Abstract
Observational studies suggest a U-shaped association between serum potassium (K⁺) levels and mortality in patients with chronic heart failure (CHF). However, the mode of death in patients with HF and K⁺ disorders remains speculative. To investigate the association between potassium disorders and the mode of death in patients with CHF. A retrospective cohort of 10,378 CHF outpatients was analyzed over an average of 3.28 ± 2.5 years. Kaplan-Meier method, Cox proportional hazards regression models, Poisson regression models adjusting for confounders, and e-value determination (e' > 1.6) were used to observe associations between potassium disorders and outcomes. Chagas etiology (p < 0.01) and triple HF therapy (p < 0.01) were associated with hyperkalemia. Atrial fibrillation was associated with hypokalemia (p < 0.01). Chronic kidney disease (CKD) (p < 0.01) and diabetes (p = 0.03) were associated with both. Hypertension was inversely related to hyperkalemia (p < 0.01); age was inversely related to hypokalemia. Associations with mortality were significant for Chagas (p < 0.01, e-value 2.16), stroke (p < 0.01, e-value 1.85), hypokalemia (p = 0.02, e-value 1.94), severe hyperkalemia (p = 0.08, e-value 1.93), and CKD (p < 0.01, e-value > 1.63). Decompensated HF or cardiogenic shock was the cause of death in 54% of patients with normokalemia, 67.8% with hypokalemia, 44.9% with mild hyperkalemia, 57.8% with moderate hyperkalemia, and 69% with severe hyperkalemia. Most patients with hypokalemia and severe hyperkalemia died from decompensated HF (p = 0.007). Data suggest hypokalemia and severe hyperkalemia, along with Chagas and CKD, are associated with death. Unexpectedly, progressive HF was the most frequent mode of death rather than arrhythmias. Further studies are needed to confirm these findings and explore the underlying mechanisms.
Similar content being viewed by others
Introduction
Chronic heart failure (CHF) is a prevalent clinical condition affecting millions globally, characterized by high morbidity and mortality rates. It imposes significant financial burdens on healthcare systems1. Potassium disorders, observed in 3 to 18% of patients in randomized clinical trials and up to 25% in observational studies, are linked to adverse outcomes in CHF, with varying impacts depending on the heart failure (HF) phenotype.
Studies have shown that serum potassium levels are independently associated with cardiovascular death, hospitalization due to HF, and heart transplantation in symptomatic CHF patients2. In HF with reduced ejection fraction (HFrEF), potassium disorders correlate with HF hospitalizations, with hypokalemia particularly associated with both cardiovascular and non-cardiovascular mortality, especially in patients with chronic kidney disease (CKD). Conversely, in HF with preserved ejection fraction (HFpEF), both hypokalemia and hyperkalemia are linked to increased risks of cardiovascular death, sudden death, and HF death3. Hyperkalemia is also a barrier to the prescription of guideline-directed medical therapy (GDMT), leading to increased cardiovascular events.
Despite these findings, questions remain about whether potassium disorders specifically increase the risk of arrhythmias or sudden death in CHF patients4,5,6. This study aims to investigate the association between potassium disorders and the mode of death in CHF patients, exploring whether hyperkalemia and hypokalemia contribute differently to this association and examining how hydroelectrolytic disorders influence etiologies prone to sudden death or arrhythmias.
Methods
Study design and patient population
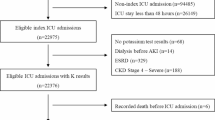
A retrospective cohort study of patients with CHF receiving outpatient care at a tertiary cardiology center between January 2013 and December 2020 was conducted. Data were collected from the electronic medical records system, encompassing patient demographics, laboratory variables (including serum potassium and other electrolytes), imaging tests, comorbidities, medication use, hospitalizations, and emergency room visits. Comorbidities were identified using ICD-10 and procedure codes, with all information verified for accuracy. Clinical data such as functional class (NYHA), weight, blood pressure, heart rate, and CHF etiology were confirmed individually in the medical records. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula within six months before 2013 to capture baseline laboratory information.
