Abstract
To evaluate the effects of different delivery methods on the pelvic floor structure and function among primiparas by transperineal ultrasound, with a view to providing guidance for early postpartum intervention. Primiparas who underwent postpartum examination with transperineal ultrasound were recruited. Subjects were divided according to mode of delivery (vaginal and cesarean delivery). General information (including age, pre-pregnancy BMI and neonatal weight) were collected, and transperineal ultrasound was performed to measure such indicators as the levator hiatus areas in resting, constrictive anal and maximum Valsalva states, the posterior vesicourethral angles and distances between vesical neck and posterior inferior margin of pubic symphysis in resting and maximum Valsalva states, as well as the vesical neck mobility and urethral rotation angle in maximum Valsalva state. The inter-group differences in ultrasound indicators between the resting and Valsalva states were compared to analyze the postpartum incidences of pelvic floor dysfunctions like stress urinary incontinence, urethral funnel formation, bladder prolapse and uterine prolapse in primiparas. The levator hiatus areas in resting, constrictive anal and maximum Valsalva states were all larger in the vaginal delivery group than in the cesarean delivery group (P < 0.05). Compared to the cesarean delivery group, the vaginal delivery group exhibited larger posterior vesicourethral angles in resting and maximum Valsalva states (P < 0.05). The distances between vesical neck and posterior inferior margin of pubic symphysis were greater in the cesarean delivery group than in the vaginal delivery group at both resting and maximum Valsalva, with that at maximum Valsalva showing significant inter-group difference (P < 0.05). The vaginal delivery group exhibited greater vesical neck mobility and urethral rotation angle at maximum Valsalva compared to the cesarean delivery group (P < 0.05). The incidences of stress urinary incontinence, urethral funnel formation, bladder prolapse and uterine prolapse were all higher in the vaginal delivery group than in the cesarean delivery group, with the stress urinary incontinence and bladder prolapse incidences showing significant inter-group differences (P < 0.05). With transperineal ultrasound, various pelvic floor indicators of primiparas can be clearly measured and, through these ultrasound indicators, the effects of different delivery methods on the pelvic floor function can be evaluated, which is conducive to early clinical detection and intervention of postpartum pelvic floor dysfunctions, thus facilitating the early postpartum treatment.
Similar content being viewed by others
Female pelvic floor dysfunction (PFD) is caused by a variety of factors, including structural relaxation of levator and ligament tissues that are responsible for pelvic floor support, as well as levator avulsion1,2, which disallows support for the normal anatomical positions of pelvic organs, leading to changes in the anatomical positions of the bladder, uterus and rectum, ultimately triggering abnormalities in the morphology, structure and function of other pelvic organs. Clinically, it manifests itself as stress urinary incontinence (SUI), pelvic organ prolapses (POP; including prolapses of anterior and posterior vaginal walls, uterine and rectal ampulla) and other diseases, which significantly affect the physical and mental health, as well as the quality of life of patients although not life-threatening3, and thus have become a public health issue of global concern. As suggested by extensive relevant epidemiological studies4,5, pregnancy and delivery are the major causes of PFD, and different methods of delivery lead to varying degrees of PFD. Pregnancy and delivery have also been considered as the independent risk factors for PFD6. With the improvement of medical level, ultrasound technology has gradually developed and matured, which has been widely applied in clinical practice. Owing to a high detection rate in the diagnosis of postpartum PFD, it seems necessary to take pelvic floor ultrasound in the early postpartum period, which facilitates the implementation of clinical treatment measures as early as possible to improve the prognosis. This study aims to observe the pelvic floor anatomical structure and various ultrasound indicators of primiparas by transperineal ultrasound, so as to evaluate the effects of different delivery methods on the pelvic floor function, with a view to providing more evidence and guidance for early clinical diagnosis and intervention of PFD, thereby lowering the risk of long-term pelvic floor injury.
