Abstract
Chlamydia psittaci pneumonia (CPP) exhibits similar characteristics as of COVID-19 with respect to clustering outbreaks and onset symptoms. This study is aimed at exploring the different clinical manifestations of both pneumonias to establish a simple nomogram to distinguish them. This multicenter, retrospective, case–control study compared two independent cohorts of patients with CPP or COVID-19. The risk factors of CPP were analyzed using multivariate logistic regression, which was used to establish the nomogram. Both patients with CPP and COVID-19 exhibited similar clinical symptoms. As compared to patients with COVID-19, a higher proportion of patients with CPP had nervous system symptoms. Patients with CPP had higher inflammatory indicators, creatine kinase, and lower lymphocyte and albumin. They also had lower proportions of ground-glass opacity and bilateral lung involvement than COVID-19 patients. Furthermore, patients with CPP had higher 30 day mortality as well as higher rates of severe pneumonia, septic shock, and ICU admission. Multivariate logistic regression showed that nervous system symptoms, lymphocytes, creatine kinase, bilateral lung lesions, and ground-glass opacity were risk factors for CPP. Incorporating these five factors, the nomogram achieved good concordance index of 0.989 in differentiating CPP from COVID-19, and had well-fitted calibration curves. Despite similar clinical characteristics, nervous system symptoms, lymphocyte, creatine kinase, lesions in bilateral lungs, and ground-glass opacity may help in differentiating the pneumonias. These were combined into a clinically useful nomogram for rapid and early identification of CPP to avoid misdiagnosis and help in the decision-making process.
Similar content being viewed by others
Introduction
Human psittacosis is a zoonotic disease caused by Chlamydia psittaci (C. psittaci), and mainly develops due to inhalation of contaminated aerosols from respiratory or eye secretions, dry feces, and urine of infected birds or asymptomatic carriers1. C. psittaci pneumonia (CPP) has been reported worldwide, including China, Australia, United States, and Europe. In addition, C. psittaci infection is believed to be the cause of 1% of community-acquired pneumonia cases2. CPP usually has mild symptoms, but some patients deteriorate rapidly and even die of severe pneumonia, acute respiratory distress syndrome (ARDS), and multiple organ failure due to delayed diagnosis and lack of appropriate anti-pathogen therapy1,3.During the COVID-19 pandemic, a number of clustering outbreaks of psittacosis in families and hospitals were reported4,5,6.Home quarantine measures and restrictions on public entertainment may have increased people’s demand for pets during the COVID-19 pandemic, especially parrots, which will increased the risk of psittacosis. The common symptoms of CPP include fever, cough, and dyspnea7,8, which are non-specific and similar to pneumonia caused by COVID-19, and can lead to misdiagnosis.
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and has spread worldwide, which has posed a serious threat to global public health. In addition, the emergence of mutant strains, such as Delta and Omicron, which are more transmissible than previous strains and may reduce the effectiveness of vaccination, are concerning9,10. Due to the strong infectivity and virulence of SARS-CoV-2, early and accurate diagnosis is important to impede the chain of transmission and deliver effective treatment. Reverse transcription polymerase chain reaction (RT-PCR) to amplify viral RNA is the gold standard for the diagnosis of COVID-19, although the test requires 3–4 h, and up to 20% to 67% of patients may have false-negative test results11,12,13. Furthermore, multiple studies have shown that C. psittaci can be transmitted among individuals in various ways, and exhibits similar characteristics as of COVID-19 with respect to clustering outbreaks, including outbreaks in families and hospitals4,5,6. Therefore, differentiating between the two diseases should be considered a matter of high biosafety due to the risk of infectious disease outbreaks. Importantly, the initial clinical symptoms of patients with COVID-19 are fever, cough, dyspnea, fatigue, and muscle soreness, which are similar to those of CPP7,11. Furthermore, previous studies have reported that laboratory results are similar in patients with CPP and COVID-19, including high levels of liver enzymes and inflammatory markers and low levels of lymphocytes and albumin1,8,11,14. Therefore, during the COVID-19 pandemic, it is possible that CPP was misdiagnosed as COVID-19.
Although CPP and COVID-19 pneumonia show similar clinical symptoms and laboratory characteristics, there are no large-sample clinical studies comparing the clinical features of the two types of pneumonia. Furthermore, the treatment and prognosis of CPP and COVID-19 are different; therefore, accurate diagnosis according to different clinical manifestations is imperative. In this study, we aimed to explore the different clinical, laboratory, and radiological characteristics of CPP and COVID-19 pneumonia to establish a simple nomogram for differentiating CPP from COVID-19 in order to guide diagnosis in clinical practice.
