Abstract
The studies evaluating the impact of Her2 levels in neoadjuvant setting have conflicting data. The aim of the study was to evaluate the prognostic impact of Her2 status in early triple negative breast cancer(TNBC). In the study TNBC patients who were treated with neoadjuvant chemotherapy (NAC) and surgery were analyzed retrospectively. The primary aim of the study was to analyze the impact of Her2 status(Her2-0 and Her2-low) on pathological complete response (pCR). The secondary objectives were disease free survival (DFS) and overall survival (OS). 620 female triple negative breast cancer patients were evaluated. 427 patients (68.9%) had Her2-0 and 193(31.1%) had her2-low pathology. The pCR rates were similar between Her2-0 and Her2-low patients (33.0% vs. 27.5%, p = 0.098). Although Her2-0 group has better DFS (106 vs. 50 months, p = 0.002), in multivariate analysis it had a HR of 0.74 (p = 0.06). In addition, OS was similar (131 vs. 105 months, p = 0.13) with a HR of 0.88 (p = 0.61). In multivariate analysis; presence of LVI (HR:2.2 (95% CI 1.1–3.5) p = 0.001), Clinical stage T1/T2 (HR:0.39 (95% CI 0.2–0.6) p < 0.001) and lymph node negativity (HR:0.35 (95% CI 0.1–0.9) p = 0.03) were independent factors for OS. Although there were pathological and clinical differences, the pCR, DFS and OS were similar between Her2-0 and Her2-low TNBC patients. The importance of Her2 status of TNBC in neoadjuvant setting should be further studied.
Similar content being viewed by others
Introduction
Cancer is a serious public health problem with increasing incidence, morbidity and mortality rates all over the world1. Breast cancer (BC) is the most common cancer in women. According to Globocan 2020, 23.9% of cancers diagnosed in women in our country are BC. The second most common cause of cancer mortality in our country and in the USA is BC1,2. Breast cancer is divided into five basic subgroups based on their estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (Her2) expressions. These groups are named as luminal A, luminal B, basal-like, claudin- low and Her2 positive3. ER, PR, and HER2 status are determined by using immunohistochemistry (IHC) and/or in situ hybridization (ISH). While Her2 3 + with IHC and ISH-positive when IHC is 2 + indicates HER2-positive; HER2 IHC 0, 1 + or IHC 2 + with ISH-negative are accepted as HER2-negative. The hormone receptor (HR)-positive, Her2(-) group accounts for 60%, the HR-positive, Her2(+) group accounts for 15%, the HR-negative, Her2(+) for 5%, and the HR-negative, HER2-negative (triple-negative) groups make up 20% of all cases. Since the treatment targets and prognoses of these groups are different, there are also variations in their treatment approaches4. Although TNBC is not a single entity and its molecular subtyping has been analyzed in many studies, the most accepted classification is basal-like 1 and 2, mesenchymal-like and luminal androgen subtypes, refining it into four subgroups4,5.
In recent years, there have been studies indicating that HER2 expression levels may present distinct characteristics in terms of treatment response and prognosis. The studies concluded that there is a significant difference between Her2-0 (IHC score of 0) and Her2-low (IHC score of 1 + or 2 + without detecting ERBB2 gene amplification) patients6,7,8,9. Her2-low tumors have Her2 proteins on their cell surface, but these levels are insufficient to classify them as HER2-positive. 45–55% of all breast cancer cases are in the HER2-low status8. HER2 amplification can be found in luminal and molecular apocrine subsets. It is associated with strong androgen receptor expression in TNBC and represents 22–33% of TNBC cases10.
