Abstract
The purpose of this study was to explore the correlation between Hashimoto’s thyroiditis and Metabolic dysfunction-associated fatty liver disease (MAFLD), and at the same time to screen high-risk groups for liver fibrosis in MAFLD, find out the high-risk related indicators. The physical examination population was included as the study subjects and was grouped according to the diagnostic criteria for MAFLD. APRI > 1 or NFS > 0.676 or FIB-4 > 2.67were used to assess people at high risk of liver fibrosis, and logistic regression analysis was used to identify risk factors associated with high risk of liver fibrosis in MAFLD. ROC curves are used to look for indicators of diagnostic value. The proportion of people with Hashimoto’s thyroiditis was lower in the MAFLD group. The MAFLD high-risk group for liver fibrosis had higher TSH levels, lower FT3 and FT4 levels, higher TGAB levels, and differences in biochemical markers. Age, BMI, FBG, and AST are risk factors for the high risk of liver fibrosis in MAFLD patients. ROC curve analysis showed that the AUC of age was 0.741 (0.721–0.761), and the optimal stage value was 57.5 years, while the AUC of AST was 0.729 (0.707–0.751), and the optimal cut-off value was 39.5 U/L. Age, BMI, FBG, and AST are risk factors for the high risk of liver fibrosis in MAFLD patients.The age is greater than or equal to 57.5 years, or the AST is greater than or equal to 39.5 U/L, indicating that the MAFLD patients are at high risk of liver fibrosis.
Similar content being viewed by others
Introduction
Metabolic dysfunction-related fatty liver disease (MAFLD) is particularly common in clinical practice, and is associated with metabolic diseases, although it is not serious in itself, but its complications include liver cirrhosis and liver cancer, which seriously affect the quality of life1. MAFLD is an umbrella term that encompasses a range of liver disease states, including hepatitis, cirrhosis, and more, and is considered a hepatic manifestation of systemic metabolic disorders2. As a multi-system clinical disease, MAFLD has many extrahepatic manifestations in addition to hepatic manifestations, including diabetes, cardiovascular disease, kidney disease, cognitive impairment and so on3. It can be seen that MAFLD includes various liver states and different extrahepatic diseases, and is a multi-system disease with a certain risk of cardiovascular disease and metabolic disorders, which is a disease that needs to be detected and managed in time.
At present, liver fibrosis caused by virus-related or metabolic liver diseases poses great challenges to global health problems, and liver fibrosis is a key factor affecting the prognosis of liver diseases and leading to the occurrence of liver cancer4. Liver fibrosis is a common occurrence in all types of chronic liver diseases, and liver biopsy is currently the gold standard for detecting liver fibrosis5, but it is an invasive procedure and is not suitable for widespread use. Fibrosis is the main tissue feature of advanced MAFLD, and its pathogenesis is related to the accumulation of triglycerides in the liver in the early stage, followed by oxidative stress, inflammation, autophagy and other links involved, and finally caused multiple blows to the liver6. At present, some researchers have explored the correlation between related indicators and liver fibrosis, and found that TyG-WHtR, TyG-BMI, TyG-WC and HOMA-IR can be used as indicators to identify intermediate and advanced fibrosis7. More and more scholars believe that MAFLD is a multifactorial disease and therefore more involved in the pathway of liver fibrosis, and the current treatment of liver fibrosis focuses on lifestyle interventions, surgery and drugs8. However, reintervention treatment is not as effective as early prevention when liver fibrosis has already occurred, so screening for people at high risk of liver fibrosis in MAFLD appears to be more in line with health strategies.
Previous studies have shown a possible link between thyroid disease and fatty liver disease, and hypothyroidism has been shown to significantly increase the risk of non-alcoholic fatty liver disease, and hypothyroidism may also be associated with non-alcoholic fatty liver disease9. Some researchers have also explored the association between Hashimoto’s thyroiditis and non-alcoholic fatty liver disease from a molecular perspective10. However, there is a lack of research on the association between Hashimoto’s thyroiditis and MAFLD. The purpose of this study was to explore the correlation between Hashimoto’s thyroiditis and MAFLD, and at the same time to screen high-risk groups for liver fibrosis in MAFLD, find out the high-risk related indicators, and apply them to clinical practice to achieve early assessment, early intervention and early prevention.
Methods
Study design and population
The population included in this study was from people who participated in physical examinations at health promotion centers from January 2019 to July 2023. The study has been reviewed by the Ethics Committee (Grant No. 2024 Yan No. 0296).