Inclusion and exclusion criteria
Patients were included if they had at least one serum potassium measurement within six months of the study’s start. If this test was conducted before 2013, that date was considered the index consultation. Patients lacking baseline variables in medical records or those participating in other institutional protocols were excluded.
Laboratory tests
Chronic kidney disease (CKD) was classified into five stages according to the eGFR determined by KDIGO guidelines7, without considering albuminuria due to its low testing frequency in this population. Hyperkalemia was categorized as mild (5.0 < K⁺ < 5.5 mEq/L), moderate (5.5 < K⁺ < 6.0 mEq/L), and severe (K⁺ > 6.0 mEq/L). Hypokalemia was classified as mild (3.0 < K⁺ < 3.5 mEq/L) and severe (K⁺ < 3.0 mEq/L).
Outcomes
The primary outcome was mortality from any cause, obtained from the Death Information System (SIM) of the Health Department, adhering to confidentiality and data protection laws, This study was conducted with the approval of the Institutional Ethics Committee of the Heart Institute (InCor) at the Hospital das Clínicas, Faculty of Medicine, University of São Paulo. Mortality records from death certificates were used, with diseases or injuries contributing to death listed hierarchically. The underlying cause of death was identified, and comorbidities contributing to death were reported. Deaths were classified by ___location (hospital or out-of-hospital) and analyzed according to underlying potassium levels and the last potassium measurement before death. Causes of death were categorized using ICD-10 codes, focusing on cardiogenic shock, decompensated HF, and arrhythmias. All research was conducted in accordance with relevant guidelines and regulations and received ethical approval.
Control measures
Given the retrospective nature of this study, we implemented rigorous statistical controls to mitigate potential biases and confounding factors: To assess the robustness of our findings against unmeasured confounding, we performed an E-value calculation. The E-value quantifies the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome to explain away the observed association fully8. We adjusted for known confounders such as age, sex, comorbidities, medication use, and clinical variables.
Statistical analysis
Patient characteristics were summarized using descriptive statistics. Categorical variables were presented as frequencies and percentages, while continuous variables were summarized using means and standard deviations or medians and interquartile ranges, as appropriate. Univariate analyses were conducted to examine the association between baseline variables and the outcomes of interest. Variables considered in the univariate analysis included age, etiology, NYHA functional class, previous stroke, hypertension, diabetes, atrial fibrillation, use of triple therapy, CKD stages, and serum potassium levels. The associations were evaluated using chi-square tests for categorical variables and t-tests or Mann-Whitney U tests for continuous variables. To control for potential confounding factors, multivariate regression models were employed. Cox proportional hazards regression was used to assess the association between serum potassium levels and mortality. Time-to-event data were analyzed using Kaplan-Meier survival curves, and differences between groups were evaluated using the log-rank test. Variables with a p-value < 0.10 in univariate analysis were included in the multivariate Cox regression models. Logistic regression was employed to evaluate the association between potassium disorders (hyperkalemia and hypokalemia) and various covariates. The results were presented as odds ratios (ORs) with 95% confidence intervals (CIs). Given the longitudinal nature of the data, time-dependent Cox regression models were applied to account for changes in exposure status over time. This approach allowed for the assessment of how variations in serum potassium levels over time influenced mortality risk. Missing data were addressed using multiple imputation techniques. Multiple imputed datasets were created, each replacing missing values with plausible values based on observed data. The results from these datasets were combined to produce final estimates that account for the uncertainty associated with the missing data. Mortality rates were calculated using Poisson regression models, presented as deaths per person-year. This approach accommodated varying follow-up times among patients, providing a standardized mortality rate for comparison. To assess the potential impact of unmeasured confounders, an E-value calculation was performed. The E-value quantifies the minimum strength of association that an unmeasured confounder would need to have with both the exposure (serum potassium levels) and the outcome (mortality) to explain away the observed association9. All statistical analyses were conducted using R version 4.2.010. A two-tailed significance level of 5% was applied for all tests, with p-values < 0.05 considered statistically significant.
