Abstract
This study aimed to analyze the complications and long-term survival outcomes in patients who underwent radical gastrectomy for gastric cancer, as well as to identify the risk factors associated with postoperative complications. After conducting a comprehensive search within the medical records system, a total of 2508 patients who underwent radical gastrectomy and met the inclusion criteria were enrolled. Of the 2508 patients, 301 were diagnosed with postoperative complications. The pathological data, postoperative recovery, and survival outcome were compared between complication and control group. Subsequently, univariate and multivariate logistic regression analyses were conducted to identified the risk factors. According to the Clavien-Dindo grading criteria for postoperative complications, the proportions of grade I, II, III, IV, and V complications following radical gastrectomy were observed to be 28.2%, 42.9%, 19.6%, 8.0%, and 1.3%, respectively. The presence of postoperative complications significantly prolonged the duration of gastrointestinal decompression (P < 0.001), catheter retention (P < 0.001), fasting (P < 0.001), and hospitalization (P < 0.001). Additionally, it had a detrimental impact on survival outcomes. Age > 65years [odds ratio (OR) = 1.378, P = 0.020], presence of diabetes (OR = 2.042, P < 0.001), operative duration > 215 min (OR = 1.450, P = 0.006), intraoperative blood loss > 275 ml (OR = 1.474, P = 0.004), and Roux-en-Y anastomosis for both whole stomach (OR = 1.567, P = 0.021) and distal gastric cancer (OR = 2.083, P = 0.003) were identified as independent risk factors for postoperative complications. This study analyzed the complications and survival outcomes following radical gastrectomy, and investigated the predictors for postoperative complications, thereby providing valuable guidance on the prevention and management of surgical complications in gastric cancer.
Similar content being viewed by others
Introduction
The prevalence of gastric cancer worldwide makes it a significant menace to human life and well-being. Although the therapeutic strategy for gastric cancer has undergone significant transformations in recent decades, surgery remains the foremost therapeutic modality for this malignancy1,2,3. The occurrence of complications following radical gastrectomy for gastric cancer remains a significant determinant that profoundly impacts surgical outcomes, postoperative rehabilitation, length of hospital stay, and even perioperative mortality. Consequently, the identification of potential risk factors for postoperative complications in patients with gastric cancer, followed by appropriate early intervention based on the related risk factors and evaluation results, as well as the development of rational and effective treatment plans, can significantly enhance the quality of medical care and ensure patients’ safety4,5.
The spectrum of surgical complications encompasses not only local complications arising from the operative procedure itself, but also systemic complications stemming from surgical invasion, underlying diseases, and physiological conditions4,5,6. Clinically, local complications following gastric cancer surgery primarily encompass anastomotic leakage, stenosis, gastrointestinal dysfunction, hemorrhage, pancreatic leakage, and intraperitoneal infection. Meanwhile, systemic complications mainly involve pneumonia, wound infection, fever, liver and kidney insufficiency, and thrombosis. Although numerous studies have been published on postoperative complications, the majority of them pertain to general abdominal and emergency surgeries, there is a dearth of specific analysis focused on patients with gastric cancer7,8,9. The reported incidence of postoperative complications for gastric cancer exhibited significant variability, and the risk factor analysis for complications based on a substantial sample of case data is also imperative10,11,12.
The Clavien-Dindo grading standard was initially developed by Clavien, Dindo et al. in 2004, and subsequently an enhanced version of the Clavien-Dindo grading standard for surgical complications was introduced in 2009. The aforementioned standard offers a dependable and valid framework for the delineation and categorization of postoperative complications. It is also widely recognized as one of the most reputable international criteria for assessing postoperative complications, including those related to gastric cancer surgery13,14. In this study, we conducted a retrospective analysis of gastric cancer cases from one of the nation’s largest medical centers. Subsequently, we utilized the Clavien-Dindo grading system (2009 version) to assess postoperative complications and investigated risk factors associated with such complications following radical gastrectomy.