Inclusion Criteria: (1) Complete liver ultrasound examination; (2) Complete the examination of thyroid function and thyroid antibodies; (3) Complete questionnaires on disease history and medication history; (4) Normal thyroid function.
Exclusion criteria: (1) Incomplete basic information such as smoking and drinking; (2) Age, blood test and other related indicators are missing; (3) Suffering from cancer, connective tissue diseases, acute inflammation, etc.; (4) Currently using antibiotics, thyroid drugs, immunosuppressants, etc.
In this study, the medical history was examined by a doctor, the blood pressure, height and other examinations were performed by a nurse, and the blood test was completed by a laboratory physician, which met the criteria for the examination carried out by the hospital.
Diagnosis of MAFLD
The diagnosis of MAFLD is confirmed according to the diagnostic criteria proposed in 202010. Diagnostic criteria11 for MAFLD are based on histologic (liver biopsy), imaging, and blood biomarker evidence of hepatic lipoaccumulation (hepatocellular steatosis) combined with one of three criteria: overweight/obesity (BMI > 23 kg/m2 in Asian population), type 2 diabetes, and metabolic dysfunction. Metabolic dysfunction is defined as a person with at least two risk factors for metabolic abnormalities. Our diagnosis is based on ultrasound suggesting fatty liver, which is considered to be the presence of fatty liver. Metabolic dysfunction is defined as the presence of at least two metabolic risk abnormalities: blood pressure ≥ 130 mmHg or specific medications; central obesity (waist circumference ≥ 90 cm in men/80 cm in women); HDL-c < 40 mg/dL for males/<50 mg/dL for females or specific medications; triglycerides ≥ 150 mg/dL or specific medications; CRP > 2 mg/L; prediabetes (fasting blood glucose:100–125 mg/dL/HbA1c: 5.7–6.4%)11.Although the steady-state model assesses the insulin resistance score as an abnormality in metabolic risk in the MAFLD criteria, insulin is absent from our examination and therefore not available11.
Indicators included
The indicators to be included in the study include the following: (1) General information: gender, age, smoking, alcohol consumption, height, weight; (2) Biochemical parameters: triglycerides (TG), cholesterol (TC), high-density lipoprotein (HDL-c), low-density lipoprotein (LDL-c), fasting blood glucose(FBG), glycosylated hemoglobin (HbA1c), hypersensitive C-reactive protein (CRP), liver function, uric acid (UA); (3) Thyroid markers: TSH, TT3, TT4, FT3, FT4,TGAB, TPOAB; (4) Imaging indicators: abdominal ultrasound; (4) BMI = weight (kg)/ height^2 (m2).
Liver fibrosis related indicators and high-risk group grouping
The 2018 practice guidelines of the United States Association for the Study of Liver Disease (AASLD) recommend the use of the NAFLD fibrosis score (NFS) and FIB-4 index for fibrosis assessment12. Non-alcoholic fatty liver disease fibrosis score (NFS), AST/platelet ratio index (APRI) and fibrosis 4 index (FIB-4) have been clinically accepted for assessing liver fibrosis risk stratification, and APRI > 1 or NFS > 0.676 or FIB-4 > 2.67 were considered at high risk of advanced fibrosis13.
Statistical analysis
SPSS 26.0 was used for statistical analysis. Firstly, the test of normal distribution was carried out, and the mean ± standard deviation (Mean ± SD) was used for the normally distributed continuous data, the median ± interquartile range (IQR) was used for the non-normally distributed continuous data, and the rate was used for the counting data. The T-test was used for comparison of continuous data with normal distribution, and the Kruskal-Wallis rank-sum test or U-test was considered for comparison between groups with continuous data with normal distribution. The chi-square test or the Fisher exact test were performed to compare the sample rates. Univariate logistic regression analysis and multivariate logistic regression analysis were used to explore the risk factors of high risk of liver fibrosis. The ROC curve is used to determine the diagnostic value of important indicators. A value of P less than 0.05 is considered statistically significant.
Results
Sample population inclusion process
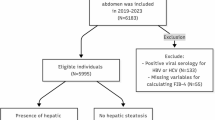
A total of 53,484 patients who had completed liver ultrasound, thyroid function and liver function were included. The people with missing age, smoking data, drinking data, and blood test index values were deleted, and the people with abnormal thyroid function were deleted, and finally 39,442 physical examiners were included in the study (Fig. 1).
