Abstract
Anecdotal evidence from preliminary observations has noted multiple instances where osteoporosis is present in elderly patients before the clinical detection of bowel disease, even in the absence of overt gastrointestinal symptoms. However, any potential association between these conditions remains to be further investigated. This computed tomography (CT) study investigates whether patients with gastrointestinal (GI) perforation have lower bone mineral density (BMD) than age and sex matched controls. BMD was measured by drawing 3D regions of interest in the bone marrow of the L1–L3 vertebral bodies on CT scans of each of 37 GI perforations and matched controls. Spectrometric calibration of Hounsfield units to the mineral scale was performed with density measurements in the paravertebral muscles (erector spinae) and subcutaneous adipose tissue. The mean BMD of patients with GI perforation (135.9 ± 24.3 mg/ml) was significantly lower than that of controls (96.9 ± 27.5 mg/ml, p < 0.05). The calculated T-and Z-scores of bone mineral density were also significantly different between the two groups (p < 0.05 for each) and were − 2.9 (± 0.90) and − 0.8 (± 0.91) in patients with GI perforation and − 1.6 (± 0.83) and 0 (± 0.96) in the control group, respectively. The results imply that patients with gastrointestinal (GI) perforation have lower bone mineral density (BMD) than age-and sex-matched controls, posing the question whether the screening and aggressive management of osteoporosis is high-risk populations for gastrointestinal perforation can prevent gastrointestinal complications in targeted populations.
Similar content being viewed by others
Introduction
Gastrointestinal perforation is a serious but rare life-threatening condition requiring emergency surgery. Despite recent improvements in surgical and medical management, the overall mortality rate is 30% and can rise to 70% in cases with diffuse peritonitis1.
Osteoporosis is characterized by a decrease in bone mass and density, making bones more fragile and susceptible to fractures. It is primarily a skeletal disorder that is also seen in association with several GI disorders, particularly those associated with malabsorption and maldigestion2.
In osteoporosis, there is evidence that systemic changes in bone density are causally related to bone collagen content, particularly type I collagen, which is a major component of the extracellular matrix in various connective tissues in the body, including the gut3.
In addition, chronic inflammation is a hallmark of both osteoporosis and chronic bowel disease as recent evidence suggests both innate and adaptive immune cells contribute to the pathogenesis osteoporosis2,4. For instance, patients with inflammatory bowel disease are at higher risk of developing osteoporosis and osteopenia than the general population, with relative risk of fracture up to 40% higher in patients with inflammatory bowel disease5. Inflammatory processes can affect collagen metabolism in various tissues, including the gut.
Above this, the key role of various collagens on the skin, skeletal as well as the gastrointestinal system is illustrated by a wide range of connective tissue disorders such as osteogenesis imperfecta, many chondrodysplasias and several subtypes of Ehlers-Danlos syndrome3,6,7.
Moreover, osteoporotic fractures can lead to reduced mobility and physical activity. Prolonged immobility or inactivity can affect intestinal motility, which in turn can affect intestinal mucosal integrity and collagen synthesis.
In this study we hypothesized that patients with reduced bone mineral density, particularly osteoporosis, are at higher risk of gastrointestinal perforation. The present study compares the bone mineral density (BMD) of patients with gastrointestinal perforation and sex- and age-matched controls using phantomless volumetric bone mineral density (BMD) assessment on routine CT scans.
Methods
This is a retrospective comparative study analyzing patients with surgically-confirmed GI perforation who underwent CT evaluation at two German centers between January 2021 and June 2023.
Inclusion criteria were (a) available contrast-enhanced CT scan of the abdomen at hospital admission and (b) CT morphological evidence of perforation such as extraluminal gas, oral contrast air, and bowel wall discontinuity. Included patients were manually matched 1:1 with controls for age and sex who underwent CECT without evidence of GI perforation within the same study period originating from the same cultural milieu and analogous environmental settings.
Control patients in the study presented to the emergency department with abdominal pain and, based on clinical evaluation, were deemed eligible for CECT by the attending physician.