Materials and methods
Study participants
The patients who underwent gastrectomy for gastric cancer at our medical center between January 2011 and December 2015 were selected as study population. Patients were additionally assessed for eligibility based on predetermined inclusion and exclusion criteria. The inclusion criteria were as follows: (1) patients underwent a radical resection of the proximal, distal, or total stomach, whatever open, laparoscopic, or robot-assisted surgery; (2) no evidence of tumors invading the adjacent organs or distant metastasis; (3) the required data of outcome indicators were fully comprehensive. The exclusion criteria included (1) patients with gastroesophageal junction cancer who underwent thoracotomy; (2) gastric stump surgery, recurrent cancer surgery, or combined with other organ surgery; (3) special gastric tumors, including lymphoma, liomyoma, neuroendocrine, or stromal tumor, etc. The study was reviewed and approved by the Medical Ethics Committee and was performed in accordance with the Declaration of Helsinki.
Surgical procedures
Patients with gastric cancer included in this study underwent radical proximal, distal, or total gastrectomy (D2 lymph node dissection) based on tumor ___location, size, and stage. The achievement of R0 resection was confirmed by intraoperative gross observation and postoperative pathology, demonstrating the absence of any residual tumor. The surgical procedures were performed by a group of highly experienced chief or deputy chief physicians, each with over 100 surgeries under their belt, in the general surgery department of our center. Additionally, the distribution of surgical approaches was as follows: open accounted for 59.8%, laparoscopic accounted for 33.0%, and robotic accounted for 7.2%.
Data collection and outcome evaluation
The case data to be observed and collected in this study encompassed three components: pathological data, postoperative recovery and survival outcomes, as well as risk factors associated with postoperative complications. The pathological data included tumor ___location, number of tumors, histological types, TNM stage, and presence of ulceration, neuroendocrine differentiation (NED), lymphovascular invasion (LVI), and nerve invasion. The variables related to postoperative recovery and survival encompassed the utilization and quantity of jejunal nutrition tube and peritoneal drainage, the duration of gastrointestinal decompression, indentation catheterization, fasting, and hospitalization, as well as survival outcomes at 1-, 3-, and 5-year. Moreover, the risk factors collected and evaluated, which may be associated with postoperative complications, encompassed sex, age, body mass index (BMI), presence of hypertension, diabetes, cardia-cerebrovascular disease, anaemia, hypoalbuminemia, neoadjuvant chemotherapy, pyloric obstruction, gastrointestinal hemorrhage, or abdominal operation history, preoperative American society of Aneshesiologists (ASA) score, operation start time, digestive tract reconstruction method, operative approach, operative duration, intraoperative blood loss, postoperative intensive care unit (ICU) transition, tumor size, and tumor stage.
The postoperative complications were assessed according to the Clavien-Dindo classification system for surgical complications (2009 version)14. For patients with concurrent multiple complications, the grading was based on the severity of the most critical complication. The term “postoperative ICU transition” referred to the direct transfer of patients to the ICU immediately after surgery, as opposed to transferring them for ICU treatment due to postoperative complications. The tumor stages were assessed in this study using the 8th edition TNM staging criteria designed jointly by the Union Internationale Against cancer (UICC) and the American joint Committee on cancer (AJCC), based on the pathological findings from the patient’s surgical specimens15. The tumor size for a single lesion refers to the longest diameter of the tumor, while for multiple lesions, it is calculated as the sum of the longest diameters of each individual lesion. The serological examination was conducted for all admitted cases. For serum hemoglobin (HGB) examination, male ≤ 120 g/L or female ≤ 110 g/L was defined as preoperative anemia. Serum albumin (ALB) < 35 g/L was defined as preoperative hypoalbuminemia.