Methods
Study protocol and selection of patients
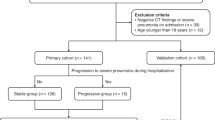
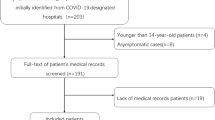
This was a retrospective case–control study. All patients with COVID-19 pneumonia were hospitalized at the West Court of Union Hospital of Huazhong University of Science and Technology between January 29, 2020, and April 8, 2020. All patients were diagnosed with COVID-19 according to the WHO Interim Guidelines. All the patients with COVID-19 by positive Reverse Transcription Polymerase Chain Reaction (RT-PCR) tests for SARS-CoV-2 from nasal and pharyngeal swab specimens. The patients with CPP were hospitalized in 10 tertiary general hospitals in Central-South China between June 21, 2018, and June 6, 2022. CPP was diagnosed on the basis of positive results of metagenomic next-generation sequencing (mNGS) of respiratory or blood samples, combined with clinical presentation. According to previous studies15,16,17, the diagnostic criteria of Chlamydia psittaci pneumonia are as follows:(1)Patients met the diagnostic criteria of community-acquired pneumonia18, including clinical symptoms (such as fever, cough, sputum, chest pain, etc.) and radiographic changes (demonstrable infiltration of the lungs). (2) The specific DNA fragment of Chlamydia psittaci was detected by mNGS. (3) The results of routine etiological pathogen examination of blood, sputum, and bronchoalveolar lavage fluid were negative and no other pathogenic microorganisms were found.
The methods and steps of mNGS detection are based on our previous research19,20. All patients in the study met the diagnostic criteria of community-acquired pneumonia (CAP)18 and underwent routine microbial etiological examination after admission, including culture (fungi and bacteria), serological tests (for influenza A virus [FluA], influenza B virus [FluB], Legionella pneumophila, Coxiella burnetii, Mycoplasma pneumoniae, and Chlamydia pneumoniae), and PCR (for SARS-CoV-2, influenza viruses, adenovirus, respiratory syncytial virus [RSV], coronaviruses, enterovirus, parainfluenza viruses [PIV], rhinovirus [RV], and metapneumovirus [MPV]). We excluded underage patients (< 18 years old), pregnant women, patients who were co-infected with bacteria, fungi, and other respiratory viruses on admission, and patients with missing medical records. A total of 81 patients with CPP and 450 patients with COVID-19 pneumonia were included in this study. The flowchart of the study is shown in Fig. 1. The Ethics Committee of Xiangya Hospital, Central South University (No. 202104005) and Union Hospital of Huazhong University of Science and Technology (No. 202003049) approved the collection and analysis of the clinical data of patients infected with C. psittaci and COVID-19, respectively. Due to the retrospective nature of the study and the anonymous processing of data prior to analysis, the Ethics Committee waived the requirement for informed consent. The study strictly complied with the Declaration of Helsinki.
Data collection
Baseline clinical features, laboratory test results, and imaging data of each patient were extracted from the electronic medical systems. A team of experienced respiratory clinicians independently collected, reviewed, and analyzed clinical data. The baseline characteristics of the patients included demographic characteristics (age and sex), underlying diseases, clinical symptoms and signs, and disease severity scores (CURB-65 and Sequential Organ Failure Assessment [SOFA]). Treatment data included respiratory support mode, life support technology, and drug therapy. Laboratory data included routine blood tests, blood biochemistry, liver and kidney function, myocardial injury mediators, inflammatory mediators, blood coagulation, and procalcitonin levels. Two senior radiologists reviewed the radiological images of lung lesions, including the ___location and characteristics of the lesions. All baseline laboratory and imaging examinations were completed within 24 h after admission.
Chest CT
All chest CT images were obtained on SOMATOM Definition (SIEMENS AG, Germany) with patients in the supine position. The main scanning parameters were as follows: tube voltage = 120 kV, tube current modulation (100–300 mAs), slice thickness = 1.0 mm, field of view = 1200 mm × 600 mm.