For both groups, the following exclusion criteria applied: (a) acute abdominal pathology other than perforation (e.g. bowel neoplasia), (b) acute or older spinal fractures and pathologies such as malignant bone lesions and high-grade average degenerative/arthrotic lesions involving more than 50% of the vertebral body of L1–L3, (c) lumbar spondylodesis or other lumbar implants, and (d) presence of beam hardening artefacts d) patients with a prior history of ulcerative colitis and Crohn’s disease (e) patients currently receiving glucocorticosteroid therapy.
This retrospective study was approved by the institutional review board of the University of Cologne. Informed patient consent was waived in accordance with the Ethics Committee policy. All investigations in this study involving human participants were conducted in accordance with the ethical standards of the institutional and national research committee and the Declaration of Helsinki (1964).
Scanning protocol CECT and image reconstruction
All scans analyzed for this study were performed on two multidetector CT scanners (Discovery CT 750 HD 64-slices, GE Healthcare corp., Chicago, USA and Ingenuity System 128 slices, Philips, Amsterdam, Netherlands).
Patients were placed in the supine position, head first. After administration of IV contrast, CT images were acquired in the portal venous phase.
The entire abdomen was scanned from the diaphragmatic dome to the pelvic floor. The scan parameters were: current 29.4 ± 9.4 mAs, collimation 40 mm, pitch 0.984, tube voltage 120 kV, mean CTDIvol 2.1 ± 0.6 mGy, and mean DLP 86.2 ± 26.8 mGy*cm.
Bone mineral measurements
Vertebral bone density measurement without the use of an external reference phantom was performed using IntelliSpace software (Philips, Amsterdam, The Netherlands), which is a well-established standardized method8,9,10. For BMD measurements, an ellipsoid volume of interest (VOI) of 9 mm thickness was placed in the axial plane in the central trabecular portions of each L1–L3 lumbar vertebral body. Sagittal reconstructions were used to align the transverse plane parallel to the endplate at each level.
Spectrometric calibration of Hounsfield units to the mineral scale was performed with density measurements in the paravertebral muscles (erector spinae muscle) and subcutaneous adipose tissue (see Fig. 1).
Example of intellispace workflow. Above: Axial CT image at L1 level. An ellipsoid volume of interest (VOI) placed in the central trabecular portions of L1–L3 lumbar vertebral bodies as well as the erector spinae muscle and subcutaneous fat. Below: Example of a calibration curve depicting the Houndsfield Units (HU) distribution of the consecutive VOI-associated pixels.
The VOIs were placed in the anterior trabecular bone of the vertebral body, avoiding the internal vertebral venous plexus, the surrounding cortical bone and any focal lytic or sclerotic lesion.
In total 2 different radiologists interpreted the images, one with 4 years experience in CT imaging and 2 years in musculoskeletal imaging and one with 10 years experience in CT imaging and 3 years in musculoskeletal imaging. To determine inter- and intrarater reliability, 10 patients were randomly selected from the data set and measured twice by reader 1 and reader 2.
Statistics
Statistical data analysis was performed using R, version 3.6.2, on RStudio, version 2023.03.0 + 386 (https://cran.r-project.org/). Independent samples Mann-Whitney U test (Wilcoxon rank sum test) was used for group comparisons, as normal distribution and variance homogeneity were not confirmed by the Shapiro-Wilk and Levene tests. For binary outcome groups such as gender the chi square test was used, as the patient frequency count in all cells of the expected contingency table were above five.
Propensity score matching with the MatchIt package was performed in R with the setting method = ‘optimal’ to ensure that the age and gender distribution between the GI perforation and control group were optimally balanced in the sense that that sum of the absolute pairwise distances in the matched sample was as small as possible.
Intra- and inter-reader reliability was tested using the intraclass correlation coefficient in a two-way random-effects model (< 0.5 poor, > 0.5 moderate, > 0.75 good, > 0.9 excellent) using the irr package.