Handling missing data
Patients without potassium measurements, missing demographic variables, participation in other protocols, or duplicate registrations were excluded. Laboratory variables with less than 20% missing data were adjusted as covariables. Multiple imputations handled missing data by creating multiple imputed datasets, substituting missing values with plausible values based on observed data. These datasets were analyzed separately and combined to account for the uncertainty caused by missing data.
Results
Baseline characteristics
The study sample consisted of patients who were followed in a heart failure clinic, with data collected from their clinical records. Data from 10,423 CHF patients were analyzed, with a mean follow-up of 3.28 ± 2.5 years. Baseline laboratory tests were available for 92% of the patients, with an average serum potassium level of 4.5 ± 0.5 mEq/L. Hypokalemia was present in 1.6% of patients, while 13% had hyperkalemia. Most patients had HF with reduced ejection fraction (HFrEF) and were symptomatic at their first visit. Common comorbidities included coronary artery disease (CAD), dyslipidemia, hypertension, atrial fibrillation (AF), diabetes mellitus (DM), smoking, alcoholism, and acute myocardial infarction (AMI). Chagas disease accounted for 5.3% of cases. As shown in Tables 1 and 80% of the patients had a left ventricular ejection fraction (LVEF) below 50%, indicating a significant proportion of patients with reduced ejection fraction. (Table 1).
For hypokalemia recurrence, the Poisson model indicated associations with valvular etiology (aRR 1.59, 95% CI: 1.07-2.37, p < 0.01), AF (aRR 1.71, 95% CI: 1.39-2.10), and moderate to severe CKD (aRR 2.61-6.08, 95% CI: 1.77-6.94, p < 0.01). Age was weakly protective against hypokalemia recurrence (Tables 2 and 3).
Chagas etiology was associated with higher mortality risk (p < 0.01), hyperkalemia, and recurrence. E-value analysis yielded a value of 2.16, indicating a significant risk associated with this etiology, likely not due to unmeasured factors. Valvular and idiopathic etiologies were weakly associated with hypokalemia. In valvular cardiomyopathy patients, the risk of hypokalemia recurrence was higher (p < 0.01).
Outcomes
Out of 10,378 patients, 2,311 died, resulting in a mortality rate of 22.2%. The mortality incidence rate was 7.44 per 100 patient-years (95% CI: 7.16-7.74). Hospitalization incidence was 10.71 per 100 patient-years (95% CI: 10.32-11.11). First hospitalization incidence was 5.08 per 100 patient-years (95% CI: 4.83-5.34). First-time emergency room access incidence was 9.53 per 100 patient-years (95% CI: 9.17-9.9).
Independent mortality risk factors in the Cox-adjusted model included Chagas etiology, age, stroke, hypokalemia, and all levels of hyperkalemia. Adjusting for CKD, hypokalemia and severe hyperkalemia remained significant risk factors, while hypertension was weakly protective (Table 4). The Kaplan-Meier curve (Fig. 1) demonstrated cumulative mortality adjusted for baseline potassium levels and potassium ranges. The multivariate model showed the effect of potassium disorders on mortality: hypokalemia (aHR 1.96, 95% CI: 1.52-2.54, p < 0.01), mild hyperkalemia (aHR 1.19, 95% CI: 1.05-1.36, p = 0.01), moderate hyperkalemia (aHR 1.33, 95% CI: 1.04-1.70, p = 0.02), and severe hyperkalemia (aHR 1.49, 95% CI: 1.0-2.21, p = 0.05).