The prognostic properties of Her2-low group have been studied in several studies and Her2-low BC has been associated with a better overall survival (OS) when compared to her2-0 BC11. In addition, the analysis of its association with histopathological characteristics revealed that it is associated with hormone receptor positivity, lower grade, lower Ki67 and lower pCR rates12. However, in most of the studies in the literature, there is no difference in pCR rates between her2-0 and her2-low groups5,13,14,15. The study by Miglietta et al. showed more pCR rates in her2-0 (33.6%) vs. Her-2 low group (21.4%)16. In addition, in TNBC patients, a 10.4% conversion from Her2-0 to her2-low and 24.0% from Her2-low to Her2-0 were reported. In the study by Kang et al. after NAC there was a 18% conversion from Her2-0 to Her2-low and a 40% conversion from Her2-low to Her2-013. In the literature the prognostic impact of Her2-low group has been broadly studied in heterogenous groups and most of the data was explorated from the early BC series. However, the inclusion of all BC subgroups and the heterogeneity in chemotherapeutics used are important limitations of these studies. In most of the studies in the literature, ER positive and negative patients have been included. As a result, there are numerous confounders in them. TNBC patients are unique to study the prognostic impact of Her2 levels because excluding the heterogeneity from the ER/PR-positive BC characteristics and treatment modalities makes TNBC patients an ideal sample. Furthermore, the broad use of neoadjuvant strategies in TNBC and the use of pathological complete response (pCR) as a primary endpoint make them well-suited for testing the prognostic impact of HER2 levels. The only study in pure TNBC patients was performed by Demogue et al. They concluded that HER2 status was not significantly associated with pCR( 41.8% vs. 35.7%, p = 0.28)5. The aim of our study was to evaluate the prognostic impact of Her2 status in early TNBC.
Material/method
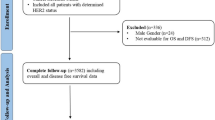
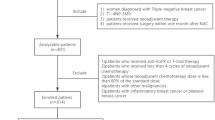
In the study TNBC patients who were treated with neoadjuvant chemotherapy (NAC) and surgery were analyzed retrospectively. The inclusion criteria were being over the age of 18, a diagnosis of breast cancer with TNBC histology (defined as ER and PR status less than 1% and HER2 0, 1 positive, or HER2 2 + with negative FISH/ISH), early stage disease (T1-4a, N0-3, M0 disease according to UICC criteria), history of neoadjuvant systemic therapy and operated after neoadjuvant therapy between January 2015- June 2016. The Exclusion criteria were metastatic disease, the patients who were not amenable to surgery after NAC, history of preoperative radiotherapy, patients with a previous cancer diagnosis or concurrent second primaries, male patients, patients with incomplete pathological data.
The study was approved by the Muğla Sıtkı Koçman University school of Medicine Clinical Research Ethical committee on 07.06.2023 with approval number of E-728855364-050.01.04-616132. After receiving approval from institutional review boards as an extension study, the data from 25 oncology centers in Turkey was collected. The data from the centers was collected between July 2023 and January 2024. Due to its retrospective design of the study informed consent was not obtained and this was also approved by Muğla Sıtkı Koçman University school of Medicine Clinical Research Ethical committee. All methods were performed in accordance with the relevant guidelines and regulations. During data collection, the patient clinical parameters, disease parameters, treatment characteristics and data for endpoints were collected. The age, ECOG performance score, menopausal status, comorbidities of the patients were noted. In terms of disease characteristics; tumor ___location, initial clinical TNM staging and histopathological properties (Her2, lymphovascular invasion (LVI), perineural invasion (PNI), androgen receptor (AR), ki67, grade) were recorded. As treatment characteristics; the NAC regimens, presence of neoadjuvant immunotherapy, the type of surgery, axillary approach, adjuvant treatment modalities were collected. The primary aim of the study was to analyze the impact of Her2 status on pCR. Her2 evaluation was conducted on biopsy specimens before neoadjuvant treatment by pathologists. HER2-low was defined as IHC 1 + or IHC 2 + without Her2 gene amplification, as measured by in situ hybridization9. IHC 0 was defined as Her2-0. Pathological complete response(pCR) was the primary objective and defined as [ypT0 or ypTis] and [pN0sn or ypN0] in pathological evaluation after surgery. The secondary objectives were disease-free survival (DFS) and overall survival (OS) of the patients. DFS was defined as the time from diagnosis to the earliest locoregional or distant disease recurrence and OS was defined as the time from diagnosis to death from any cause17.
In addition to the impact of Her2 status on pCR, DFS and OS; other clinical and histopathological parameters were included in the analysis. For the statistical purposes, they were categorized. The ages of the patients were grouped with a cutoff of 65. Menopausal status was recorded according to the “menopause” definition criteria18. Because of the high frequency of invasive ductal pathology, histopathological findings were grouped as invasive ductal vs. other. As in most studies, the Ki-67 cut-off value was set at 20%. Most patients received either conventionally scheduled or intensified dose-dense anthracycline-taxane-based neoadjuvant chemotherapy. Because dose-dense(DD) regimens are the preferred standard regimens, the analysis was performed as standard vs. others. Since the PDL-1 status was available in only 28 patients and AR positivity was present in only 126 patients, they were not included in the statistical analysis. In addition, the tumor infiltrating lymphocyte status and BRCA status were not used in the analysis due to inadequate data.