Clinical outcomes
The primary clinical outcome of this study was 30 day mortality after admission. Secondary outcomes included acute respiratory distress syndrome (ARDS), which was based on the Berlin definition21, severe pneumonia (based on the criteria for severe community-acquired pneumonia18), septic shock (based on Sepsis-322), ICU admission, and length of hospital stay of survivors.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and were compared using the Student’s t-test for normally distributed data or as median (interquartile range [IQR]) and tested using the Mann–Whitney U test for non-normal distribution. Categorical variables were presented as frequencies and percentages and compared using the Chi-square test or Fisher’s exact test. Furthermore, risk factors were evaluated using univariate logistic analysis, and variables with statistical significance (P value < 0.05) in the univariate analysis were included in the multivariate logistic regression analysis using forward stepwise selection based on the likelihood ratio to calculate independent risk factors. A final multivariate model was used to develop the nomogram. The nomogram prediction accuracy was assessed using a calibration curve. The model discriminative ability was assessed by calculating Harrell’s concordance index(C-index) and the area under the receiver operating characteristic (AUROC) curves. A C-index and AUROC of 0.5 indicates the absence of discrimination, whereas a C-index and AUROC of 1.0 indicates a perfect separation of CPP patients from COVID-19. Bootstrapping with 1000 repetitions was used to calculate a corrected C-index and AUROC to confirm the stability and performance of the model. We performed a decision curve analysis to assess the net benefit of using the CPP prediction model. Statistical analyses were performed using SPSS version 26.0 (IBM Corporation, Armonk, NY, USA), GraphPad Prism version 9.0 software (GraphPad Software Inc., San Diego, CA, USA), or R-4.1.2 software (R Foundation for Statistical Computing, Vienna, Austria). Differences were considered statistically significant at p < 0.05.
Results
Demographic and clinical characteristics in C. psittaci and COVID-19 pneumonia
Based on the inclusion and exclusion criteria, 450 patients with COVID-19 and 81 patients with CPP were included in our study (Fig. 1). In terms of demography and underlying diseases (Table 1), the age and sex distributions of CPP and COVID-19 were similar and differences were not significant (p > 0.05). Moreover, the distribution of most comorbidities in the two groups was similar. There was no significant difference in the history of malignancy, cerebrovascular disease, coronary heart disease, chronic kidney disease, diabetes or chronic obstructive lung disease between the two groups (p > 0.05). However, compared to patients with COVID-19 pneumonia, patients with CPP had higher rates of chronic liver disease and lower rates of hypertension. CPP and COVID-19 usually show similar clinical symptoms and signs (Table 1), such as fever, cough, dyspnea, and fatigue. However, a higher proportion of patients with CPP were found to have the following: fever (96.3% vs. 77.3%, p < 0.001), chills (9.9% vs. 1.6%, p < 0.001), sputum (58.0% vs. 25.1%, p < 0.001), and nervous system symptoms (46.9% vs. 9.6%, p < 0.001) as compared to those with COVID-19 pneumonia. Patients with CPP had a higher oxygen saturation(SpO2) and pulse, as well as a lower mean arterial pressure (MAP) (p < 0.05). There were no significant differences in respiration rate, cough, dyspnea, sore throat, myalgia, fatigue, or gastrointestinal symptoms (p > 0.05) between the two groups. Patients with CPP had higher SOFA scores (3.0 [2.0, 5.0] vs. 2.0 [2.0, 3.0], p < 0.001) and CURB-65 scores (1.0 [0.0, 2.0] vs. 1.0 [0.0, 1.0], p = 0.001) as compared to patients with COVID-19 pneumonia.
Laboratory examinations and radiological characteristics at admission
In terms of blood biochemical tests (Table 2), both patients with C. psittaci and COVID-19 pneumonia had high C-reactive protein (CRP), D-dimer levels and lactate dehydrogenase (LDH), and low levels of lymphocytes and albumin. However, patients with CPP had a higher CRP, white blood cell count (WBC), neutrophil percentage, blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), LDH, total bilirubin, creatine kinase, D-dimer, and procalcitonin as compared to patients with COVID-19 pneumonia. They also had a lower lymphocyte percentage, platelet count, and albumin level (p < 0.05), as compared to patients with COVID-19 pneumonia. In terms of the ___location of radiological lesions (Table 2, Fig. 2), a higher proportion of patients with COVID-19 had right (98.0% vs. 79.0%, p < 0.001), left (96.9% vs. 70.4%, p < 0.001), and bilateral lung involvement (95.8% vs. 50.6%, p < 0.001) as compared to those with CPP. However, in terms of the characteristics of the lesions (Table 2, Fig. 2), a higher proportion of patients with CPP had consolidation (87.7% vs. 27.1%,p < 0.001) and pleural effusion (59.3% vs. 3.1%,p < 0.001) and a lower proportion of patients had ground-glass opacity (13.6% vs. 96.4%, p < 0.001) as compared to patients with COVID-19 pneumonia.