Continuous variables were expressed as mean ± standard deviation (SD). Statistical significance was defined as p < 0.05.
Results
Demographic data
The propensity score matched study cohort consisted of 37 patients in the GI perforation group (12 females, 25 males) and 37 patients in the control group (14 females, 23 males). With propensity score matching in total n = 14 perforation cases from the initial cohort were omitted.
The perforation and control groups were balanced with regard to gender (P = 0.81), as shown in Table 1. The mean age in the perforation group (55.2 ± 9.3 years) was comparable to that of the control group (54.2 ± 10.0 years, p = 0.63).
In the total GI perforation group n = 32/37 patients were known with diverticulosis, while in the control group no patients were noted to have diverticulosis in their medical history.
In the perforation group n = 35 patients had a large bowel perforation, with n = 1 case of stomach and n = 1 case of small bowel perforation. Specifically, 30 patients had a sigmoid perforation and 6 patients had a perforation of the ascending colon, transverse colon and/ or descending colon (from which one case suffered both colon and sigmoid perforation).
In our population the mean age in the control group of females is 54.64 years and of males 55.60 years. A bigger discrepancy is found in the perforation group, where mean female age is 49.71 years compared to 56.91 years in the male subgroup.
Bone mineral density measurements
Measurements of BMD by both readers provided a good inter- and intrareader reliability (both > 0.75) and therefore can be considered reproducible and robust.
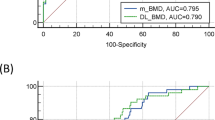
The median BMD of patients with GI perforation (104.0 ± 28.01 mg/ml) was significantly lower than that of the controls (135.9 ± 24.3 mg/ml, p = 3.6 × 10 − 7 as shown in Table 1; Fig. 2). Women had higher median BMD than men in both, the perforation group (118.7 ± 21.3 vs. 95.8 ± 30.3, p = 0.17) and the control group (142.7 ± 28.6 vs. 133.8 ± 20.85, p = 0.37). BMD was also inversely correlated with patient age in both, the perforation group (RPearson= −0.53, p = 8.3 × 10 − 4) and the control group (RPearson= −0.12, p = 0.49).
The calculated bone mineral density T-scores and Z-scores also differed significantly (p = 6.9 × 10 − 7 and p = 1.2 × 10 − 5 respectively) between the two groups, being − 1.6 ± 0.83 and 0 ± 0.96, respectively, in the control group and − 2.6 ± 0.93 and − 0.82 ± 0.88, respectively, in the GI perforation group. Like for the BMD, T-scores and Z-scores surprisingly were increased in women (T-score: −1.95 ± 0.68 vs. −2.9 ± 0.96, p = 0.02; Z-score: −0.9 ± 1.08 vs. −1.1 ± 0.76, P = 0.47) and T-scores correlated inversely with patient age (T-score: RPearson= −0.53, p = 8.3 × 10 − 4 for the perforation group and RPearson= −0.18, p = 0.27 for the control group; Z-score: RPearson=0.15, p = 0.38 for the perforation group and RPearson= 0.56, p = 2.8 × 10 − 4 for the control group).
Discussion
Osteoporosis and bowel perforation share a complex interplay, often linked by disruptions in collagen, a crucial component of bone and connective tissues2,6,7.
The concomitant role of increased collagen turnover in both GI perforation as well as osteoporosis and hence the postoperative risk for GI perforation in elderly osteopenic patients has thus far not yet been elucidated in clinical studies.
In this retrospective cohort study we have found through a phantomless BMD quantitative CT workflow method that BMD was significantly (p < 0.05) reduced in GI perforation patients (n = 37) compared to matched controls (n = 37).
The finding that the women had higher BMD, T-scores, and Z-scores compared to men is likely due to factors such as smaller, denser bones, protective effects of estrogen before menopause, and lifestyle differences like physical activity and diet. In our study, the mean age difference between females and males, particularly in the perforation group, may also contribute to this finding.