Statistical analysis
All the statistical analyses in this study were performed using SPSS software (version 26.0). Q-Q plots were utilized to assess normal distribution for continuous variables, and mean with the standard deviation (SD) or median with the interquartile range (IQR) was calculated to present the data. Subsequently, intergroup comparison was conducted using independent-samples T test or Mann-Whitney U test. Receiver operating characteristic (ROC) curves were plotted for operative duration, intraoperative blood loss, and tumor size, and the thresholds corresponding to the maximum sum of sensitivity and specificity were determined as the cut-off values. The continuous variables were subsequently converted into binary variables for further analysis based on the specified cut-off values. Univariate analyses were conducted to examine the correlations between covariates and dependent variables, and the covariates with P < 0.1 would be included in a multivariate logistic regression model to identify the independent predictors. All tests were considered statistically significant if P values < 0.05.
Results
Patient selection and complication rate
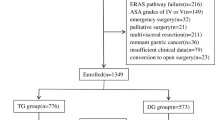
Through the medical records system, a total of 2973 patients who underwent radical gastrectomy between January 2011 and December 2015 were retrieved. Of these, 287 patients with gastroesophageal junction cancer undergoing thoracotomy, 106 underwent gastric stump surgery, recurrent cancer surgery, or combined with other organ surgery, 56 underwent palliative surgery, and 16 without cancerous pathological reports. After rigorous screening, 2508 patients with comprehensive outcome indicators were selected as the subjects for this study. The detailed process of patient recruitment and screening was illustrated in Fig. 1. Of the 2508 patients, 301 (12.0%) were diagnosed with postoperative complications. The incidence of complications in patients undergoing surgery from 2011 to 2015 was as follows: 13.1% (65/496), 11.1% (52/470), 12.1% (52/430), 13.6% (69/506), and 10.4% (63/606) respectively. The results of comparative analysis revealed no statistically significant variation in the occurrence of complications across different years (χ2 = 3.726, P = 0.444). According to the Clavien-Dindo grading criteria for postoperative complications, the proportions of grade I, II, III, IV, and V complications following radical gastrectomy were observed to be 28.2%, 42.9%, 19.6%, 8.0%, and 1.3%, respectively. The comprehensive details regarding the gastrointestinal complications was provided in Table 1.
Tumor characteristics between complication and control groups
The enrolled patients were categorized into complication group (n = 301) and control group (n = 2207) based on the incidence of postoperative complications. Firstly, the pathological data of patients in the complication and control group were compared, and the analysis results indicated no statistically significant differences between the two groups in terms of tumor ___location, number, histological types, T and N stage, presence of ulceration, NED, LVI, or nerve invasion (P > 0.05) (Table 2).
Postoperative complications and survival outcomes
Subsequently, the postoperative recovery and survival outcomes of the complication and control group were compared. The comparative results demonstrated that the presence of postoperative complications not only increased the likelihood and quantity of jejunal nutrition tube (P = 0.001) and abdominal drainage usage (P < 0.001), prolonged the duration of gastrointestinal decompression (P < 0.001), catheterization (P < 0.001), fasting (P < 0.001), and hospital stay (P < 0.001), but also decreased the 1- (P < 0.001), 3- (P = 0.001), and 5-year (P = 0.002)survival rates of patients (Table 3). During the follow-up period, a total of 121 patients in the complication group and 655 patients in the control group succumbed to mortality. The Kaplan-Meier curve illustrating the overall survival (OS) revealed a statistically significant difference between the complication and control group (P < 0.001). The mean OS for the complication group was 67.3 months, while it was 79.4 months for the control group (Fig. 2).
Logistic regression analysis of risk factors for postoperative complications
Univariate and multivariate logistic regression analyses were conducted between 301 patients with postoperative complications and 2207 control patients. Based on the area under the curve (AUC) of ROC curves, 275 ml, 215 min and 5 cm for blood loss, operative duration and tumor size were determined with higher sensitivity and specificity and they were converted to binary variables (0 for below and 1 for above the value suggested by the ROC curves). The univariate analyses showed that the occurrence of postoperative complications was significantly correlated with the age (P = 0.001), presence of diabetes (P < 0.001), pyloric obstruction (P = 0.016), digestive tract reconstruction method (P < 0.001), operative duration > 215 min (P < 0.001), intraoperative blood loss > 275 ml (P < 0.001), postoperative ICU transition (P = 0.008), and tumor size > 5 cm (P < 0.001) (Table 4).