Data and methods
General information
A total of 92 primiparas, who gave birth in the Maternal and Child Health Hospital Affiliated to Nantong University from August 2022 to August 2023 and underwent postpartum reexamination and transperineal pelvic floor ultrasound at the outpatient department 6–8 weeks after delivery, were enrolled. The modes of delivery include vaginal delivery and elective cesarean delivery. Selective cesarean section refers to the termination of pregnancy by cesarean section due to maternal or fetal factors before the start of labor. The inclusion criteria were as follows: (1) Primiparas with full-term single birth; (2) No history of genitourinary tract infection or pelvic surgery; (3) Thoroughly eliminate lochia; (4) Cooperative in completing effective Valsalva maneuver. Exclusion criteria: (1) Those complicated by urinary, reproductive infections and diagnosed with PFD before delivery; (2) Those with grade III or above perineal laceration during labor and those with protracted or prolonged second stage of labor7.(The total stage of labor exceeds 24 h or the second stage of labor for primiparous women exceeds 3 h and primiparous women with Painless delivery exceeds 4 h); (3) A previous history of pelvic surgery; (4) A history of chronic cough and constipation; (5) Those incapable of completing the effective Valsalva maneuver (lasting at least six seconds) and the anal constriction (lasting less than three seconds) after doctor’s guidance; (6) Those complicated by other systemic diseases. The 92 primiparas enrolled were divided into the vaginal delivery group (52 cases, 1 case of forceps assisted delivery and 3 cases of episiotomy) and the cesarean delivery group (40 cases) depending on the method of delivery. Basic information, including age, pre-pregnancy BMI, neonatal weight, delivery method and presence or absence of urinary incontinence during and after pregnancy, were recorded.
Methods
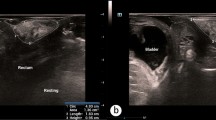
NuewaR9 T color Doppler ultrasound system (Mindray Bio-Medical Electronics, Shenzhen) was utilized for performing ultrasound examination. Transabdominal 3D volumetric SD8-1U probe was used for scanning over a frequency range of 1–8 MHz, and the maximum scanning angle was 85°. The primiparas were instructed to empty their bladders 10 min before the examination. During the examination, the primiparas were placed in the bladder lithotomy position, the transabdominal 3D volumetric probe, whose surface was applied with coupling agent and covered with a condom, was placed between the labia majora on both sides of the perineum, and the standard images for median sagittal plane of pelvic floor were obtained by using the lower margin of pubic symphysis as the reference line. The 2D, 3D and 4D volumetric images were collected, measured and stored separately in the resting, maximum Valsalva and constrictive anal states, and then the obtained images were transferred to the workstation for analysis of images and data information via the 4D Pelvic Floor software. All the operations were completed by practitioners who have passed the training for pelvic floor ultrasound, and all the data were measured in triplicate and averaged to serve as the final results.
Ultrasound measures
(1) The levator hiatus areas in resting, constrictive anal and maximum Valsalva states; (2) The posterior vesicourethral angles and the distances between vesical neck and posterior inferior margin of pubic symphysis in resting and maximum Valsalva states ; (3) The vesical neck mobility and urethral rotation angle in maximum Valsalva state; (4) Inter-group comparison of PFD was made, the funnel-like changes in internal urethral orifice were observed, and the presence or absence of SUI, POP were recorded.
Normal reference values
The normal ranges of posterior vesicourethral angle were set to be < 140° (Valsalva state) and < 110° (resting state), respectively. When the angle exceeded 140°, the posterior vesicourethral angle was considered open, which was often accompanied by the funnel-like changes in internal urethral orifice. The normal range of levator hiatus area was < 20 cm2 in maximum Valsalva state, and the levator hiatus expansion was ≥ 20 cm² during maximum Valsalva maneuver, which reflected the weakening of levator functional status and elasticity. The normal range of vesical neck mobility was <20 mm, and the mobility was considered slightly large when its range was 20–25 mm. A vesical neck within 10 mm of the marker line was defined as mild vesicocele. A vesical neck mobility of > 25 mm was considered large and was defined as evident vesicocele. The normal range of urethral rotation angle was < 45°. The normal uterus, bladder and rectum were all located above the reference line of the posterior inferior margin of pubic symphysis8.
Statistical processing
Data were statistically analyzed via the SPSS 22.0 software. Measurement data tested by P-P chart and conforms to a normal distribution were presented as \(\:\stackrel{-}{x}\)± s, and their inter-group comparison was made by independent t-test. Count data were expressed as n (%), and their inter-group comparison was made by χ2 test. Differences were considered statistically significant when P < 0.05.
Results
No significant differences in the age, pre-pregnancy BMI or neonatal weight were found between the primiparas in the two groups (P > 0.05). Details are listed in Table 1.