Statistical analysis
Baseline characteristics of the patient group were described using proportions for dichotomous and categorical variables. Differences between continuous variables were assessed with the Student t-test and non-parametric tests for repeated measures (Friedman Test). The chi-square or Fisher exact tests were used to analyze the relationship between parameters and pCR cases. The statistically significant factors were further tested. The multivariate analysis was performed by logistic regression modeling. The effects of factors on DFS and OS were investigated by using the log ranks test. The Kaplan- Meier survival estimates were calculated. The multivariate analysis of statistically significant parameters was further performed by Cox-regression modeling.
All analyses were performed using SPSS 22.0 for Windows (IBM Corp., Armonk, NY). P- value of less than 0.05 was considered as statistically significant.
Results
In the analysis; 620 female triple negative breast cancer patients were evaluated. The median age was 49(20–85) and 48.5% of them were post-menopausal (Table 1). 219(35.3) of them had at least one comorbidity, the most common comorbidity was hypertension (22.4%) and 98.9% of the patients had good performance scores at the initial presentation. The most common histopathology was invasive ductal carcinoma and half of the tumors originated from upper- outer quadrant. The clinical staging revealed that 64% of them were T2 and 81.1% had axillary lymph node metastases. While 338 (54.5%) patients had clinical stage II BC, 249(33.4%) had stage III BC. The pathological evaluation revealed that half of the cases were grade III and in accordance with it 87.3% of the cases had ki67 of more than 20%. There were LVI in 27.6% and PNI of 11.6% of the study population.
In the study cohort; 427 patients (68.9%) had Her2-0 and 193(31.1%) had her2-low IHK. The clinicopathological comparison of the two groups showed that there were more elderly patients (16.6 vs. 8.7, p = 0.003) and more lymph node positive patients (86.5% vs. 78.7%, p = 0.013) in her2-low group. However, PNI was less documented in her2-low patients (7.3% vs. 13.6%, p = 0.014). All other clinical and pathological features were similar between the groups. In terms of neoadjuvant treatment modalities, the patients treated with dose-dense anthracycline + Taxane regimens were more in Her2-0 group (52.2% vs. 42.5, p = 0.039) (Table 2). Only a minority of them had neoadjuvant immunotherapy(12, 1.9%). While Breast conserving surgeries were more in Her2-0 patients (47.1% vs. 33.2%, p = 0.005), lymph node dissections were similar between groups (54.9 vs. 45.1, p = 0.11).
In the analysis of pathological results; pCR rates were similar, 33.0% vs. 27.5%(p = 0.098) in Her2-0 and Her2-low groups, respectively (Fig. 1). pCR rates were similar in different her2 groups (33%, 26.8% and 28.1% in Her2-0, Her2-1 + and Her2-2+, respectively, p = 0.37). The other clinical and histopathological factors associated with pCR rates revealed that low grade (grade 1) TNBC patients had more pCR when compared to grade 2–3. (54.2% vs. 30.4, p = 0.015) (Table 3). In addition, absence of LVI or PNI were found to have more pCR rates, 37.0% and 33.0%, respectively. T1-2 tumors (34.8%) and, consistent with it, stage 1–2 TNBC cases (34.8%) had more pCR rates. For the analysis the neoadjuvant regimens were divided in to groups to compare the standard of care to others. In terms of neoadjuvant regimens, DD-regimens provided more pCR rates (38.6% vs. 24.3%, p < 0.001). 2 weekly DD-regimens were more effective when compared with 3 weekly anthracycline plus taxane combination (38.6% vs. 26.6%, p = 0.001). In the multivariate analysis of factors associated with pCR revealed a OR of 1.15 (95%CI 0.7–1.7, p = 0.47) for Her2-0 cases. Invasive ductal carcinoma histopathology (OR of 1.63, 95% CI 1.0- 2.5, p = 0.03), Grade 1 histology (OR of 2.63, 95% CI 1.08–6.3, p = 0.032), Absence of LVI (OR of 2.80, 95% CI 1.6–4.6, p < 0.01) and treatment with DD- regimen(OR of 2.06, 95%CI 1.4–2.9, p < 0.01) were associated with higher pCR rates(Table 3).