The variables that exhibited statistically significant differences in the univariate analysis, along with gender and cardia-cerebrovascular disease, were subsequently included in the multivariate analysis. Age > 65years [odds ratio (OR) = 1.378, P = 0.020], presence of diabetes (OR = 2.042, P < 0.001), operative duration > 215 min (OR = 1.450, P = 0.006), intraoperative blood loss > 275 ml (OR = 1.474, P = 0.004), and Roux-en-Y anastomosis for both whole stomach (OR = 1.567, P = 0.021) and distal gastric cancer (OR = 2.083, P = 0.003) were identified as independent risk factors for postoperative complications (Table 5). Additionally, the study population was further stratified into different subgroups based on the year of surgery, and univariate logistic regression analysis was employed to investigate the association between independent risk factors and complications within each subgroup (Fig. 3). The subgroup analysis results indicated that there was no statistically significant difference in the impact of risk factors on complications across different years, thereby suggesting the validity and reliability of the regression analysis findings.
Discussion
The present study demonstrated that age > 65years, presence of diabetes, operative duration > 215 min, intraoperative blood loss > 275 ml, and Roux-en-Y anastomosis were independently associated with postoperative complications. The accuracy, techniques, and equipment of gastric cancer surgery have experienced a remarkable advancement in recent years, leading to a significant reduction in the incidence of postoperative complications2,16. The occurrence of postoperative complications, however, remains a significant contributor to perioperative mortality and has a profound impact on both surgical outcomes and patients’ quality of life. Chen’s research revealed that metabolic syndrome (MetS), Billroth II anastomosis, age, and Charlson score independently contribute to the risk of complications following gastrectomy8. Lou et al. discovered that sarcopenia serves as an autonomous predictor of postoperative complications in overweight or obese patients diagnosed with gastric cancer17. The limited number of participants included in the published studies and the lack of long-term follow-up, however, contributed to the findings’ limited strength18.
The findings of Yasuda’s study indicated that operation time and intraoperative bleeding were independent risk factors associated with postoperative complications following radical gastrectomy, which was consistent with our own results19. Intraoperative massive bleeding may result in gastrointestinal ischemia surrounding the anastomosis and duodenal stump, thereby leading to delayed wound healing and even gastrointestinal necrosis. Additionally, postoperative anemia, hypoproteinemia, and malnutrition associated with blood loss can contribute to tissue edema and infection, which hinders the growth of granulation tissue20,21.
The release of tumor necrosis factor in substantial amounts can occur during the progression of tumors, thereby amplifying the local inflammatory response. Interestingly, our findings revealed that tumor size > 5 cm was significantly associated with an elevated rate of complications in the univariate analysis, while no significant association was observed between tumor stage and complications. The above results suggest that postoperative complications are primarily influenced by tumor volume, while showing no significant correlation with depth of tumor invasion and lymph node metastasis. Additionally, the lack of impact on complication from N status-based tumor staging may be attributed to the extensive experience in D2 lymph node dissection among surgeons at our center. Large tumors are frequently accompanied by more severe inflammation, necessitating extensive surgery, increased surgical complexity, and posing an elevated risk of postoperative complications. Moreover, the performance of complex surgical procedures often leads to extensive tissue damage, prolonged operative duration, and increased intraoperative blood loss22,23. The extensive tissue damage and prolonged exposure time increase the likelihood of postoperative local infection, edema in the gastrointestinal tube, and damage to gastrointestinal blood supply vessels, thereby elevating the risk of complications such as anastomotic leakage, dysfunction, bleeding, abdominal effusion and infection20,24.