Based on whether the levator ani muscle is damaged, the incidence of levator ani muscle damage primarily occurs in the vaginal delivery group, with a rate of 15.38%. There is a close correlation between newborn weight and levator ani muscle damage, where a higher newborn weight increases the risk of developing levator ani muscle damage.Details are listed in Table 2.
The vaginal delivery group exhibited significantly larger levator hiatus areas in the resting, constrictive anal and maximum Valsalva states compared to the cesarean delivery group (P < 0.05). Details are listed in Table 3.
Compared to the cesarean delivery group, the vaginal delivery group exhibited significantly greater posterior vesicourethral angle and distance between vesical neck and posterior inferior margin of pubic symphysis at both resting and maximum Valsalva (P < 0.05). The distances between vesical neck and posterior inferior margin of pubic symphysis at both resting and maximum Valsalva were greater in the cesarean delivery group than in the vaginal delivery group, with that at maximum Valsalva showing significant inter-group difference (P < 0.05). Details are listed in Tables 4 and 5.
The vaginal delivery group exhibited greater vesical neck mobility and urethral rotation angle at maximum Valsalva compared to the cesarean delivery group, showing statistically significant differences (P < 0.05). Details are listed in Table 6.
Comparison of pelvic floor functional injury in parturient women under Valsalva condition, the incidence of pressure incontinence/urethral infundibulation, bladder prolapse and uterine prolapse in the vaginal delivery group were higher than those in the cesarean section group, but there was no statistical significance in the incidence of urethral infundibulation and uterine prolapse between the two groups (P > 0.05). There were significant differences in the incidence of stress incontinence and bladder prolapse between the two groups (P < 0.05). Details are listed in Table 7.
Discussion
Female pelvic floor is an integral structure comprising pelvic muscle group, bone, connective tissue, nerves and organs. Damage to any of these tissues and structures will affect the pelvic floor function to cause PFD. During pregnancy, the weights of fetus and fetal appendage gradually increase over time, leading to increased weight of the uterus, the pelvic floor tissue will be compressed, leading to its stretching to result in the relaxation and gradual weakening of connective tissue ligament. During delivery, the fetal pressure on the pelvic floor supporting tissue increases, and the pelvic floor tissue expands continuously, resulting in mechanical injury. All the above factors are risk factors for PFD. Pregnancy and delivery, as the major causes of structural and functional damage to the maternal pelvic floor support, may further lead to the occurrence of PFD, resulting in a series of symptoms like SUI, POP, sexual dysfunction and fecal incontinence9,10,11,12,13. During pregnancy14,15 and delivery, structural and functional damage to the pelvic floor is inevitable regardless of what kind of pregnancy method is adopted. However, there is no unified conclusion in the clinical setting regarding the degrees of influences of these two methods. In this study, transperineal ultrasound was employed to quantitatively analyze the pelvic floor function of primiparas with different delivery methods, and to find the factors associated with PFD, with a view to achieving early diagnosis and intervention, delaying or avoiding PFD progression, and providing guidance for subsequent pregnancies to avoid the occurrence of severer pelvic floor injury.