A cubic polynomial model adjusted for age and year of inclusion estimated a reversed J-shaped curve for baseline potassium levels, with the highest death risk at potassium levels ≤ 3 mEq/L and ≥ 6 mEq/L and the lowest risk between 4.3 and 4.6 mEq/L. Sensitivity analysis, including the e-value, is provided in Table 4.
Decompensated HF and cardiogenic shock, followed by ischemic heart disease, were the most frequent causes of death (Table 2). Electrolyte disorders contributed to 1% of deaths, with arrhythmias accounting for 6.2%. Other significant causes included septicemia/infections, stroke, pulmonary embolism, and respiratory failure. Analyzing baseline potassium levels as a predictor of death mode revealed no significant difference between potassium levels and the place of death (p = 0.475). However, patients with severe hypokalemia and primary hyperkalemia tended to die from HF-related causes (p = 0.08). To enhance the accuracy of a potential association between potassium levels and outcomes, we opted to use the most recent potassium level measured before the event. This approach ensures that the potassium measurements are temporally closer to the outcome, thereby providing a more reliable assessment of the relationship between potassium levels and the event. The last potassium measurement before death indicated that hypokalemia and hyperkalemia were associated with higher risks of death due to HF, cardiogenic shock, or arrhythmia (p = 0.03), with these patients often dying from decompensated HF (p = 0.007) (Table 5).
Potassium disorders
The incidence rate of hyperkalemia was 22.72 per 100 patient-years (95% CI: 22.01-23.48), while hypokalemia occurred at a rate of 3.56 per 100 patient-years (95% CI: 3.32-3.81). Mild hyperkalemia had an incidence rate of 19.31 (95% CI: 18.66-19.98), moderate hyperkalemia 5.07 (95% CI: 4.78-5.37), and severe hyperkalemia 1.77 (95% CI: 1.6-1.94).
Variables associated with the incidence of hyperkalemia included the Chagas disease, age, DM, use of triple therapy, and all stages of CKD (adjusted hazard ratio [aHR] range from 1.49 to 5.53, p < 0.01). Hypertension was inversely related to hyperkalemia (aHR 0.84, 95% CI: 0.76-0.93, p < 0.01). Chagas etiology, age, DM, and use of triple therapy were positively associated with hyperkalemia (aHR 1.54, 1.02, 1.13, and 1.34 respectively, p < 0.01 for all) (Table 2).
In the Poisson model for hyperkalemia recurrence, associated variables were Chagas etiology (adjusted relative risk [aRR] 1.35, 95% CI: 1.11-1.64, p < 0.01), age (aRR 1.35, 95% CI: 1.11-1.64, p < 0.01), DM (aRR 1.35, 95% CI: 1.11-1.64, p < 0.01), use of triple therapy, and all stages of CKD (aRR 1.64-4.63, 95% CI: 1.35-6.18, p < 0.01) (Table 3).
Factors associated with hypokalemia incidence were DM (aHR 1.23, 95% CI: 1.02-1.50, p = 0.03), AF (aHR 1.67, 95% CI: 1.39-2.01, p < 0.01), and all stages of CKD (aHR 1.76-7.76, 95% CI: 1.24-11.61, p < 0.01). Age was a weak protective factor against hypokalemia incidence (Table 2).
Discussion
This study is the first to describe the association of hypokalemia and severe hyperkalemia with modes of death from heart failure or cardiogenic shock in patients with chronic heart failure. Our findings revealed an E-value greater than 1.6 for mortality associations with Chagas etiology, valvular etiology, stroke, hypokalemia, severe hyperkalemia, and chronic kidney disease (CKD). We observed the influence of specific etiologies on the development and recurrence of potassium disorders: Chagas etiology was linked to a higher incidence and recurrence of hyperkalemia. In contrast, valvar etiologies were more commonly associated with hypokalemia and its recurrence. Additionally, triple therapy, age, diabetes mellitus (DM), Chagas etiology, idiopathic etiology, and CKD were significantly associated with hyperkalemia, whereas hypertension was a protective factor. Conversely, DM, atrial fibrillation (AF), and CKD were associated with hypokalemia, while age served as a protective factor.