Chest CT images of patients with C. psittaci or COVID-19 pneumonia. (A) A 69-year-old woman with C. psittaci pneumonia exhibited unilateral left lung consolidation and pleural effusion. (B) A 69-year-old man with C. psittaci pneumonia showed unilateral right lung consolidation. (C) A 65-year-old woman with COVID-19 pneumonia showed multiple ground-glass opacities in bilateral lung. (D) A 61-year-old man with COVID-19 pneumonia exhibited diffuse ground-glass opacities in bilateral lung.
Treatment and clinical outcomes in C. psittaci and COVID-19 pneumonia
As compared to patients with COVID-19, a greater number of patients with CPP underwent invasive ventilation and were treated with high-flow nasal cannula, and fewer patients were treated with conventional oxygen therapy (Table 3; p < 0.05). A lower proportion of patients with CPP were treated with glucocorticoids and immunoglobulins and a higher proportion of patients with CPP were treated with vasopressors (p < 0.05), as compared to patients with COVID-19 pneumonia. There was no significant difference in noninvasive ventilation and continuous renal replacement therapy between the two groups (p > 0.05). In terms of clinical outcomes (Table 3), the 30 day mortality rate of patients with COVID-19 was 4.0%, while that of patients with CPP was 9.9% (p = 0.048). Furthermore, during hospitalization, patients with CPP had a higher prevalence of ARDS (32.1% vs. 9.3%, p < 0.001), severe pneumonia (55.6% vs. 38.4, p = 0.004), septic shock (23.5% vs. 8.0%, p < 0.001), and ICU admission (59.3% vs. 8.7%, p < 0.001), and a shorter length of hospital stay among survivors, as compared to patients with COVID-19 pneumonia.
Independent risk factors for differentiating CPP From COVID-19 pneumonia
The results of univariate and multivariate logistic regression analyses are presented in Table 4. Compared to COVID-19 pneumonia patients, patients with CPP had a greater tendency to exhibit nervous system symptoms (OR:4.483, 95% CI:1.119–17.959, p < 0.034) and higher creatine kinase levels (OR:1.004, 95% CI:1.002–1.006, p < 0.001) (Figs. 3, 4). Moreover, compared to patients with CPP, patients with COVID-19 pneumonia tended to present with bilateral lung lesions (OR:0.024, 95% CI:0.004–0.134, p < 0.001), ground-glass opacity (OR:0.011, 95% CI:0.003–0.037, p < 0.001), and higher lymphocyte percentage (OR:0.822, 95% CI:0.752–0.898, p < 0.001) (Figs. 3, 4).
Nomogram establishment
Multivariate logistic regression showed that nervous system symptoms, lymphocyte percentage, creatine kinase level, bilateral lung lesions, and ground-glass opacity were risk factors for patients with CPP (Table 4). A nomogram was constructed based on the five risk factors identified in the multivariate analysis (Fig. 5A). The nomogram showed good accuracy in distinguishing CCP from COVID-19, with a concordance index(C-index) of 0.9895 (95% CI 0.9804–0.9986), and was confirmed to be 0.9896 through bootstrapping validation, which suggested a nomogram with good discrimination. The receiver operating characteristic curve of the nomogram for predicting CPP and after bootstrapping was 0.9895 and 0.9896, respectively (Fig. 5B). The calibration curve overlapped with the ideal line, indicating that the predicted possibilities based on the nomogram were consistent with the actual observations (Fig. 5C). The benefits of using the nomogram in clinical practice according to decision curve analysis are shown in Fig. 5D. Threshold probability for the net benefit associated with application of the nomogram in differentiating CPP from COVID-19 pneumonia ranged from 0.01 to 0.98 (Fig. 5D).
Construction of differential diagnostic nomogram in patients with C. psittaci or COVID-19 pneumonia. (A) The nomogram composed of lesions in bilateral lungs, ground-glass opacity, nervous system symptoms, lymphocyte percentage and creatine kinase was established, which differentiate C. psittaci pneumonia from COVID-19 pneumonia. (B) The ROC for performance of the prediction nomogram to distinguish patients with C. psittaci pneumonia from COVID-19 pneumonia. (C) Calibration curves for the nomogram. (D) Decision curve analysis (DCA) for the nomogram.