Levator anal muscle is the most important muscle group in the pelvic floor supporting system, which supports pelvic organs and maintains their normal positions. The hiatus of levator anal muscle is formed by the bilateral levator anal muscles and the anterior pubic ramus. It is the largest portal in the peritoneum and the main path of pelvic organ descent. The integrity of anal levator muscle and the morphology of its hiatus can reflect the ___location and structural changes of pelvic organs. Meanwhile, the size of hiatus of levator anal muscle can reflect the compliance and elasticity of pelvic floor. In this study, it was discovered that the risk of levator ani muscle injury during vaginal delivery is significantly elevated, particularly for mothers giving birth to larger infants. This is attributed to the stretching of pelvic floor muscles during delivery, which enlarges the levator ani hiatus, potentially causing tearing or even rupture of the muscle during the birth of the fetus. Levator hiatus is composed of the pubic symphysis, the left and right pubic rami and the levator group. As a vital supporting structure of the pelvic floor, it not only supports the pelvic and abdominal organs, but also participates in the physiological functions like the excretion of pelvic organs and the coordinated control of urination. Levator hiatus is a relatively weak site in the pelvic floor supporting tissue. During labor, the pelvic floor muscles extend, and the levator hiatus enlarges to allow the delivery of the fetus. Measurement of its area can effectively reflect the levator function and the degree of pelvic floor structure relaxation. The smaller the measured value, the milder the pelvic floor relaxation and the lower the risk of PFD16. This study found that the levator hiatus area increased regardless of the delivery method chosen, which was more significant in the vaginal delivery group than in the cesarean delivery group. The inter-group differences in levator hiatus area at resting and maximum Valsalva were statistically significant, indicating that the delivery method would affect the hiatus histomorphology. Suggestively, delivery causes great positional changes in the pelvic organs, and cesarean delivery has a certain protective effect on pelvic structure and function compared to the vaginal delivery, which agree with the previous findings17,18. The mechanism whereby pregnancy and delivery cause increase in the levator hiatus area may involve the increasing pressure of the enlarging fetus on the pelvic floor tissue (affecting the blood supply of the pelvic floor connective tissue), along with the indirect effect of increasing hormones during pregnancy19, which lead to the relaxation of the pelvic floor tissue to ultimately enlarge the area of levator hiatus formed by the levator group and fascia. Another study20 found that during vaginal delivery, the fetus was delivered through the levator hiatus, and the pelvic floor muscle stretching and expansion were 1.47 times that in the non-delivery state. The levator group was overstretched during vaginal delivery, even exceeding the physiological limit, and levator tearing was detected in some women after vaginal delivery21, which subsequently developed into PFD.
In this study, statistically significant differences were found in the posterior vesicourethral angle measured at rest and in the posterior vesicourethral angle, the distance between vesical neck and posterior inferior margin of pubic symphysis, the vesical neck mobility and the urethral rotation angle measured after maximum Valsalva maneuver between the two delivery groups (P < 0.05). Women who underwent vaginal delivery exhibited lower positions of vesical neck and internal cervical orifice than those in the cesarean delivery group, as well as greater posterior vesicourethral angle, vesical neck mobility and urethral rotation angle, showing significant differences (P < 0.05). This might be associated with the greater degrees of decline in the vesical and uterine positions after vaginal delivery, which further verifies alteration in the pelvic floor muscle during the fetal passage through the birth canal due to excessive extension of its elastic function.
It was found that when the lowest point of vesical neck was closer to the horizontal line of the posterior inferior margin of pubic symphysis, the bladder descension was greater and the bladder prolapse was severer. This might be attributed to the passive stretching of pelvic muscle group supporting the bladder position by external forces due to the downward pressure of the fetal head during delivery, which significantly affected the bladder (located in the anterior pelvic cavity) alteration in the pelvic position. Vesical neck mobility is a crucial functional indicator for the supporting structures around the bladder and urethra. The smaller its value, the milder the injury of surrounding supporting tissues. This study revealed that the posterior vesicourethral angle, vesical neck mobility and urethral rotation angle at maximum Valsalva of women in the vaginal delivery group were all greater than those in the cesarean delivery group, indicating that compared to the cesarean delivery, the vaginal delivery caused severer damage to the vesical neck of primiparas. This was due to the following three reasons: Firstly, during vaginal delivery, the fetus descended through the birth canal, directly squeezing the bladder located in front of the uterus, leading to the fetal displacement and tilt to affect the pelvic floor function. Secondly, during labor, the fetal head directly acted on the pelvic floor muscle group such as the levator muscle, so that the entire group was stretched to cause mechanical injury. Thirdly, the prolonged compression of fetus and fetal appendage during labor might reduce the blood supply to the pelvic floor support structure, leading to its hypoxia and ischemia to affect the pelvic floor muscle group and fascia, thereby impacting the pelvic floor structure and function. During cesarean delivery, the fetus was delivered through the abdominal incision, which greatly reduced the impact on the pelvic floor tissue compared to the fetal head descension, perineal laceration and forceps traction during vaginal delivery. Thus, vaginal delivery caused greater damage to the pelvic floor than cesarean delivery. However, at the present stage, it remains controversial whether cesarean delivery can be adopted for protecting the pelvic floor function to lower the occurrence of long-term PFD.