Despite the commonly described significance of osteopenia in elderly patients undergoing emergency gastrointestinal surgery this study is as far as we can discern one of the first QCT imaging studies to compare bone mineral density in GI perforation patients versus healthy controls.
Several studies have investigated the role of sarcopenia, one of the most important contributing factors related to osteopenia as well as directly of osteopenia in the prediction of postoperative complications and mortality11,12,13.
In one retrospective cohort study (n = 103) on elderly patients that underwent emergency gastrointestinal surgery, univariate analysis revealed that an American Society of Anesthesiologists Physical Status of ≥ 3 (P = 0.0084), hemoglobin levels (P = 0.0026), albumin levels (P < 0.001), sarcopenia (P = 0.015), and osteopenia (P < 0.001) were significantly associated with severe postoperative complications (≥ grade III according to Clavien-Dindo grading system)12. Collagen diseases, such as Ehlers-Danlos syndrome (EDS) and osteogenesis imperfecta, exemplify how genetic abnormalities impacting collagen synthesis can lead to widespread tissue fragility. EDS, specifically, highlights the association between collagen defects and gastrointestinal complications, including an increased risk for bowel perforation.
In a systematic review on 4 retrospective case series 19 to 25% of the total number of EDS IV patients in the respective series experienced a spontaneous GI perforation or solid organ rupture (liver or spleen)6.
In another systematic review on 839 EDS cases the most common musculoskeletal complications were in order of magnitude reduced bone density (39/43, 90.7%), joint pain (217/270, 80.4%), and hypotonia/weakness (79/140, 56.4%)7.
Understanding the intricate relationship between osteoporosis, bowel perforation, and collagen may shed light on the systemic nature of collagen-related disorders, emphasizing the importance of comprehensive clinical care addressing both bone health and connective tissue integrity.
In osteoporosis, an imbalance in bone remodeling disrupts the intricate equilibrium between bone formation and resorption that often stems from decreased osteoblast activity and increased osteoclast function leading to reduced bone density. Recent research has shown the various roles that collagens play in osteoblastogenesis within the extracellular matrix (ECM)14,15.
Type I collagen accounts for 90% of the total collagen in bone tissue and forms triple helices of polypeptides which form the collagen fibrils which further interact with other (non)collagenous proteins to assemble the higher-order fibril bundles and fibers that provide the bone with support16,17.
In osteoporosis, diminished collagen synthesis and altered collagen cross-linking may contribute to weakened bone architecture, rendering the skeletal system more susceptible to fractures3,16.
Importantly, as the gastrointestinal tract also relies on collagen for structural integrity and a collagen dysregulation is not confined to bones but may extend to other tissues, including the intestinal walls. Alterations in collagen composition and organization may lead to weakening of the connective tissue matrix, therefore compromising the integrity of the bowel, rendering the intestines more susceptible to perforation. Chronic inflammation and changes in collagen turnover further exacerbate this vulnerability, creating a microenvironment conducive to bowel perforation. Moreover, alterations in bone metabolism can influence the release of signaling molecules and growth factors that impact the gastrointestinal system. For example, the imbalance in bone-derived cytokines, such as transforming growth factor-beta (TGF-β), can affect the maintenance of intestinal homeostasis and contribute to intestinal barrier dysfunction18,19.
The intricate pathophysiological mechanisms linking osteoporosis to bowel perforation involve disruptions in (type I) collagen at both skeletal and gastrointestinal levels. Understanding these complex interactions is crucial for developing approaches to manage and prevent complications in individuals affected by these conditions. In our view, future research should explore whether screening and aggressive management of osteoporosis in high-risk populations, including the use of medications like bisphosphonates, calcium, and vitamin D to improve bone density, along with strict coagulation control, can help prevent gastrointestinal complications in these targeted groups.
Quantitative CT (QCT) can currently determine BMD accurately from routine clinical CTA images in the vertebrae even when extra-osseous calcifications, osteophytes and degenerative phenomena are present. With DEXA these phenomena and the dependence on patient size have been previously shown to give potentially misleading results20. Furthermore in a comparative study of BMD accuracy using the European Spine Phantom a deviation of up to 7% in BMD values between scanners of different DXA manufacturers was highlighted21.