Discussion
CPP and COVID-19 pneumonia are characterized by overlapping clinical symptoms and laboratory results, which makes differential diagnosis challenging during the persisting COVID-19 pandemic. To date, the differences between the two conditions have only been described in case reports23. To the best of our knowledge, this is the largest case–control study of CPP and COVID-19. Our study compared the clinical, laboratory, and radiological characteristics of patients with COVID-19 and CPP. We found that patients with CPP were more likely to exhibit nervous system symptoms and higher creatine kinase levels. Moreover, patients with COVID-19 tended to have a higher lymphocyte percentage and present with lesions in bilateral lungs and ground-glass opacity on chest computed tomography (CT) scans. Finally, we developed a nomogram for early differential diagnosis of CPP and COVID-19. The nomogram, based on nervous system symptoms, lymphocyte percentage, creatine kinase, bilateral lung lesions, and ground-glass opacity, had good accuracy in differentiating CPP from COVID-19, with a C-index of 0.989 (95% CI 0.980–0.999).
The treatment and prognosis of CPP and COVID-19 pneumonia are different; therefore, it is important to differentiate these conditions based on early clinical manifestations and laboratory results. Our research showed that there are similarities and differences between CPP and COVID-19 pneumonia. In our study, the age, sex, and underlying disease distributions of CPP and COVID-19 were similar. Furthermore, our research showed that CPP and COVID-19 usually show similar clinical symptoms, such as fever, cough, dyspnea, and fatigue. Recently, studies have shown that C. psittaci can be transmitted from person to person in a variety of ways, with the same characteristics of clustering outbreaks as COVID-19, including outbreaks in families and hospitals4,5,6. During the COVID-19 pandemic, home quarantine measures and restrictions on public entertainment may have increased people’s demands for pets, especially parrots, which may have increased the risk of CPP. In addition, the continuing COVID-19 pandemic has limited people’s long-distance travel and holidays, increasing the opportunities for family members to get together, and increasing the possibility of family clustering outbreaks of CPP.
Therefore, in the context of the COVID-19 pandemic, CPP is likely to be misdiagnosed as COVID-19. However, there were some differences in the clinical symptoms between the two groups. In our study, a higher proportion of patients with CPP exhibited fever, chills, sputum, and nervous system symptoms (46.9% vs. 9.6%, p < 0.001) than those with COVID-19. Moreover, multivariate logistic regression showed that nervous system symptoms (OR:4.483, 95% CI:1.119–17.959) were risk factors for CPP. Therefore, the presence of neurological symptoms in patients may be helpful in identifying CPP. Although neurological symptoms in patients with psittacosis have been reported for a long time, their pathogenesis remains unclear. Recently, studies have found that the sequence of C. psittaci can be detected by mNGS in the cerebrospinal fluid of patients with psittacosis24,25. Nervous system symptoms may be related to the direct invasion of C. psittaci into the central nervous system.
In terms of blood biochemical tests, both C. psittaci and COVID-19 pneumonia had high CRP, LDH, and D-dimer levels, and low levels of lymphocytes and albumin. However, our study found that patients with CPP had higher inflammatory indicators (WBC, neutrophil and CRP), liver enzymes (AST, ALT, and LDH), BUN, creatinine, creatine kinase, and D-dimer levels and lower lymphocyte, platelet, and albumin levels than patients with COVID-19. Previous studies have shown that both psittacosis and COVID-19 can cause liver and kidney damages8,26,27,28. In our study, patients with psittacosis showed higher indices of liver enzymes and renal function, which may indicate that patients with psittacosis have more severe liver and kidney damage than those with COVID-19. We found that elevated creatine kinase levels were also common in patients with CPP, particularly in those with severe pneumonia and death. Recent studies have shown that elevated creatine kinase is a high-risk factor for severe pneumonia and death in patients with psittacosis8, which is consistent with our findings. In addition, the clinical inflammatory index of patients with CPP was significantly higher than that of COVID-19 patients, indicating that patients with psittacosis may have a more serious inflammatory response. Previous studies have shown that C. psittaci replicates continuously in alveolar epithelial cells after inhalation of the respiratory tract and releases a large number of pro-inflammatory chemokines, which cause inflammatory cells such as macrophages and neutrophils to migrate from the blood to the lungs and activate them, triggering an inflammatory cascade29. Our study found that hypoalbuminemia and hypolymphocytosis are more likely to occur in C. psittaci pneumonia. This may be related to more severe systemic inflammation and liver injury; however, the exact mechanism remains unclear.