Differences between MAFLD and non-MAFLD
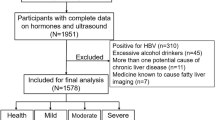
According to the diagnostic criteria for MAFLD, we divided the included sample population into two groups. Through the analysis, we found that the age was older in MAFLD group, had a larger proportion of men, a larger proportion of people who smoked and drank alcohol, and a higher BMI, with a statistically significant difference in the results (P < 0.001). The proportion of Hashimoto’s thyroiditis was lower in the MAFLD group, but there was no difference in the proportion of people at high risk of liver fibrosis. However, the values of liver fibrosis-related indicators (APRI, NFS, FIB-4) in the MAFLD group were relatively higher and statistically different (P < 0.001). There were differences in blood lipids, liver function, CRP, uric acid and other indicators between the MAFLD group and the non-MAFLD group (Table 1).
Differences in the characteristics of people at high risk of liver fibrosis of MAFLD
Patients with MAFLD who was divided into high-risk and for liver fibrosis were according to the literature (APRI > 1 or NFS > 0.676 or FIB-4 > 2.67) (Table 2). The analysis showed that the age was older at high risk of liver fibrosis, but there was no difference in gender, smoking and alcohol consumption. BMI levels were higher at high risk of liver fibrosis group, higher levels of blood pressure and blood glucose, and different liver function values compared with non-high-risk groups. Regarding the comparison of blood lipid levels, the levels of TC and LDL-C were lower in people at high risk of liver fibrosis. CRP levels were higher in people at high risk of liver fibrosis, but there was no difference in uric acid. At the same time, in the comparison of thyroid function, it was found that the levels of TSH, FT3, FT4 and TGAB were statistically different between the two groups.
Logistic regression analysis of high risk of liver fibrosis in MAFLD
Multivariate logistic regression analysis was performed further for variables that differed in univariate logistic regression analysis (Table 3). The results of the analysis showed that age, BMI, FBG, and AST were all risk factors for high-risk groups of MAFLD liver fibrosis, that is, with the increase of age, the increase of fasting blood glucose or the increase of AST, the risk of liver fibrosis also increased. HbA1c, ALT CRP, FT3 and FT4 are also associated with a high risk of liver fibrosis in MAFLD.
ROC curve analysis of MAFLD-related risk factors
Statistically significant indicators in multivariate logistic regression analysis were included in the ROC curve analysis to further clarify the diagnostic value of these indicators (Table 4; Fig. 2). AUC > 0.7 is generally considered to have some diagnostic value, in which age and AST meet this criterion, while several other indicators below 0.7 have lower diagnostic value. Therefore, further analysis was carried out, and the optimal cut-off values of two indicators were obtained, in which the nearest cut-off value for age was 57.5 and the optimal cut-off value for AST was 39.5 U/L.
Discussion
With the aging of the population and the increasing number of obese people, MAFLD has become the most prevalent chronic liver disease, and its epidemic shows no signs of abating, and the number of liver disease-related deaths due to MAFLD has increased dramatically in recent years14. The disease composition of MAFLD is not a single entity, but consists of a series of progressive liver disease stages, which initially consist of simple fatty liver, which leads to fat writing testimonials, and most always develops to fibrosis and liver cancer, MAFLD also carries a series of related comorbidities, leading to the appearance of cardiovascular disease, cognitive impairment and other complications, so that the mortality rate increases, and the quality of life decreases15. Studies have found that more than 20% of patients with steatohepatitis will develop cirrhosis in their lifetime, and liver biopsy as a diagnosis is an invasive procedure that increases the risk of bleeding and other risks, so some non-invasive biomarkers have replaced the trend of liver fibrosis prediction and diagnosis16. Liver fibrosis is characterized by excessive accumulation of extracellular matrix proteins, which is the pathophysiological result of chronic liver injury, and liver fibrosis is also a dynamic process, with various cell disturbances, among which hepatic stellate cells play an important role in the mechanism of liver fibrosis, and advanced liver fibrosis can lead to liver cirrhosis, which are high-risk factors for liver cancer17. Therefore, although MAFLD is more related to metabolic disorders, there is still the possibility of cancer, resulting in a significant decline in quality of life and death, which is a problem worthy of the attention and attention of clinicians. Our study found no difference in the proportion of people at high risk of liver fibrosis between MAFLD and non-MAFLD, but there were differences in liver fibrosis-related indicators, which were manifested in higher levels of NFS, FIB-4 and APRI in the MAFLD group, suggesting that MAFLD has a greater potential for liver fibrosis.