This study also found that the incidences of SUI, urethral funnel formation, bladder prolapse and uterine prolapse in the vaginal delivery group were all higher than those in the cesarean delivery group. Among them, the inter-group differences in SUI and bladder prolapse were statistically significant (P < 0.05), and the vaginal delivery group exhibited higher incidence of urethral funnel formation during Valsalva maneuver compared to the cesarean delivery group. Some studies have considered the urethral funnel formation as a marker of urethral sphincter dysfunction, which could evaluate and predict the occurrence of SUI22,23, showing consistency with the present results as well. In this study, the incidence of PFD was higher in the vaginal delivery group than in the cesarean delivery group. Nonetheless, when choosing the specific delivery method, the clinicians need to combine the actual situation of primiparas and various factors to choose the most appropriate delivery method, so as to minimize the delivery risk.
The limitations of this study include small sample size, Non- pregnant ultrasound indicators were not included and short follow-up duration. They are directions that need improvement and effort in later research. A larger sample size, more complete and comprehensive grouping, and longer follow-up datas will make the experimental results more credible.
Conclusion
Pelvic floor ultrasound, as a convenient, reproducible and non-radiative examination technique, is applicable for evaluating the function and anatomical structure of female pelvic floor24. By quantifying the positional changes in pelvic floor organs, it can achieve early detection of asymptomatic PFD, which has high application value in evaluating the pelvic floor function of women with different delivery methods. Transperineal pelvic floor ultrasound enables early diagnosis of diseases and thus provides a scientific basis for clinical practice.
To follow up the pelvic floor status in the later stage, we will further explore the effects of different delivery methods on the postpartum rehabilitation by enlarging the sample size, incorporating the ultrasound indicators for non-pregnant women, extending the follow-up duration and including the postpartum rehabilitation information.
Data availability
Data availability statements The author confirms that all data generated or analysed during this study are included in this published article. Furthermore, primary and secondary sources and data supporting the findings of this study were all publicly available at the time of submission.
Change history
23 October 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-76793-0
References
Pessoa, P., Carvalho, A. & Mota, P. Prevalence of levator ani muscle injuries in primiparous women after delivery and their influence on pelvic floor disorders-systematic review[J]. Neurourol Urodyn. 1. https://doi.org/10.1002/nau.25529. (2024)
DeLancey, J. O. L. et al. Pelvic floor injury during vaginal birth is life-altering and preventable: what can we do about it? [J] Am. J. Obstet. Gynecol. 2024, 230(3):279–294e2. https://doi.org/10.1016/j.ajog.2023.11.1253
Fritel,Xavier et al. VarnouxSymptomatic Pelvic Organ Prolapse at Midlife, Quality of Life, and Risk Factors[J]. Obstetr. Gynecol. https://doi.org/10.1097/aog.0b013e3181985312. (2009)
Thubert, T. et al. Postpartum pelvic floor disorders[J]. La. Revue Du Praticien. 66 (2), 207–210 (2016).
De Araujo, C. C. et al. Does vaginal delivery cause more damage to the pelvic floor than cesarean section as determined by 3D ultrasound evaluation? A systematic review[J]. Int. Urogynecol. J.29 (5), 639–645 (2018).
Zhu, L. et al. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China.[J].Menopause-the. J. North. Am. Menopause Soc.16 (4), 831–836 (2009).
Yang, F. & Hongyu, L. The Influence of Obstetric Factors on the Occurrence of Pelvic Floor Dysfunction in Women in the Early Postpartum Period[J]. International Journal of General Medicine. 25(15), 3353–3361. https://doi.org/10.2147/IJGM.S355913 (2022) (eCollection).
Zhang Xinling & Practical ultrasound diagnosis of pelvic floor [M]. first edition. Beijing: People’s Medical Publishing House, :38. (2019).
Li, M. W., Liu, X. & Zhou, P. Effect of delivery mode on early pelvic floor structure in primiparous women based on pelvic floor ultrasound analysis [J]. Chin. J. Anat. Clin. Med.26 (6), 6. https://doi.org/10.3760/cma.j.cn101202-20210415-00101 (2021).
Dietz, H. P. & Steensma, A. B. The prevalence of major abnormalities of the levator ani in urogynaecological patients[J]. Bjog Int. J. Obstet. Gynecol.113 (2), 225–230. https://doi.org/10.1111/j.1471-0528.2006.00819.x (2010).
Gyhagen, M. et al. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery.[J]. Bjog Int. J. Obstet. Gynecol.120 (2), 152–160. https://doi.org/10.1111/1471-0528.12020 (2013).