In terms of imaging, significant differences were observed between the two groups. In our study, patients with COVID-19 often showed ground-glass opacity in both lungs, whereas patients with CPP often had lung consolidation and pleural effusion. Previous studies have shown that peripheral ground-glass opacities in bilateral lungs are typical features of COVID-19 on CT scans11,14, while CPP is often characterized by consolidation and pleural effusion30, and this was consistent with our results. The histopathological characteristics of COVID-19 pneumonia include diffuse alveolar damage, vascular damage, and thrombosis, which contribute to ground-glass opacity lesions on chest CT31. However, there is still a lack of autopsy results to explain pulmonary imaging lesions in patients with psittacosis. In addition, the chest CT images of both patients were non-specific and overlapped with other bacterial, viral, and atypical pathogens. In the early stage of the disease, some patients diagnosed with COVID-19 exhibited normal chest CT findings, and no lesions were found32. Therefore, the value of chest CT in the differential diagnosis of these two conditions is limited.
The results of etiological detection are significant for differential diagnosis. Using RT-PCR to amplify viral RNA is the gold standard for the diagnosis of COVID-19, although it is time consuming (requiring 3–4 h to complete the test), and up to 20% to 67% of patients may have false-negative test results11,12,13. The factors that lead to false-negative results include the standardization of sample collection techniques11, time from exposure33, and sample type34. For example, the positive rate of RT-PCR test was highest in bronchoalveolar lavage fluid (BALF, 91.8%), followed by rectal swabs (87.8%), sputum (68.1%), nasopharyngeal swabs (45.5%), and oropharyngeal swabs (7.6%)34. Therefore, the etiological diagnosis of COVID-19 still presents many challenges. For C. psittaci, etiological laboratory detection methods include serology, etiological culture, and PCR. Culture of C. psittaci from sputum, BALF, and blood is the theoretical gold standard for the diagnosis of psittacosis. However, cultivation needs to be conducted in a biosafety III laboratory and can only be performed in a limited number of laboratories, which limits its clinical application1. Common commercial serological tests for the diagnosis of C. psittaci can easily produce false-negative or false-positive results1,7,35. Moreover, the sensitivity and specificity of nucleic acid detection based on PCR were higher than those of serological detection, although sensitivity was high in the acute phase and rapidly decreased with the progression of the disease35. Recently, many studies have shown that mNGS can rapidly obtain etiological results, adjust tetracycline-based antibacterial therapy, reduce the time needed to diagnose psittacosis, and shorten the course of psittacosis6,28. However, at present, there are still some shortcomings of mNGS, such as false positives, low detection rates of some pathogens, difficulty in interpreting the results, and elevated costs36. In particular, the high price greatly increases the economic burden on patients and limits its clinical application as a routine examination. Based on the limitations of the above tests, the detection of C. psittaci is not included in routine etiological examinations in most hospitals, which often leads to misdiagnosis and omission of diagnosis.
In the current study, the clinical data of 81 CPP cases and 450 COVID-19 cases were described, and the clinical features of the two groups were compared and analyzed. Multivariate logistic regression showed that nervous system symptoms, lymphocyte count, creatine kinase level, bilateral lung lesions, and ground-glass opacity were risk factors for patients with CPP. Based on these five risk factors, we developed a nomogram to differentiate CPP from COVID-19. The nomogram exhibited good accuracy in distinguishing CPP from COVID-19 with a good C-index of 0.989. The calibration curve overlapped with the ideal line, demonstrating that the predicted possibilities based on the nomogram were consistent with the actual observations. In terms of clinical utility, the decision curve analysis showed that the nomogram was feasible for clinical work. This was reflected by the threshold probability for the net benefit associated with application of the nomogram in differentiating CPP from COVID-19, which ranged from 0.01 to 0.98. There are many similarities between CPP and COVID-19 in terms of clinical symptoms and laboratory examinations. At present, there is still a lack of specific, simple, and reliable diagnostic methods. The nomogram that we proposed combines patient clinical symptoms as well as readily available laboratory and imaging results to reasonably predict CPP, and allows for rapid or even direct diagnosis possible.