The median follow-up was 31 months (95%CI 28.3–33.6)). The median DFS was 77 months (95%CI 53.0- 100.9). 2-year and 5-year DFS rates were 72.5% and 55.5%, respectively. The median DFS was 50.0 months (95%CI 28.9–89.0) in Her2-low group and 106 months (95%CI 79.5- 132.4) in Her2-0 group (p = 0.002) (Fig. 2). 5- year DFS rates were 44.8% and 57.8% in her2-low and Her2-0 groups, respectively. Cox regression analysis showed that Her2-0 doesn’t have statistically significant impact on DFS (HR: 0.74(95% CI 0.5–1.01) p = 0.063) (Table 4). In multivariate analysis; clinical Lymph node negativity (HR: 0.48 (95% CI 0.2–0.8) p = 0.01), Ki67 levels (> 20%)(HR:0.63 (95%Cı 0.4–0.9, p = 0.016) and treatment with DD- regimen (HR:0.69 (95%CI 0.5–0.9), p = 0.02) are independent factors for DFS.
Pathological complete response was an important predictor of DFS. While mDFS of pCR patients had not been reached, non-PCR had 56 months (95% CI 44.7–67.2) of mDFS. Both in Her2-low and Her2-0 groups pCR provided better mDFS. In her2-low patients; mDFS of 32 months vs. NR and in her2-0 patients 58 months vs. NR (p < 0.001) (Fig. 3).
The median OS was 131 months (95% CI 97.1- 164.8). The 5-year and 10-year OS rates were 74.6% and 34.3%, respectively. The median OS was 105 months (95%Cı 95.3- 114.6) in Her2-low group and 131 months (95% CI 95.7- 166.2) in Her2-0 group(p = 0.13). 5-year OS rates were 62.8% and 75.7% in Her2-low and Her2-0 groups, respectively. In the multivariate analysis of the factors associated with OS revealed a HR:0.88 (95%CI 0.5–1.4, p = 0.61) for Her2-O group (Table 4). Presence of LVI (HR:2.2 (95% CI 1.1–3.5) p = 0.001), Clinical stage T1/T2 (HR:0.39 (95% CI 0.2–0.6) p < 0.001) and lymph node negativity (HR:0.35 (95% CI 0.1–0.9) p = 0.03) were independent factors for OS.
Discussion
Recently there have been numerous data indicating that Her2-low BC is a heterogenous group in terms of prognosis and systemic treatment response8. With the documentation of anti-her2 therapies in Her2-low metastatic BC, more data has emerged and directed our attention to neoadjuvant setting. Until now, the HER2 test has been examined in all patients as it is a prognostic and predictive marker in patients diagnosed with invasive BC. Indeed, the impressive outcomes of clinical trials involving new antibody-drug conjugates like trastuzumab deruxtecan and trastuzumab duocarmazine have sparked increased interest in the HER2-low subgroup19. This has opened up a field of research to explore whether this group may actually represent a distinct biological subtype. Therefore, we aimed to investigate the effect of HER2 status on pCR in locally advanced BC patients who received neoadjuvant chemotherapy. In our study, we aimed to analyze the prognostic impact of Her2 status in 620 early triple negative breast cancer patients. The baseline characteristics of Her2-0 and Her2-low patients were similar, except the percentage of elderly patients, clinical lymph node positive patients and PNI present patients. The pCR rates were similar between Her2-0 and Her2-low patients (33.0% vs. 27.5%, p = 0.098). Although Her2-0 group has better DFS (106 vs. 50 months, p = 0.002), in multivariate analysis it had a HR of 0.74 (p = 0.06). In addition, OS was similar (131 vs. 105 months, p = 0.13) with a HR of 0.88 (p = 0.61).
Many studies have defined the Her2-low group as a distinct subgroup with different characteristic features14,15. The analysis of histopathological characteristics revealed that it is associated with more hormone receptor positivity15,20,21,22,23,24, lower grade15,20,21,22,23,24,25,26, lower Ki6712,15,20,23,24,25, more IDC histopathology20,23,27, more LVI25. In terms of clinical characteristics, Her2 low pathologies were associated with postmenopausal patients28, more T1/T2 disease25,26, lower N0 frequency at the initial diagnosis23,25,26 and obese patients25. In our cohort, there were more elderly patients (16.6% vs. 8.7%, p = 0.003) and LN positive patients (86.5% vs. 78.7%, p = 0.013) in her2-low group. In addition, they have less PNI in the initial pathological evaluation (7.3% vs. 13.6%, p = 0.014).