At the same time, our study suggests that the MAFLD group had higher levels of CRP. Recent studies have found that patients with fatty liver disease, including MAFLD and non-alcoholic fatty liver disease, have higher levels of inflammation, and the inflammation level is the highest in the MAFLD group alone, and the inflammatory indicators include CRP18; Therefore, this result is consistent with ours. A complication of diabetes mellitus (diabetic nephropathy) is associated with chronic, low-grade inflammation and is therefore characterized by an elevated C-reactive protein to serum albumin ratio19. It has also been suggested that the ratio of CRP to lymphocyte count can assess the inflammatory complex and is associated with fibrosis of hepatitis20. This shows that the increase in CRP confirms that MAFLD also has a chronic inflammatory state and may be related to MAFLD fibrosis, and our study found that the CRP of people with MAFLD risk of fibrosis is significantly increased. Our study also found that uric acid levels were significantly higher in patients with MAFLD, but there was no significant association with fibrosis. Studies have found that uric acid is associated with a variety of chronic diseases, such as diabetes and hypertension, and recent studies have used the ratio of uric acid to HDL cholesterol as a metabolic marker, and found that the ratio is significantly higher in patients with non-alcoholic fatty liver disease, and it is believed that this ratio can be used as a marker of hepatic steatosis21. Blood uric acid levels are associated with various metabolic conditions, and studies have found that higher levels of uric acid are associated with a significantly higher risk of hypertension and diabetes22. It can be seen that there is a correlation between the increase of uric acid level and MAFLD, and uric acid may also be one of the factors for the increased risk of MAFLD.
Previous studies have found that thyroid hormone is involved in the occurrence and development mechanism of MAFLD, especially hypothyroidism, which is closely related to MAFLD and leads to an increased risk of liver fibrosis, and also pointed out that there is a direct correlation between serum T3 level and MAFLD progression23. Hashimoto’s thyroiditis is a common autoimmune disease, its onset is related to the environment, epigenetics, etc., and can be clinically manifested as symptoms caused by thyroid damage, which can develop into hypothyroidism24. Recently, some researchers have explored the potential relationship between Hashimoto’s thyroiditis and non-alcoholic fatty liver disease from the perspective of genetics, and found genes that have a causal relationship between the two10. At present, there are few studies on the relationship between Hashimoto’s thyroiditis and MAFLD, and based on the association between hypothyroidism and MAFLD, and the potential genetic association between Hashimoto’s thyroiditis and non-alcoholic fatty liver disease, we screened the population with normal thyroid function to investigate its association with MAFLD and high risk of liver fibrosis. Our study found that the proportion of people with Hashimoto’s thyroiditis in MAFLD was lower and statistically different. In the study of the high risk of liver fibrosis in MAFLD, it was found that TSH, FT3, FT4 and TGAB were associated with the high risk of liver fibrosis, among which FT4 and FT3 may be the protective factor for liver fibrosis, that is, within the normal range of thyroid function, the higher the FT4 or FT3 level, the lower the risk of liver fibrosis in MAFLD patients.
Our analysis found that age, BMI, FBG, and AST were all risk factors for the high risk of liver fibrosis, and that the risk of liver fibrosis in MAFLD increased significantly with age, with an increase in body mass index, higher fasting blood glucose levels, and higher AST levels. In our ROC curve analysis, I found two diagnostic indicators that suggest that patients with MAFLD are at high risk of liver fibrosis: age and AST. The results suggest that for MAFLD populations, if the age is greater than or equal to 57.5 years, or the AST level is greater than 39.5 U/L, it indicates that this population is at high risk of liver fibrosis and should be further diagnosed and managed. The results suggest that for MAFLD populations, if the age is greater than or equal to 57.5 years, or the AST level is greater than 39.5 U/L, it indicates that this population is at high risk of liver fibrosis and should be further diagnosed and managed. It has been found that in Asians, liver fibrosis in patients with MAFLD varies depending on the subgroup classification of diabetes, body mass index, and metabolic risk factors25. Our study also confirmed a clear association between blood glucose and BMI and the high risk of liver fibrosis, and the results were consistent. Our study found that with increased FT3 and FT4 levels, the high risk of liver fibrosis in MAFLD is less likely, which is consistent with previous studies that hypothyroidism increases the risk of liver fibrosis. Some researchers have proposed that thyroid dysfunction may be associated with the risk of liver fibrosis, and researchers have found that the ratio of FT3 and FT4 can be used as a predictor of liver fibrosis in nonalcoholic fatty liver disease26.Studies have found that MAFLD can be characterized by low-grade inflammation, and inflammation also has an impact on the cardiovascular system, and inflammation is an important mechanism for regulating the body’s metabolism, and our study also found that CRP, an inflammation-related index, is associated with a high risk of liver fibrosis in MAFLD27. Basic research has found that during the progression of MAFLD, damaged hepatocytes release DAMP, which is recognized by PRR and further recruits immune cells to initiate inflammatory responses in the oxidative stress microenvironment28. Recent studies29 have also found that people with MAFLD who are hypertensive or normotensive are at higher risk of developing liver fibrosis, which is consistent with our findings, and the mechanism may be related to the regulatory role of the renin-angiotensin-aldosterone system. A United States study of middle-aged and older adults also showed a clear association between elevated liver enzymes, diabetes, hypertension, hepatitis virus infection, and steatosis and the risk of severe liver fibrosis30.Studies have found a clear association between liver fibrosis and an increased risk of all-cause mortality and cardiovascular mortality in patients with coronary artery disease31. Therefore, early screening, early assessment and early management are very important for people at high risk of liver fibrosis in MAFLD.
