. http://www.cpcb.nic.in (2006).
Tang, X. Y., Yang, Y., Tam, N. F., Tao, R. & Dai, Y. N. Pesticides in three rural rivers in Guangzhou, China: spatiotemporal distribution and ecological risk. Environ. Sci. Pollut Res. 26, 3569–3577 (2019).
Ccanccapa, A., Masiá, A., Navarro-Ortega, A., Picó, Y. & Barceló, D. Pesticides in the Ebro River basin: occurrence and risk assessment. Environ. Pollut. 211, 414–424. https://doi.org/10.1016/j.envpol.2015.12.059 (2016).
Ali, D. et al. Assessment of genotoxic and mutagenic effects of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem. Toxicol. 47 (3), 650–656. https://doi.org/10.1016/j.fct.2008.12.021 (2009).
Hladik, M. L., Main, A. R. & Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Tech. 52 (6), 3329–3335. https://doi.org/10.1021/acs.est.7b06388 (2018).
Jabber, S. M., Khan, Y. S. & Rahman, M. S. Levels of organochlorine pesticide residues in some organs of the Ganges perch, lates calcarifer, from the Ganges–Brahmaputra–Meghna estuary, Bangladesh. Mar. Pollut Bull. 42, 1291–1296 (2001).
Yahia, D. & Elsharkawy, E. E. Multi pesticide and PCB residues in Nile tilapia and catfish in Assiut city, Egypt. Sci. Total Environ. 466, 306–314. https://doi.org/10.1016/j.scitotenv.2013.07.002 (2014).
Tomizawa, M. & Casida, J. E. Neonicotinoid insecticides: highlights of a symposium on strategic molecular designs. J. Agric. Food Chem. 59 (7), 2883–2886. https://doi.org/10.1021/jf103856c (2011).
Singh, P. B., Sharma, S., Saini, H. S. & Chadha, B. S. Biosurfactant production by Pseudomonas sp. and its role in aqueous phase partitioning and biodegradation of chlorpyrifos. Lett Appl Microbiol49(3), 378–83. https://doi.org/10.1111/j.1472-765X.2009.02672.x (2009).
Hussain, B. et al. Fish eco-genotoxicology: Comet and micronucleus assay in fish erythrocytes as in situ biomarker of freshwater pollution. Saudi J. Biol. Sci. 25 (2), 393–398. https://doi.org/10.1016/j.sjbs.2017.11.048 (2018).
Vieira, C. E., Pérez, M. R., Acayaba, R. D. & Raimundo, C. C. & Dos Reis Martinez, C.B. DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the neotropical fish Prochilodus lineatus. Chemosphere. https://doi.org/10.1016/j.chemosphere.2017.12.077 (2018). 195,125 – 34.
Bolognesi, C. & Hayashi, M. Micronucleus assay in aquatic animals. Mutagenesis. 26 (1), 205–213. https://doi.org/10.1093/mutage/geq073 (2011).
Mohanty, D. & Samanta, L. Multivariate analysis of potential biomarkers of oxidative stress in Notopterus notopterus tissues from Mahanadi River as a function of concentration of heavy metals. Chemosphere. 155, 28–38. https://doi.org/10.1016/j.chemosphere.2016.04.035 (2016).
Obiakor, M. O., Okonkwo, J. C. & Ezeonyejiaku, C. D. Genotoxicity of freshwater ecosystem shows DNA damage in preponderant fish as validated by in vivo micronucleus induction in gill and kidney erythrocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 775, 20–30. https://doi.org/10.1016/j.toxrep.2017.12.019 (2014).
Hossain, M. A., Sutradhar, L., Sarker, T. R., Saha, S. & Iqbal, M. M. Toxic effects of chlorpyrifos on the growth, hematology, and different organs histopathology of Nile tilapia, Oreochromis niloticus. Saudi J. Biol. Sci. 29 (7), 103316. https://doi.org/10.1016/j.sjbs.2022.103316 (2022).
Bhatnagar, A., Yadav, A. S. & Cheema, N. Genotoxic effects of chlorpyrifos in freshwater fish Cirrhinus mrigala using micronucleus assay. Adv. Biol. https://doi.org/10.1155/2016/9276963 (2016).
Viana, L. F. et al. The response of neotropical fish species (Brazil) on the water pollution: metal bioaccumulation and genotoxicity. Arch. Environ. Contam. Toxicol. 75, 476–485. https://doi.org/10.1007/s00244-018-0551-9 (2018).