Previous studies have identified risk factors for hyperkalemia and cardiovascular mortality, including CKD, diabetes, and medication use11,12High-dose loop diuretics, CKD, and neurohormonal activation have been associated with hypokalemia and death. Our study found a higher incidence of mild to severe hyperkalemia (22%) and hypokalemia (3.5%) compared to previous reports13,14, likely due to a higher percentage of patients in NYHA III/IV functional class, renal dysfunction, diuretic use, and RAASi use. Furthermore, we identified Chagas and valvular etiologies as novel risk factors for potassium disorders in this population.
Contrary to the traditional view that arrhythmias predominantly cause death in patients with potassium disorders, our findings indicate that HF or cardiogenic shock was the most frequent mode of death in patients with hypokalemia and severe hyperkalemia. Both hyperkalemia and hypokalemia were associated with a higher risk of hospitalization for HF and death from HF compared to normokalemia.
The mechanisms underlying the association between potassium disorders and death from HF and cardiogenic shock remain speculative. Potassium disorders may serve as markers of greater disease severity in CHF15. Hypokalemia might indicate disease progression, associated with more frequent diuretic use and insufficient neurohormonal inhibition, leading to increased activation of angiotensin II, aldosterone, and norepinephrine16. Severe hemodynamic disturbances may cause a catecholamine-induced drop in serum potassium15. Hyperkalemia, resulting from decreased potassium elimination, could be a marker of cardiorenal syndrome and other comorbidities like Chagas disease and older age, which were identified as risk factors for mortality17,18,19. Alternatively, potassium disorders could contribute to increased mortality through their effects on myocardial function, the cardiovascular system, neurohormonal activation, coagulation, and endothelial function20,21,22,23. Potassium can stimulate aldosterone production independently of the renin-angiotensin-aldosterone system and reduce angiotensin II-induced vasoconstriction, especially when there is low nitric oxide availability24. Animal and human studies suggest that low serum potassium may increase thrombosis rates, endothelial dysfunction, and platelet aggregation25. Additionally, serum potassium levels may influence cardiac systolic function by affecting the Na+/K+ ATPase pump, essential for maintaining cellular ion balance and myocardial contractility26.
In agreement with these mechanisms, triple therapy medications that increase serum potassium levels and improve survival in HFrEF were also associated with hyperkalemia in our study. The suspension or dose reduction of triple therapy might explain increased mortality, although sub-analyses of the PARAGON-HF and PARADIGM-HF studies suggest that hyperkalemia-related mortality occurred despite constant sacubitril-valsartan doses5,6.
The association between Chagas etiology and potassium disorders is significant given its prevalence in endemic regions and higher mortality compared to other CHF etiologies. Potassium disorders have not been previously identified as contributors to high mortality in Chagas disease, necessitating further research to elucidate the pathophysiological mechanisms involved27,28.
Limitations
This retrospective observational study has limitations such as selection bias, confounding, and misclassification. However, it addresses important clinical practice issues not evaluable in clinical trials. We included various patient characteristics to reduce confounding bias and performed sensitivity analyses and e-value calculations to mitigate limitations. The database reflects real-world practice standards, limiting misclassification by reducing human interference in data capture and implementing quality control measures29.
While the e-value has limitations, it was used to assess causality in observational studies. Retrospective data may be incomplete and non-standardized, but our missing data rate was below 20%, with all patients having at least one potassium measurement. Clinical decisions determine the variables obtained, reflecting real-world practice but potentially limiting the accuracy of certain questions. The use of death certificates to determine the mode and cause of death may introduce inaccuracies, but they provide the most accurate information closest to the time of death. The sample size was large, adjustments were made for measured confounders, and sensitivity analyses were performed for unmeasured confounders, enhancing internal validity and generalizability of results.