Limitations of this study are (1) the use of different contrast media and flow rates compared to the original standardized algorithm, leading to a potential over or underestimation of BMD (2) small sample size, leading to high sensitivity in the analysis for BMD outliers (3) not taking into account relevant covariates such as BMI, DMII and number of packyears in both perforation and osteoporosis formation.
The absence of BMD measurement from DEXA scans in this study may be a limitation; however, recent studies demonstrate a significant correlation between areal BMD measurements with DEXA at the lumbar spine and quantitative CT methods for volumetric BMD-evaluation at the lumbar spine as well as the thoracic spine22,23. Volumetric trabecular BMD measurement offers further advantages over DEXA by detecting low bone mass earlier in the spine, especially in cases of trabecular bone loss24, and avoiding artificially high BMD readings caused by obesity25, disc space narrowing or degenerative diseases26, aortic calcification27, and osteophytes28.
Future studies should in our opinion address the role of covariates such as previous intra-abdominal surgery, cardiovascular disease, DMII and nicotine abuse on the association between BMD and GI perforation and further clarify the underlying pathophysiological mechanisms that are in common between osteopenia/ osteoporosis and GI perforation formation in order to improve the detection rate and early treatment of these two apparently distinct entities.
Data availability
The code in R and data that support the findings of this study will be openly available in Github following publication at: https://github.com/Radiology4U/BMD-measurement-GI.
Abbreviations
- GP:
-
Gastrointestinal perforation
- BMD:
-
Bone mineral density
- VOI:
-
Volume of interest
References
Shin, R. et al. Predictors of morbidity and mortality after surgery for intestinal perforation. Ann. Coloproctol. 32(6), 221–227. https://doi.org/10.3393/ac.2016.32.6.221 (2016).
Katz, S. & Weinerman, S. Osteoporosis and gastrointestinal disease. Gastroenterol. Hepatol. (N Y) 6(8), 506–517 (2010).
Viguet-Carrin, S., Garnero, P. & Delmas, P. D. The role of collagen in bone strength. Osteoporos. Int. 17, 319–336. https://doi.org/10.1007/s00198-005-2035-9 (2006).
Saxena, Y., Routh, S. & Mukhopadhaya, A. Immunoporosis: Role of innate immune cells in osteoporosis. Front. Immunol. 12, 687037. https://doi.org/10.3389/fimmu.2021.687037 (2021).
Compston, J. E. et al. Osteoporosis in patients with inflammatory bowel disease. Gut 28(4), 410–415 (1987).
El Masri, H., Loong, T. H. & Meurette et al. Bowel perforation in type IV vascular Ehlers-Danlos syndrome. A systematic review. Tech. Coloproctol. 22(5), 333–341 (2018). https://doi.org/10.1007/s10151-018-1783-4
Doolan, B. J., Lavallee, M. E., Hausser, I. et al. Extracutaneous features and complications of the Ehlers-Danlos syndromes: A systematic review. Front. Med. (Lausanne) 10, 1053466. https://doi.org/10.3389/fmed.2023.1053466 (2023).
Engelke, K. et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J. Clin. Densitom. 11(1), 123–162. https://doi.org/10.1016/j.jocd.2007.12.010 (2008).
Abdullayev, N. et al. Effects of contrast enhancement on in-body calibrated phantomless bone mineral density measurements in computed tomography. J. Clin. Densitom. 21(3), 360–366. https://doi.org/10.1016/j.jocd.2017.10.001 (2018).
Mueller, D. K. et al. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur. J. Radiol. 79(3), 375–381. https://doi.org/10.1016/j.ejrad.2010.02.008 (2011).
Simonsen, C. et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann. Surg. 268(1), 58–69. https://doi.org/10.1097/SLA.0000000000002679 (2018).