The method of digestive tract reconstruction was found to be another important factor affecting the incidence of postoperative complications. Compared to direct esophagogastric anastomosis in proximal gastric surgery, Roux-en-Y anastomosis in distal and total gastrectomy entails greater surgical trauma, a broader operative scope, and more extensive digestive anastomoses. Additionally, the Roux-en-Y anastomosis procedure involves the incision of the jejunum, resulting in significant structural and functional alterations to the digestive tract. Complex procedures often result in increased indentation of the abdominal drainage catheter and heightened postoperative discomfort and pain for patients, which hinders early postoperative mobility and elevates the risk of complications such as pulmonary infection, abdominal effusion, lower limb venous thrombosis, and gastrointestinal dysfunction24,25.
The proportion of gastric cancer patients who are elderly or diabetic has gradually increased due to population aging, changes in lifestyle, and dietary structure. The physiological characteristics of the elderly, such as cellular senescence, reduction in cell count, muscle atrophy and loss, result in compromised compensatory function of the body’s organs, inadequate stress response, and self-regulation ability. The presence of sarcopenia and malnutrition have been reported as significant risk factors for postoperative complications in elderly patients with gastric cancer26,27,28. The elderly patients are commonly afflicted with cardiovascular and cerebrovascular diseases, hypertension, diabetes, and other comorbidities. Consequently, their tolerance to anesthesia and surgery is decreased, leading to a higher frequency of postoperative ICU transfers. Moreover, they face an elevated risk of thrombosis, cardio-cerebral ischemic disease, and organ dysfunction29,30.
The diagnostic criteria for hypoalbuminemia was set at < 30 g/L and the results were re-analyzed, but no significant association with complications was observed. Regardless of the ASA score, all patients in this study underwent radical surgery. In addition, to mitigate the potential impact of ASA score on digestive tract reconstruction, we conducted a correlation analysis between the aforementioned variables. The ASA I-II and ASA III-IV groups did not exhibit a statistically significant difference in terms of the patients’ receipt of billroth or roux-en-y reconstructions. The present study, in summary, failed to establish a significant correlation between preoperative hypoalbuminemia or ASA scores with surgical complications. The limited occurrence of hypoalbuminemia and ASA score III-IV has somewhat impacted the precision and persuasiveness of the analysis findings, necessitating further investigation in subsequent studies.
The influence of diabetes on postoperative complications is multifaceted and intricate. Firstly, diabetic patients experience prolonged immune stress due to systemic inflammatory response, leading to a reduction in the body’s resistance and weakened phagocytic ability of cells, resulting in delayed regeneration of anastomotic granulation tissue. Furthermore, diabetic patients often present with microvascular lesions, resulting in heightened wound exudation, susceptibility to infection, and impaired healing. Thirdly, the blood glucose of diabetic patients fluctuates significantly due to surgical stimulation, postoperative fasting, and parenteral nutrition support, which is also detrimental to the recovery from trauma30,31,32.
The present study also revealed that the occurrence of postoperative complications significantly prolonged the recovery process, resulting in increased treatment duration and medical expenses. After excluding the influence of malignant degree of tumor, the occurrence of postoperative complications significantly decreased both the survival duration and quality of patients. Consequently, the early identification, prevention, and treatment of patients with associated risk factors hold immense significance. The elderly patients with diabetes, malnutrition, and other underlying conditions should actively enhance their physical fitness during the perioperative period. Additionally, the endocrinology department should be consulted to adjust medication and closely monitor perioperative blood glucose changes for diabetic patients with inadequate blood glucose control, while appropriately increasing the dosage of parenteral nutrition insulin4,33,34.The operation should be performed in the setting of well-controlled underlying disease and optimal nutritional status, in accordance with the principles and protocols of Enhanced Recovery After Surgery (ERAS)35. Furthermore, the operation should be performed with utmost care to minimize potential damage to blood vessels and nerves, control bleeding effectively, and optimize the duration of the procedure. After the surgical procedure, it is imperative to minimize the retention of the peritoneal drainage tube, prioritize anti-infection treatment, facilitate tissue healing, and mitigate the risk of complications. It is also crucial to closely monitor the patient’s physical condition, abdominal drainage, and test results in order to promptly identify potential complications such as abdominal infection, bleeding, or leakage. Depending on the severity of these complications, appropriate measures including anti-infection treatment, abdominal irrigation, endoscopy, intervention procedures or surgical interventions should be taken5,16,36. For patients without complications, the abdominal drainage, gastric and nutrition tube should be promptly removed in order to facilitate early eating and exercise, thereby promoting recovery and reducing hospitalization duration37.