Conclusion
Our study underscores the importance of monitoring potassium levels in CHF patients, as both severe hypokalemia and hyperkalemia were associated with increased mortality risk. The most common causes of death related to potassium disorders were the progression of HF and cardiogenic shock. Potassium disorders may serve as therapeutic targets for managing CHF, especially in patients with Chagas, valvar disease, elderly, diabetic, chronic kidney disease, and those on triple therapy. Close monitoring and management of potassium disorders could help identify patients with a high risk of death from decompensated HF or cardiogenic shock. Further research is needed to confirm these findings and explore the underlying mechanisms.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Chioncel, O. et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart failure Long-Term Registry. Eur. J. Heart Fail. 19 (12), 1574–1585. https://doi.org/10.1002/ejhf.813 (2017).
Urbich, M. et al. A systematic review of medical costs associated with heart failure in the USA (2014–2020). PharmacoEconomics. 38, 1219–1236. https://doi.org/10.1007/s40273-020-00952-0 (2020).
Ferreira, J. P. et al. Abnormalities of potassium in heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 75 (22), 2836–2850. https://doi.org/10.1016/j.jacc.2020.04.021 (2020).
Toledo, C. C. et al. Serum potassium levels provide prognostic information in symptomatic heart failure beyond traditional clinical variables. ESC Heart Fail. 8 (3), 2133–2143. https://doi.org/10.1002/ehf2.13295 (2021). Epub 2021 Mar 18. PMID: 33734611; PMCID: PMC8120348.
Ferreira, J. P. et al. Serum potassium in the PARADIGM-HF trial. Eur. J. Heart Fail. 22 (11), 2056–2064. https://doi.org/10.1002/ejhf.1987 (2020).
Ferreira, J. P. et al. Serum potassium and outcomes in heart failure with preserved ejection fraction: A post-hoc analysis of the PARAGON-HF trial. Eur. J. Heart Fail. 23 (5), 776–784. https://doi.org/10.1002/ejhf.2134 (2021).
Ketteler, M. et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. ;92(1):26–36. (2017). https://doi.org/10.1016/j.kint.2017.04.006. Erratum in: Kidney Int. 2017;92(6):1558.
VanderWeele, T. J. & Ding, P. Analysis in observational research: Introducing the E-value. Ann. Intern. Med. 167, 268–274 (2017).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 321, 602–603 (2019).
Mathur, M. B., Ding, P., Riddell, C. A., & Vander Weele, T. J. Website and R package for computing E-values. Epidemiology. 29, e45–e47 (2018).
Henrysson, J., Thunström, E., Chen, X., Fu, M. & Basic, C. Hyperkalaemia as a cause of undertreatment with mineralocorticoid receptor antagonists in heart failure. ESC Heart Fail.https://doi.org/10.1002/ehf2.14137 (2022).
James, G. et al. Serum potassium variability as a predictor of clinical outcomes in patients with cardiorenal disease or diabetes: A retrospective UK database study. Clin. Kidney J. 15 (4), 758–770 (2022).
Aldahl, M. et al. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur. Heart J. 38 (38), 2890–2896 (2017).
Cooper, L. B. et al. Association between potassium level and outcomes in heart failure with reduced ejection fraction: A cohort study from the Swedish Heart failure Registry. Eur. J. Heart Fail. 22 (8), 1390–1398. https://doi.org/10.1002/ejhf.1757 (2020).
Núñez, J. et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 137 (13), 1320–1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576 (2018).
Linde, C. et al. Serum potassium and clinical outcomes in heart failure patients: Results of risk calculations in 21 334 patients in the UK. ESC Heart Fail. 6 (2), 280–290. https://doi.org/10.1002/ehf2.12402 (2019).