Our study suggests that age and AST are important indicators of high risk of liver fibrosis, and in many cases, we do not pay attention to middle-aged people or people with mild abnormalities in liver function, and our results give more clinical guidance. However, our study still has certain limitations, first, it is a cross-sectional study, and the causal relationship cannot be clarified; Second, the group for liver fibrosis did not use a liver biopsy, and the results were not the gold standard. Therefore, in future research, I hope to establish a cohort of MAFLD and further diagnose liver fibrosis by standard means, in order to provide an evaluation method for the early detection of MAFLD liver fibrosis in high-risk groups, and also provide strategies for clinical chronic disease management.
Conclusion
Age, BMI, FBG, and AST are risk factors for the high risk of liver fibrosis in MAFLD patients, and age and AST have diagnostic value, that is, the age is greater than or equal to 57.5 years, or the AST is greater than or equal to 39.5 U/L, indicating that the MAFLD patients are at high risk of liver fibrosis.
Data availability
The data can be searched by contacting the correspondng author (Guanqun Chao).
References
Ji, J., Wu, L., Wei, J., Wu, J. & Guo, C. The gut microbiome and ferroptosis in MAFLD. J. Clin. Transl. Hepatol. 11(1), 174–187 (2023).
Bae SDW, George J, Qiao L. From MAFLD to hepatocellular carcinoma and everything in between. Chin. Med. J. (Engl). 135(5):547–556 (2022).
Kaya, E. & Yilmaz, Y. Metabolic-associated fatty liver disease (MAFLD): A multi-systemic disease beyond the liver. J. Clin. Transl. Hepatol. 10(2), 329–338 (2022).
Roehlen, N., Crouchet, E. & Baumert, T. F. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells. 9(4), 875 (2020).
Lai, M. & Afdhal, N. H. Liver fibrosis determination. Gastroenterol. Clin. N. Am. 48(2), 281–289 (2019).
Qu, W. et al. Liver fibrosis and MAFLD: From molecular aspects to novel pharmacological strategies. Front. Med. (Lausanne) 8, 761538 (2021).
Xue, Y., Xu, J., Li, M. & Gao, Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index-related parameters. Front. Endocrinol. (Lausanne) 13, 951689 (2022).
Dong, Q. et al. Liver fibrosis and MAFLD: The exploration of multi-drug combination therapy strategies. Front. Med. (Lausanne) 10, 1120621 (2023).
Zhang, H. F., Zhang, Q. X., Feng, Y. H., Li, S. J. & Xie, B. Y. Association of thyroid disease and risk of fatty liver disease: An exposure-wide mendelian randomization study. Eur. Rev. Med. Pharmacol. Sci. 27(23), 11402–11411 (2023).
Li, D., Zhang, Z., Zhang, C., Guo, Q., Chen, C., Peng, X. Unraveling the connection between Hashimoto’s Thyroiditis and non-alcoholic fatty liver disease: exploring the role of CD4(+)central memory T cells through integrated genetic approaches. Endocrine. (2024).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73(1), 202–209 (2020).
Sumida, Y. et al. First in the diagnostic algorithm of metabolic-dysfunction-associated fatty liver disease in the era of the global metabodemic. Life (Basel) 11(2), 143 (2021).
Li, L. et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: Results from the NHANES 1999–2004. Nutrients 14(6), 1224 (2022).