Mir, M. I. et al. Scenario of genotoxicity in fishes and its impact on fish industry. IOSR J. Environ. Sci. Toxicol. Food Technol. 8 (6), 2319–2402. https://doi.org/10.9790/2402-08626576 (2014).
Ayala, A., Muñoz, M. F. & Argüelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev.https://doi.org/10.1155/2014/360438 (2014).
Fritz, K. S. & Petersen, D. R. Exploring the biology of lipid peroxidation-derived protein carbonylation. Chem. Res. Toxicol. 24 (9), 1411–1419. https://doi.org/10.1021%2Ftx200169n (2011).
Vieira, C. E. et al. Multiple biomarker responses in Prochilodus lineatus subjected to short-term in situ exposure to streams from agricultural areas in Southern Brazil. Sci. Total Environ. 542, 44–56. https://doi.org/10.1016/j.scitotenv.2015.10.071 (2016).
Soorya, S. R. et al. Quantitative changes in antioxidant enzyme activities, glutathione content and malondialdehyde in a freshwater fish, Anabas testudineus (Bloch), exposed to sewage. J. Aquat. Biol. Fish. 1, 68–76 (2013).
Benli, P. & Çelik, M. Glutathione and its dependent enzymes’ modulatory responses to neonicotinoid insecticide sulfoxaflor induced oxidative damage in zebrafish in vivo. Sci. Prog. 104 (2). https://doi.org/10.1177/00368504211028361 (2021).
Bagnyukova, T. V., Chahrak, O. I. & Lushchak, V. I. Coordinated response of goldfish antioxidant defenses to environmental stress. Aquat. Toxicol. 78 (4), 325–331 (2006).
Cong, B., Liu, C., Wang, L. & Chai, Y. The impact on antioxidant enzyme activity and related gene expression following adult zebrafish (Danio rerio) exposure to dimethyl phthalate. Animals. 10 (4), 717 (2020).
Bouraoui, Z. et al. Acute effects of cadmium on liver phase I and phase II enzymes and metallothionein accumulation on sea bream Sparus aurata. Fish. Physiol. Biochem. 34, 201–207 (2008).
Printes, L. B., Fernandes, M. N. & Espíndola, E. L. G. Laboratory measurements of biomarkers and individual performances in Chironomus xanthus to evaluate pesticide contamination of sediments in a river of southeastern Brazil. Ecotoxicol. Environ. Saf. 74 (3), 424–430. https://doi.org/10.1016/j.ecoenv.2010.10.033 (2011).
Shah, Z. U. & Parveen, S. Oxidative, biochemical and histopathological alterations in fishes from pesticide contaminated river Ganga, India. Sci. Rep. 12 (1), 3628. https://doi.org/10.1038/s41598-022-07506-8 (2022).
Clasen, B. et al. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Sci. Total Environ. 626, 737–743. https://doi.org/10.1016/j.scitotenv.2018.01.154 (2018).
Majumder, R. & Kaviraj, A. Cypermethrin induced stress and changes in growth of freshwater fish Oreochromis niloticus. Int. Aquat. Res. 9, 117–128. https://doi.org/10.1007/s40071-017-0161-6 (2017).
Boelsterli, U.A., & Boelsterli, U.A. Mechanistic Toxicology: The Molecular Basis of How Chemicals Disrupt Biological Targets. https://doi.org/10.3109/9780203361764 (CRC Press, London, 2002).
Costa, L. G., Giordano, G., Guizzetti, M. & Vitalone, A. Neurotoxicity of pesticides: a brief review. Front. Biosci. 13 (4), 1240–1249 (2008).
Garawani, I. M. et al. The effect of neonicotinoids exposure on Oreochromis niloticus histopathological alterations and genotoxicity. Bull. Environ. Contam. Toxicol. 109 (6), 1001–1009 (2022).
Salunke, A., Pandya, P. & Parikh, P. Behavioral alterations and neurotoxicity of Imidacloprid on freshwater Teleost Oreochromis mossambicus. Int. Res. J. Sci. Eng. 9, 23–30 (2020).
Desai, B. & Parikh, P. Behavioural responses to acute exposure of Imidacloprid and Curzate on Labeo rohita (Hamilton, 1822). Int. J. Sci. Res. 2 (1), 1–2 (2014).
Zhang, J. G. et al. Imidacloprid and Thiamethoxam affect synaptic transmission in zebrafish. Ecotoxicol. Environ. Saf. 227, 11291–11297. https://doi.org/10.1016/j.ecoenv.2021.112917 (2021).