Takano, Y. et al. Significance of osteopenia in elderly patients undergoing emergency gastrointestinal surgery. Ann. Gastroenterol. Surg. 6(4), 587–593. https://doi.org/10.1002/ags3.12558 (2022).
Erős, A. et al. Sarcopenia as an independent predictor of the surgical outcomes of patients with inflammatory bowel disease: a meta-analysis. Surg. Today. 50(10), 1138–1150. https://doi.org/10.1007/s00595-019-01893-8 (2020).
Yano, H. et al. Sp7/Osterix induces the mouse pro-α2(I) collagen gene (Col1a2) expression via the proximal promoter in osteoblastic cells. Biochem. Biophys. Res. Commun. 452(3), 531–536. https://doi.org/10.1016/j.bbrc.2014.08.100 (2014).
Volk, S. W. et al. Type III collagen regulates osteoblastogenesis and the quantity of trabecular bone. Calcif. Tissue Int. 94(6), 621–631. https://doi.org/10.1007/s00223-014-9843-x (2014).
Lin, X. et al. The bone extracellular matrix in bone formation and regeneration. Front. Pharmacol. 11, 757. https://doi.org/10.3389/fphar.2020.00757 (2020).
Varma, S., Orgel, J. P. & Schieber, J. D. Nanomechanics of type I collagen. Biophys. J. 111(1), 50–56. https://doi.org/10.1016/j.bpj.2016.05.038 (2016).
Hahm, K. B. et al. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut 49(2), 190–198. https://doi.org/10.1136/gut.49.2.190 (2001).
Ke, K., Arra, M. & Abu-Amer, Y. Mechanisms underlying bone loss associated with gut inflammation. Int. J. Mol. Sci. 20(24), 6323. https://doi.org/10.3390/ijms20246323 (2019).
Bolotin, H. H. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 41(1), 138–154. https://doi.org/10.1016/j.bone.2007.02.022 (2007).
Larkin, A. et al. QA/acceptance testing of DEXA X-ray systems used in bone mineral densitometry. Radiat. Prot. Dosim. 129(1–3), 279–283. https://doi.org/10.1093/rpd/ncn086 (2008).
Chen, L. et al. Using QCT to evaluate bone mineral and abdominal adipose changes in patients with primary hyperparathyroidism and comparing it to DXA for bone status assessment: a retrospective case-control study. Ann. Transl. Med. 10(10), 606. https://doi.org/10.21037/atm-22-1827 (2022).
Te Beek, E. T. et al. Quantitative CT evaluation of bone mineral density in the thoracic spine on 18F-fluorocholine PET/CT imaging in patients with primary hyperparathyroidism. J. Clin. Densitom. 27(1), 101464. https://doi.org/10.1016/j.jocd.2023.101464 (2024).
Adams, J. E. Quantitative computed tomography. Eur. J. Radiol. 71(3), 415–424 (2009).
Yu, E. W. et al. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 27(1), 119–124 (2011).
Guglielmi, G. et al. Effect of spinal degenerative changes on volumetric bone mineral density of the central skeleton as measured by quantitative computed tomography. Acta Radiol. 46(3), 269–275 (2005).
Smith, J. A., Vento, J. A., Spencer, R. P. & Tendler, B. E. Aortic calcification contributing to bone densitometry measurement. J. Clin. Densitom. 2(2), 181–183 (1999).
Liu, G. et al. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos. Int. 7(6), 564–569 (1997).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: S.S., J.K., L.G., N.A. ; data collection: K.E., T.D., M.E., N.H. ; administrative support and supervision: J.B., N.T., C.B., D.M., N.A., K.E., V.V., N. N. ; analysis and interpretation of results: S.S., L.G., J.K., N.A.; draft manuscript preparation: S.S., L.G., J.K., N.A. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sanduleanu, S., Ersahin, K., Kottlors, J. et al. Investigating the association between osteopenia and bowel perforation through a multicenter radiologic analysis. Sci Rep 14, 23625 (2024). https://doi.org/10.1038/s41598-024-74549-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74549-4