Although this study analyzed the complications and survival outcomes following radical gastrectomy based on a sample of 2508 cases from one of the largest medical centers in China, and identified associated risk factors, it still possesses certain limitations. Firstly, the level of evidence was limited by administrative and technical disparities between retrospective cohort studies and cases originating from a single center. Secondly, the postoperative complications were influenced to a certain extent by the variations in surgical skills and practices among different surgeons, despite their extensive experience as chief physicians in this study. Thirdly, the correlation between postoperative complications and survival outcomes could be influenced by factors such as age and diabetes, both of which could impact the occurrence of postoperative complications and overall survival. Subsequently, multi-center, large-scale, and prospective studies focused on specific gastric cancer populations are needed to validate and augment the findings of this study.
Conclusions
The occurrence of postoperative complications significantly prolonged the recovery process, resulting in increased treatment duration and medical expenses. After excluding the influence of malignant degree of tumor, the occurrence of postoperative complications had a detrimental impact on patients’ survival outcomes. Age > 65years, presence of diabetes, operative duration > 215 min, intraoperative blood loss > 275 ml, and Roux-en-Y anastomosis were identified as independent risk factors for postoperative complications. The present study conducted an analysis of complications and survival outcomes following radical gastrectomy, while also exploring the risk factors associated with these complications. This research provides valuable insights for the prevention and management of surgical complications in gastric cancer. The findings of this study enable surgeons to identify relevant risk groups and implement ERAS protocols for perioperative intervention, thereby mitigating the risk of complications following radical gastrectomy and enhancing survival prognosis.
the corresponding author on reasonable request.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ajani, J. A. et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN. 20(2), 167–192. https://doi.org/10.6004/jnccn.2022.0008 (2022).
Sugawara, K., Kawaguchi, Y., Seto, Y. & Vauthey, J. N. Multidisciplinary treatment strategy for locally advanced gastric cancer: A systematic review. Surg. Oncol. 38, 101599. https://doi.org/10.1016/j.suronc.2021.101599 (2021).
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet (Lond. Engl.). 396(10251), 635–648. https://doi.org/10.1016/S0140-6736(20)31288-5 (2020).
Kanda, M. Preoperative predictors of postoperative complications after gastric cancer resection. Surg. Today. 50(1), 3–11. https://doi.org/10.1007/s00595-019-01877-8 (2020).
Kubota, T. et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann. Surg. Oncol. 21(3), 891–898. https://doi.org/10.1245/s10434-013-3384-9 (2014).
Kanda, M. et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med. 8(11), 5194–5201. https://doi.org/10.1002/cam4.2439 (2019).
Chen, X. et al. Metabolic syndrome predicts postoperative complications after gastrectomy in gastric cancer patients: Development of an individualized usable nomogram and rating model. Cancer Med. 9(19), 7116–7124. https://doi.org/10.1002/cam4.3352 (2020).
Gurunathan, U. et al. Association of obesity with septic complications after major abdominal surgery: A secondary analysis of the RELIEF Randomized Clinical Trial. JAMA Netw. open. 2(11), e1916345. https://doi.org/10.1001/jamanetworkopen.2019.16345 (2019).
Lasithiotakis, K. et al. The Hellenic Emergency Laparotomy Study (HELAS): A prospective Multicentre Study on the Outcomes of Emergency Laparotomy in Greece. World J. Surg. 47(1), 130–139. https://doi.org/10.1007/s00268-022-06723-6 (2023).
Orsenigo, E. et al. Duodenal stump fistula after gastric surgery for malignancies: A retrospective analysis of risk factors in a single centre experience. Gastric cancer. 17(4), 733–744. https://doi.org/10.1007/s10120-013-0327-x (2014).