Our study had some limitations. First, due to the limitations of commercial clinical diagnostic kits, all patients with CPP in our study were diagnosed using mNGS, which may underestimate the incidence of CPP. Second, as this was a retrospective study, we did not establish an external verification cohort to evaluate the predictive accuracy and clinical usefulness of our nomogram. However, our nomogram showed a satisfactory C-index, and was in good consistency with the actual observations reflected by the calibration curve. It also exhibited good clinical utility verified by the decision curve analysis. Third, although the nomogram can rapidly and accurately distinguish between CPP and COVID-19 pneumonia, the clinical benefits of the clinical management strategy based on our nomogram need to be confirmed by a large sample size and prospective studies. Finally, the data of the CPP cohort were generated over 5 years, while that of the COVID-19 cohort were generated within a 3-month period, which may have affected the results of our study.
In conclusion, although there are many similarities in the clinical symptoms and laboratory results of both types of pneumonias, there are some characteristic manifestations that may help in differentiating CPP from COVID-19 pneumonia. Patients with CPP are more likely to exhibit nervous system symptoms and higher creatine kinase levels. However, patients with COVID-19 pneumonia had a greater disposition to present with bilateral lung lesions, ground-glass opacity, and a higher lymphocyte percentage. Moreover, we established a nomogram for the differential diagnosis of CPP and COVID-19 pneumonia, which will help in avoiding misdiagnosis and allowing effective clinical decision making.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author (Pinhua Pan,Phone: + 86-0731-89753287, E-mail: [email protected]) on reasonable request.
References
Beeckman, D. S. & Vanrompay, D. C. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. 15(1), 11–17 (2009).
Hogerwerf, L. et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol. Infect. 145(15), 3096–3105 (2017).
Wang, L. et al. extracorporeal membrane oxygenation in severe acute respiratory distress syndrome caused by Chlamydia psittaci: A case report and review of the literature. Front. Med. (Lausanne). 8, 731047 (2021).
Zhang, Z. et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: An epidemiological and aetiological investigation. Lancet Microbe. 3(7), e512–e520 (2022).
Lei, J. H. et al. Clustering cases of Chlamydia psittaci pneumonia in coronavirus disease 2019 screening ward staff. Clin. Infect. Dis. 73(9), e3261–e3265 (2021).
Li, N. et al. Metagenomic next-generation sequencing in the family outbreak of psittacosis: The first reported family outbreak of psittacosis in China under COVID-19. Emerg. Microbes Infect. 10(1), 1418–1428 (2021).
Stewardson, A. J. & Grayson, M. L. Psittacosis. Infect. Dis. Clin. N. Am. 24(1), 7–25 (2010).
Yang, F. et al. Clinical symptoms and outcomes of severe pneumonia caused by Chlamydia psittaci in Southwest China. Front. Cell. Infect. Microbiol. 11, 727594 (2021).
Khan, W. H. et al. COVID-19 pandemic and vaccines update on challenges and resolutions. Front. Cell. Infect. Microbiol. 11, 690621 (2021).
Choi, J. Y. et al. Neutralizing activity against SARS-CoV-2 delta and omicron variants following a third BNT162b2 booster dose according to three homologous or heterologous COVID-19 vaccination schedules. Front. Cell. Infect. Microbiol. 12, 948014 (2022).
Wiersinga, W. J. et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 324(8), 782–793 (2020).
Ai, T. et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 296(2), E32–E40 (2020).
Pecoraro, V. et al. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur. J. Clin. Investig. 52(2), 13706 (2022).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382(18), 1708–1720 (2020).
Yin, Q. et al. Atypical pneumonia caused by Chlamydia psittaci during the COVID-19 pandemic. Int. J. Infect. Dis.122, 622–627 (2022).
Tang, J. et al. Application of metagenomic next-generation sequencing in the diagnosis of pneumonia caused by Chlamydia psittaci. Microbiol. Spectr. 10(4), e238421 (2022).
Chen, X. et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection 48(4), 535–542 (2020).
Mandell, L. A., Wunderink, R. G., & Anzueto, A., et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 44 Suppl 2(Suppl 2):S27–S72 (2007).
Wu, X. et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: A prospective multicenter study. Infect Dis. Ther. 9(4), 1003–1015 (2020).
Jiang, J. et al. Metagenomic next-generation sequencing for the diagnosis of pneumocystis jirovecii pneumonia in non-HIV-infected patients: A retrospective study. Infect. Dis. Ther. 10(3), 1733–1745 (2021).
Ranieri, V. M. et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 307(23), 2526–2533 (2012).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8), 801–810 (2016).
Zhao, W. et al. Clustering cases of Chlamydia psittaci pneumonia mimicking COVID-19 pneumonia. World J. Clin. Cases 9(36), 11237–11247 (2021).