Dietz, H. P. & Simpson, J. M. .Levator trauma is associated with pelvic organ prolapse[. J] BJOG: Int. J. Obstet. Gynecol., 2008(8):115 .https://doi.org/10.1111/j.1471-0528.2008.01751.x
Kreft, M. et al. The evolution of levator ani muscle trauma over the first 9months after vaginal birth[J]. Int Urogynecol J 33(9), 2445–2453. https://doi.org/10.1007/s00192-021-05034-z (2022).
Filippova, R. D., Stepanova, N. R. & Nikiforova V N. Health status of pregnant women[J]. Wiad Lek. :596–597. (2015).
[1]He, Z. X. et al. Effect of protein or energy restriction during late gestation on hormonal and metabolic status in pregnant goats and postnatal male offspring[J]. Animal. 9 (11), 1843–1851. https://doi.org/10.1017/S1751731115001147 (2015).
Youssef, A. et al. The maternal pelvic floor and labor outcome[J]. Am. J. Obstet. Gynecol. MFM, 2021(246):100452. :https://doi.org/10.1016/j.ajogmf.2021.100452
Postpartum sexual function. ; the importance of the levator ani muscle[J]. Int. Urogynecol. J.31 (11), 2261–2267. https://doi.org/10.1007/s00192-020-04250-3 (2020).
Blomquist, J. L. C. & AlvaroHanda, M. M. Victoria L.Pelvic floor muscle strength and the incidence of pelvic floor disorders after vaginal and cesarean delivery[J]. Am. J. Obstet. Gynecol.222 (1), 62 (2020).
Wang, C. Y., Wang, Y. C. & Yu, H. X. Correlation study between serum relaxin levels in late pregnancy and postpartum pelvic floor dysfunction and pelvic floor ultrasound parameters [J]. Chin. J. Reprod. Contracept.42 (03), 261–267. https://doi.org/10.3760/cma.j.cn101441-20200810-00435 (2022).
Héctor, R. T. et al. Comprehensive evaluation of the effect of bariatric surgery on pelvic floor disorders[J]. Surg Obes Relat Dis Official J Am Soc Bariatr Surg 12(1), 138–143. https://doi.org/10.1016/j.soard.2015.08.499 (2016).
Guo, X. F. & Ding, C. W. Zhang.4D Transperineal Ultrasound for the diagnosis and classification of stress urinary incontinence in Postmenopausal Women[J]. Coll. Physicians Surg. Pak. 33 (4), 438–442. https://doi.org/10.29271/jcpsp.2023.04.438 (2023).
Lou, Y. L. et al. Establishment and validation of a risk column chart for postpartum stress urinary incontinence [J]. Chin. J. Urol.42 (8), 6. https://doi.org/10.3760/cma.j.cn112330-2020422-00320 (2021).
Zhao, B. H. et al. QL Pelvic floor ultrasound study of the relationship between urethral morphology and motility and female stress urinary incontinence. Chinese J Ultrasound Imaging 30(7), 5. https://doi.org/10.3760/cma.j.cn131148-2021224-00968 (2021).
Bahrami, S. et al. Pelvic floor ultrasound: when, why, and how?[J]. Abdominal Radiol 45(7). https://doi.org/10.1007/s00261-019-02216-8. (2020).
Acknowledgements
We appreciated Jie Zhang for Language polish for the manuscript.
Statement
I promise that all experimental protocols were approved by the institutional review board of Affiliated Matern&Child Care Hospital of Nantong University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by Nantong Science and Technology Bureau Social Livelihood Science and Technology Plan Project (Grant No: MSZ2022035)and Nantong Youth Talent Project(Grant No: YQY202310)and Nantong Innovation Team Project(Grant No: NTXK202302).
Author information
Authors and Affiliations
Contributions
Authors’ contributions:Shengnan Cai: Experimental design, case collection and article writing.Mengchu Xia:Ultrasound examination and collection of ultrasound dataYiqian Ding: Case collection.Li Zeng: Case collection and data analysis.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the order of the author names, which was incorrectly given as Li Zeng, Shengnan Cai, Mengchu Xia,Yiqian Ding.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, S., Xia, M., Ding, Y. et al. Clinical value of transperineal ultrasound in evaluating the effects of different delivery methods on the primipara pelvic floor structure and function. Sci Rep 14, 23980 (2024). https://doi.org/10.1038/s41598-024-75014-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75014-y