Shi, J. et al. Early diagnosis of anastomotic leakage after gastric cancer surgery via analysis of inflammatory factors in abdominal drainage. Ann. Surg. Oncol. 29(2), 1230–1241. https://doi.org/10.1245/s10434-021-10763-y (2022).
Tu, R. et al. Development of a nomogram for predicting the risk of anastomotic leakage after a gastrectomy for gastric cancer. Eur. J. Surg. Oncol. 43(2), 485–492. https://doi.org/10.1016/j.ejso.2016.11.022 (2017).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240(2), 205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae (2004).
Clavien, P. A. et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 250(2), 187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2 (2009).
He, X. et al. Validation of the American Joint Committee on Cancer (AJCC) 8th edition stage system for gastric cancer patients: A population-based analysis. Gastric cancer. 21(3), 391–400. https://doi.org/10.1007/s10120-017-0770-1 (2018).
Dai, J., Jiang, Y. & Fu, D. Reducing postoperative complications and improving clinical outcome: Enhanced recovery after surgery in pancreaticoduodenectomy - A retrospective cohort study. Int. J. Surg. (Lond. Engl.). 39, 176–181. https://doi.org/10.1016/j.ijsu.2017.01.089 (2017).
Lou, N. et al. Sarcopenia in overweight and obese patients is a predictive factor for postoperative complication in gastric cancer: A prospective study. Eur. J. Surg. Oncol. 43(1), 188–195. https://doi.org/10.1016/j.ejso.2016.09.006 (2017).
Bian, L. et al. Associations of radiological features of adipose tissues with postoperative complications and overall survival of gastric cancer patients. Eur. Radiol. 32(12), 8569–8578. https://doi.org/10.1007/s00330-022-08918-w (2022).
Yasuda, T. et al. Ten cases of gastro-tracheobronchial fistula: A serious complication after esophagectomy and reconstruction using posterior mediastinal gastric tube. Dis. Esophagus. 25(8), 687–693. https://doi.org/10.1111/j.1442-2050.2011.01309.x (2012).
Koyanagi, K. et al. Association between indocyanine green fluorescence blood flow speed in the gastric conduit wall and superior mesenteric artery calcification: Predictive significance for anastomotic leakage after esophagectomy. Esophagus. 18(2), 248–257. https://doi.org/10.1007/s10388-020-00797-8 (2021).
Yu, Z. et al. Gastrointestinal fistula in Radical Distal Gastrectomy: Case-control study from a high-volume hospital. J. Laparoendosc. Adv. Surg. Tech. Part A. 33(12), 1154–1161. https://doi.org/10.1089/lap.2023.0259 (2023).
Kim, S. G., Eom, B. W., Yoon, H., Kim, Y. W. & Ryu, K. W. Prognostic value of preoperative systemic inflammatory parameters in Advanced Gastric Cancer. J. Clin. Med. 11(18), 5318. https://doi.org/10.3390/jcm11185318 (2022).
Koerner, A. S., Moy, R. H., Ryeom, S. W. & Yoon, S. S. The present and future of neoadjuvant and adjuvant therapy for locally advanced gastric cancer. Cancers. 15(16), 4114. https://doi.org/10.3390/cancers15164114 (2023).
Ellis, R. J. et al. Risk factors for post-pancreaticoduodenectomy delayed gastric emptying in the absence of pancreatic fistula or intra-abdominal infection. J. Surg. Oncol. 119(7), 925–931. https://doi.org/10.1002/jso.25398 (2019).
Yu, Z. et al. Risk factor analysis of Gastroparesis Syndrome in 2652 patients with radical distal Gastrectomy. J. Gastrointest. Surg. 27(8), 1568–1577. https://doi.org/10.1007/s11605-022-05538-z (2023).
Zhou, C. J. et al. Sarcopenia: A new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J. Surg. Res. 211, 137–146. https://doi.org/10.1016/j.jss.2016.12.014 (2017).