Tian, X. et al. Chronic brain toxicity response of juvenile Chinese rare minnows (Gobiocypris rarus) to the neonicotinoid insecticides imidacloprid and nitenpyram. Chemosphere. 210, 1006–1012. https://doi.org/10.1016/j.chemosphere.2018.06.083 (2018).
Halappa, R. & David, M. Behavioral responses of the freshwater fish, Cyprinus carpio (Linnaeus) following sublethal exposure to chlorpyrifos. Turk. J. Fish. Aquat. Sci. 9 (2). https://doi.org/10.4194/trjfas.2009.0218 (2009).
Topal, A., Şişecioğlu, M., Atamanalp, M., Işık, A. & Yılmaz, B. The in vitro and in vivo effects of chlorpyrifos on acetylcholinesterase activity of rainbow trout brain. J. Appl. Anim. Res.44 (1), 243–247. https://doi.org/10.1080/09712119.2015.1031776 (2016).
Braunbeck T., Hinton D.E., Strett B. Eds. Fish Ecotoxicology, Environmental Practice, Birkhaeuser Verlag, Basel, Switzerland. https://doi.org/10.1017/S1466046600001198 (1998).
Agus, H. H., Erkmen, B., Sümer, S., Sepici-Dinçel, A. & Erkoç, F. Impact of DBP on histology and expression of HSP 70 in gill and liver tissue of Cyprinus carpio. Mol. Biol. Rep. 42, 1409–1417. https://doi.org/10.1007/s11033-015-3920-8 (2015).
Veldhoen, N. et al. Gene expression profiling and environmental contaminant assessment of migrating Pacific salmon in the Fraser River watershed of British Columbia. Aquat. Toxicol. 97 (3), 212–225. https://doi.org/10.1016/j.aquatox.2009.09.009 (2010).
Jin, Y. et al. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere. 78(7), 846 – 52. (2010). https://doi.org/10.1016/j.chemosphere.2009.11.044
Hansen, B. H., Rømma, S., Garmo, Ø. A., Olsvik, P. A. & Andersen, R. A. Antioxidative stress proteins and their gene expression in brown trout (Salmo trutta) from three rivers with different heavy metal levels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 143 (3), 263–274. https://doi.org/10.1016/j.cbpc.2006.02.010 (2006).
Acknowledgements
The authors acknowledge the Head of the Department of Zoology at the University of North Bengal for providing the Departmental Central Instrument Facility in support of the Fund for Improvement of Science and Technology Infrastructure Program (FIST), Department of Science and Technology, New Delhi, India, and the organization that provided the funding as National Fellowship for Schedule Cast, University Grants Commission, New Delhi. The authors are thankful to the team of Food Safety Referral Lab, ICAR-IIHR, Bangalore for the pesticide residue analysis. The authors are also thankful to the people who contributed to this research. Mr. Subhajit Das, Department of Zoology, University of North Bengal, is also acknowledged for his assistance in building Map.
Funding
This work was partially supported by UGC- National Fellowship for Schedule Cast.
Author information
Authors and Affiliations
Contributions
There are no disagreements of interest among the writers. D. Dutta, (A) Ray, and E. Bhattacharya performed the original research. D. Dutta and (B) Ghosh carried out the statistical analyses. M. Bahadur authored the manuscript as the supervisor of the Genetics and Molecular Biology Laboratory at the Department of Zoology at the University of North Bengal. M. Bahadur edited and reviewed the work. Aathira U. carried out the sample preparation, and Abhishek Mandal conducted a multi-residue analysis of LC-MS/MS and GC-MS/MS. Partha P. Choudhury supervised the pesticide residue analysis and reviewed the manuscript. UGC partially funded this research. The data generated during this investigation are accessible from the corresponding author upon request. The authors have no financial or non-financial interests to report.
Corresponding author
Ethics declarations
Ethical permission
The present study has been authorized by the Institutional Animal Ethics Committee of the University of North Bengal, vide ref No. IAEC/NBU/2022/30 dated 23.09.2022 (Proposal No. 12).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in Figures 5 and 6 where “Site II” was incorrectly labelled as “Site III” and Vice-versa.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dutta, D., Bhattacharya, E., Ray, A. et al. Genotoxic impact of agricultural insecticides as contaminants of river Teesta on the resident fish Pethia Conchonius. Sci Rep 14, 28283 (2024). https://doi.org/10.1038/s41598-024-74434-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74434-0