Moriyama, H., Kohno, T. & Kohsaka, S. Letter regarding the article ‘Effects of hyperkalaemia and non-adherence to renin-angiotensin-aldosterone system inhibitor therapy in patients with heart failure in Italy: A propensity-matched study’. Eur. J. Heart Fail. 23 (3), 495–496. https://doi.org/10.1002/ejhf.2081 (2021).
Srivastava, T. N. & Young, D. B. Impairment of cardiac function by moderate potassium depletion. J. Card Fail. 1, 195–200 (1995).
Desai, A. S. et al. Eur. Heart J. ;36(30):1990–1997. (2015).
Sudhir, K., Kurtz, T. W., Yock, P. G., Connolly, A. J. & Morris, R. C. Potassium preserves endothelial function and enhances aortic compliance in Dahl rats. Hypertension. 22, 3 (1993).
Parksook, W. W. & Williams, G. H. Aldosterone and cardiovascular diseases. Cardiovasc. Res. 2022 Apr 7:cvac027. https://doi.org/10.1093/cvr/cvac027. Epub ahead of print.
Taddei, S. et al. Effect of potassium on vasodilation to acetylcholine in essential hypertension. Hypertension. 23, 485–490 (1994).
Young, D. B., Lin, H. & McCabe, R. D. Potassium’s cardiovascular protective mechanisms. Am. J. Physiol. 268, R825–R837 (1995).
Alper, A. B. et al. A propensity-matched study of low serum potassium and mortality in older adults with chronic heart failure. Int. J. Cardiol. 137 (1), 1–8. https://doi.org/10.1016/j.ijcard.2008.05.047 (2009).
Shapiro, J. I., Banerjee, A., Reiss, O. K. & Elkins, N. Acute and chronic hypokalemia sensitize the isolated heart to hypoxic injury. Am. J. Physiol. 274 (5), H1598–H1604. https://doi.org/10.1152/ajpheart.1998.274.5.H1598 (1998).
Barri, Y. M. & Wingo, C. S. The effects of potassium depletion and supplementation on blood pressure: A clinical review. Am. J. Med. Sci. 314, 37–40 (1997).
Issa, V. S. et al. The course of patients with Chagas heart disease during episodes of decompensated heart failure. ESC Heart Fail. 8 (2), 1460–1471. https://doi.org/10.1002/ehf2.13232 (2021).
Mocelin, A. O. et al. The influence of aetiology on inflammatory and neurohumoral activation in patients with severe heart failure: A prospective study comparing Chagas’ heart disease and idiopathic dilated cardiomyopathy. Eur. J. Heart Fail. 7 (5), 869–873. https://doi.org/10.1016/j.ejheart.2004.10.014 (2005).
Boyko, E. J. Observational research — opportunities and limitations. J. Diabetes Complicat. 27 (6), 642–648 (2013).
Acknowledgements
The authors would like to express their sincere gratitude to all the participants who generously contributed their time and effort to this study. We would also like to acknowledge the support and resources provided by AstraZeneca. We extend our appreciation to the Instituto do Coração staff and the InCor -HF research team for their invaluable assistance in data collection and analysis. Special thanks go to Professor Edimar Bocchi for their valuable insights and expertise. Lastly, we thank our families and loved ones for their unwavering support throughout this research endeavor.
Author information
Authors and Affiliations
Contributions
I.G.C.V.L. and J.T.N. conceptualized, designed the study, wrote the initial manuscript, and collected the data. I.H.O collected the data. L.P.D performed the statistical analysis, S.M.A.F., and R.T.M. P.R.C., B.B., B.R.G., F.R., A.S., F.R, and E.A.B. critically reviewed the final manuscript and provided significant intellectual input. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lima, I.G.C.V., Nunes, J.T., de Oliveira, I.H. et al. Association of potassium disorders with the mode of death and etiology in patients with chronic heart failure: the INCOR-HF study. Sci Rep 14, 30167 (2024). https://doi.org/10.1038/s41598-024-74928-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74928-x