Huang, D. D. et al. Measurement of muscle quantity/quality has additional predictive value for postoperative complications and long-term survival after gastrectomy for gastric cancer in patients with probable Sarcopenia as defined by the new EWGSOP2 consensus: analysis from a large-scale prospective study. Nutrition. 86, 111156. https://doi.org/10.1016/j.nut.2021.111156 (2021).
Lin, J. X. et al. Which nutritional scoring system is more suitable for evaluating the short- or long-term prognosis of patients with gastric Cancer who underwent Radical Gastrectomy? J. Gastrointest. Surg. 24(9), 1969–1977. https://doi.org/10.1007/s11605-019-04360-4 (2020).
Miao, X. et al. Exploration of frailty trajectories and their associations with health outcomes in older gastric cancer survivors undergoing radical gastrectomy: A prospective longitudinal observation study. Eur. J. Surg. Oncol. 50(2), 107934. https://doi.org/10.1016/j.ejso.2023.107934 (2024).
Chen, M. et al. Risk factors for surgical site infection in patients with gastric cancer: A meta-analysis. Int. Wound J. 20(9), 3884–3897. https://doi.org/10.1111/iwj.14264 (2023).
van Kooten, R. T. et al. Patient-related prognostic factors for anastomotic leakage, Major complications, and short-term mortality following esophagectomy for Cancer: A systematic review and Meta-analyses. Ann. Surg. Oncol. 29(2), 1358–1373. https://doi.org/10.1245/s10434-021-10734-3 (2022).
Zhao, T., Li, L., Wang, Y., Xie, W. & Liu, Q. Prognostic nutritional index combined with carcinoembryonic antigen and carbohydrate antigen 242 for early prediction of anastomotic leakage after radical gastrectomy for gastric cancer. Am. J. Transl. Res. 15(7), 4668–4677 (2023).
Takeuchi, D. et al. Postoperative complications in elderly patients with gastric cancer. J. Surg. Res. 198(2), 317–326. https://doi.org/10.1016/j.jss.2015.03.095 (2015).
Fiorillo, C. et al. Postoperative hyperglycemia in nondiabetic patients after gastric surgery for cancer: Perioperative outcomes. Gastric cancer. 20(3), 536–542. https://doi.org/10.1007/s10120-016-0621-5 (2017).
Wee, I. J. Y., Syn, N. L., Shabbir, A., Kim, G. & So, J. B. Y. enhanced recovery versus conventional care in gastric cancer surgery: A meta-analysis of randomized and non-randomized controlled trials. Gastric Cancer. 22(3), 423–434. https://doi.org/10.1007/s10120-019-00937-9 (2019).
D’Souza, J., McCombie, A. & Roberts, R. The influence of short-term postoperative outcomes on overall survival after gastric cancer surgery. ANZ J. Surg. 93(12), 2875–2884. https://doi.org/10.1111/ans.18613 (2023).
Wong-Lun-Hing, E. M. et al. Abandoning prophylactic abdominal drainage after hepatic surgery: 10 years of No-Drain policy in an enhanced recovery after surgery environment. Dig. Surg. 34(5), 411–420. https://doi.org/10.1159/000455246 (2017).
Acknowledgements
We thank all the patients whose data were used for the study. Our greatest acknowledgement goes to the authors who made detailed data available for this study and to all our colleagues in this study for their hard work.
Funding
None.
Author information
Authors and Affiliations
Contributions
X.Z., S.Z., P.L. and W.L. designed study and edited the original draft. Z.Y., C.L. and Q.X. participated in writing the manuscript. Z.Y., R.L., J.G., Y.G. and Q.X. collected and analyzed the case data. C.L. and Z.Y. produced the tables and figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the First Medical Center of the Chinese PLA General Hospital (Approval No. S2021-022-01) and was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants during the follow-up period.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, Z., Liang, C., Xu, Q. et al. Analysis of postoperative complications and long term survival following radical gastrectomy for patients with gastric cancer. Sci Rep 14, 23869 (2024). https://doi.org/10.1038/s41598-024-74758-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74758-x