Shi, Y. et al. A case of chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: A case report and literature review. BMC Infect. Dis. 21(1), 621 (2021).
Davar, K. et al. A rare bird: Diagnosis of Psittacosis meningitis by clinical metagenomic next-generation sequencing. Open Forum Infect. Dis. 8(12), b555 (2021).
Tian, D. & Ye, Q. Hepatic complications of COVID-19 and its treatment. J. Med. Virol. 92(10), 1818–1824 (2020).
Han, X. & Ye, Q. Kidney involvement in COVID-19 and its treatments. J. Med. Virol. 93(3), 1387–1395 (2021).
Tang, J., Tan, W., & Luo, L., et al. Application of metagenomic next-generation sequencing in the diagnosis of pneumonia caused by Chlamydia psittaci. Microbiol. Spectr. 238421 (2022).
Knittler, M. R. et al. Chlamydia psittaci: New insights into genomic diversity, clinical pathology, host-pathogen interaction and anti-bacterial immunity. Int. J. Med. Microbiol. 304(7), 877–893 (2014).
Kong, C. Y. et al. Clinical characteristics of Chlamydia psittaci pneumonia. Chin. Med. J. (Engl). 134(3), 353–355 (2021).
Kianzad, A. et al. COVID-19: Histopathological correlates of imaging patterns on chest computed tomography. Respirology. 26(9), 869–877 (2021).
Shi, H. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet. Infect. Dis. 20(4), 425–434 (2020).
Kucirka, L. M. et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med. 173(4), 262–267 (2020).
Bwire, G. M. et al. Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis. J. Med. Virol. 93(2), 719–725 (2021).
Nieuwenhuizen, A. A. et al. Laboratory methods for case finding in human psittacosis outbreaks: A systematic review. BMC Infect. Dis. 18(1), 442 (2018).
Zheng, Y. et al. The diagnostic value of metagenomic next-generation sequencing in lower respiratory tract infection. Front. Cell. Infect. Microbiol. 11, 694756 (2021).
Acknowledgements
We thank all the patients and their families involved in the study. We thank all the medical staff who work in ten tertiary general hospitals(Xiangtan Central Hospital, First People’s Hospital of Huaihua, Yueyang Central Hospital, First People’s Hospital of Chenzhou, Hunan Provincial People’s Hospital, First Hospital of Changsha, Yiyang Central Hospital, Changsha Central Hospital, Second Affiliated Hospital, Hengyang Medical School, University of South China).
Funding
This study was supported by Key R&D Program of Hunan Province (No. 2022SK2038),The National Key Clinical Specialist Construction Programs of China (No.z047-02), Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant No. 2020LNJJ05), National Natural Science Foundation of China (No.82000089, No.82100100, No.82200099), Science and Technology Projects in Guangzhou(No.202201010012),Hunan Natural Science Youth Foundation(No.2022JJ40810,No.2022JJ40775)and Scientific research project of Hunan Health Commission(No.202103020612).
Author information
Authors and Affiliations
Contributions
Rongli Lu, Fengyu Lin, Yi Li and Pinhua Pan conceived and designed the research. Yi Li and drafted Fengyu Lin the manuscript. Jiefeng Luo was responsible for revising the article. Jiefeng Luo reviewed the article data. Duoduo Han, Haitao Li, Wen Li, Gang Chen, and Chao Song collected the data. Li Sha and Liu Ben analyzed the imaging data. Yi Li and Fengyu Lin analyzed the data. Yi Li and Fengyu Lin prepared the figures and tables. Yi Li, Jiefeng Luo, Rongli Lu, Pinhua Pan, Yanhui Cui and Yanjun Zeng edited and revised the manuscript. Yi Li and Pinhua Pan approved the final version of manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was approved by the Ethics Committee of Xiangya Hospital, Central South University (No. 202104005) and Union Hospital of Huazhong University of Science and Technology (No. 202003049). Due to the nature of the retrospective study and the anonymous processing of data prior to analysis, the Ethics Committee of Xiangya Hospital, Central South University and the Ethics Committee of Union Hospital of Huazhong University of Science and Technology approved the waiver of informed consent. The study we carried out strictly complies with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, R., Luo, J., Lin, F. et al. Comparison of clinical, laboratory and radiological characteristics between COVID-19 and Chlamydia psittaci pneumonia: a multicenter retrospective study. Sci Rep 14, 23790 (2024). https://doi.org/10.1038/s41598-024-74708-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74708-7