Crane, H., Gofton, C., Sharma, A. & George, J. MAFLD: An optimal framework for understanding liver cancer phenotypes. J. Gastroenterol. 58(10), 947–964 (2023).
Pal, S. C. & Méndez-Sánchez, N. Screening for MAFLD: Who, when and how?. Ther. Adv. Endocrinol. Metab. 14, 20420188221145650 (2023).
Segura-Azuara, N. L. Á., Varela-Chinchilla, C. D. & Trinidad-Calderón, P. A. MAFLD/NAFLD biopsy-free Scoring systems for hepatic steatosis, NASH, and fibrosis diagnosis. Front. Med. (Lausanne) 8, 774079 (2022).
Dhar, D., Baglieri, J., Kisseleva, T. & Brenner, D. A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. (Maywood) 245(2), 96–108 (2020).
Liu, Q. et al. Shift in prevalence and systemic inflammation levels from NAFLD to MAFLD: A population-based cross-sectional study. Lipids Health Dis. 22(1), 185 (2023).
Bilgin, S. et al. Does C-reactive protein to serum albumin ratio correlate with diabetic nephropathy in patients with type 2 dIabetes mellitus? The care time study. Prim. Care Diabetes 15(6), 1071–1074 (2021).
Demirkol, M. E. et al. C-reactive protein to lymphocyte count ratio is a promising novel marker in hepatitis C infection: The clear hep-c study. Rev. Assoc. Med. Bras. (1992) 68(6), 838–841 (2022).
Kosekli, M. A. et al. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: The abund study. Rev. Assoc. Med. Bras. (1992) 67(4), 549–554 (2021).
Kosekli, M. A. & Aktas, G. Serum uric acid to HDL cholesterol ratio is associated with diabetic control in new onset type 2 diabetic population. Acta Clin. Croat. 62(2), 277–282 (2023).
Marschner, R. A., Arenhardt, F., Ribeiro, R. T. & Wajner, S. M. Influence of altered thyroid hormone mechanisms in the progression of metabolic dysfunction associated with fatty liver disease (MAFLD): A systematic review. Metabolites 12(8), 675 (2022).
Ralli, M. et al. Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 19(10), 102649 (2020).
Sohn, W., Kwon, H. J., Chang, Y., Ryu, S. & Cho, Y. K. Liver fibrosis in asians with metabolic dysfunction-associated fatty liver disease. Clin. Gastroenterol. Hepatol. 20(5), e1135–e1148 (2022).
Türker, F. et al. Does the FT3-to-FT4 ratio easily predict the progression of NAFLD and NASH cirrhosis?J. Int. Med. Res. 49(11), 3000605211056841 (2021).
Liang, D. et al. Associations of life’s essential 8 with MAFLD and liver fibrosis among US adults: A nationwide cross-section study. Front. Nutr. 11, 1403720 (2024).
Wang, Y. F. et al. METTL14 downregulation drives S100A4(+) monocyte-derived macrophages via MyD88/NF-kappaB pathway to promote MAFLD progression. Signal Transduct. Target Ther. 9(1), 91 (2024).
Liu, J. et al. Blood pressure stratification for predicting liver fibrosis risk in metabolic dysfunction associated fatty liver disease. Ann. Hepatol. 28(2), 100892 (2023).
Yang, S. et al. Metabolic dysfunction-associated fatty liver disease and liver fibrosis: Prevalence and associated factors in the middle-aged and older US population. Hepatol. Res. 52(2), 176–186 (2022).
Lu, C., Chen, Y., Zhang, Y. & Zhao, X. Liver fibrosis scores and coronary artery disease: novel findings in patients with metabolic dysfunction-associated fatty liver disease. Diabetes Metab. Syndr. Obes. 16, 2627–2637 (2023).
Acknowledgements
N/A.
Funding
Zhejiang Provincial Clinical Research Program of Traditional Chinese Medicine (2024ZL103).
Author information
Authors and Affiliations
Contributions
Guanqun Chao wrote the article, Yue Zhu and Yang Bao did the analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study has been reviewed by the Ethics Committee of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, and the approval number is Sir Run Run Shaw Hospital Ethics Review 2024 Yan No. 0296. The data of this study were obtained from the database of physical examination centers and the consent of the physical examiners.
Consent for publication
N/A.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chao, G., Zhu, Y. & Bao, Y. A screening study of high-risk groups for liver fibrosis in patients with metabolic dysfunction-associated fatty liver disease. Sci Rep 14, 23714 (2024). https://doi.org/10.1038/s41598-024-74792-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74792-9