In early TNBC, the standard treatment strategy has been neoadjuvant therapy for many years. Residual cancer burden and pCR are highly prognostic for disease recurrence and survival. As a result, pCR is the primary objective in most of the neoadjuvant trials29. The impact of pCR on DFS has been clearly documented in our study (Fig. 3). In the trials evaluating the impact of Her2 status in neoadjuvant setting, pCR has been used as the primary objective. However, nearly all neoadjuvant studies have included both the HR positive and negative patients. Because of the heterogeneity of the cohorts, the statistical powers of the studies are low. In our study, especially including triple negatives, even excluding the ER 1–10% patients, we have provided an important data for TNBC.
In the literature, the rates of pathological complete response (pCR) between the HER2-low and HER2-zero groups are contradictory. However, in most of the studies in the literature, there is no difference in pCR rates between her2-0 and her2-low groups5,13,14. When triple negative subgroups are analyzed in those studies, in the majority of them pCR are rates are similar between Her2-0 and Her2-low patients6,15,20,21,22,28,30,31. In these studies, pCR rates ranges between 14.2 and 51%. Because all of these studies were in retrospective design, the heterogeneity in treatment modalities which are missing in most of them, may be the cause of this broad range of pCR.
After the integration of DD- regimens into practice, we have obtained pCR rates of > 50%. When we have a look at the highest pCR studies, Leite et al. showed 51% vs. 47% pCR in Her2-low and Her2-0, respectively(p = 0.64)30. 52.7% of the patients were treated with standard DD regimens. Ilie et al. reported 47% vs. 35% pCR rates in Her2-0 and Her2-low cases, respectively(p = 0.08)31. In this study, DD- regimens were not mentioned. 46% of them were treated with neoadjuvant anthracycline + taxanes. In the study by Domergue et al., in which solely TNBC patients were included, pCR rates were 35.7% vs. 41.8% in her2-low and Her-0 patients, respectively(p = 0.28)5. In this cohort, only 13% of the patients were treated with DD- regimens. In our analysis, DD regimens were found to be independent predictor of pCR. In consistent with the previous data, we had also similar pCR rates in Her2-0 and Her2-low patients (33.0% vs. 27.5%, p = 0.098). Although %41.5 of the patients were treated with DD regimens and 40.8% with 3 weekly anthracycline- taxane combination, our pCR rates were low as expected. This could be related with the histopathological differences of our patients when compared to the literature. Only 1.3% of the patients had immunotherapy which has not been discussed in the previous studies. However, it is very low to be included in the analysis. In multivariate analysis of factors predicting pCR, IDC histology, grade 1 cases, absence of LVI and treatment with DD regimens were found to be independent factors.
The impact of Her2 status on DFS and OS was the secondary objectives of our study. Similar analyses have been performed in previous mentioned studies. In most of the studies, the multivariate analyses concluded that DFS and OS were similar between Her2-0 and Her2-low patients15,22,23,28,30,31. Some of the studies showed a better DFS and OS in Her2-low patients in multivariate analysis20,21,23,25. Till now, there are two meta-analysis that have analyzed these heterogenous studies. The meta-analysis by Tang et al. revealed that Her2-low HR negative patients had better DFS and pCR rates. But, the OS was similar11. The meta-analysis by Ergun et al. showed that there was no difference between HER2-low and HER2-zero in terms of pCR in early-stage TNBC. However, HER2-low was found to be associated with prolonged DFS and OS26. When compared to data, in our cohort pCR rates were higher in Her2-0 group, but statistically non-significant. It was same in the study which was held only in TNBC by Domergue et. al5. They have also presented statistically non-significant better DFS and OS in Her2-0 cases. Our data confirmed the results of this unique study with a higher sample size. In the multivariate analysis of factors predicting OS, presence of LVI showed poor survival outcomes, as expected. In addition, T1/T2 stage and absence of clinical lymph nodes at the initial evaluation resulted in better OS. The history of DD- regimen was statistically insignificant (HR:1.1, p = 0.49).
Anthracyclines and taxanes were the most preferred chemotherapy regimen, with dose-dense regimens being slightly more preferred in HER2-0 compared to the HER2-low group (52% vs. 41.5%). We believe that patients with Her2 0 were in more advanced stages at diagnosis, which is why clinicians preferred dose-dense treatments more in these patients. Although this may introduce a bias in the HER2-0 group, it has not resulted in a difference in terms of pCR between the HER2-0 and Her2-low groups. Since the responses to neoadjuvant treatment were not different between the two groups, there was no difference between the groups in terms of the preferred surgery and LND, but HER2-0 patients had slightly more breast conserving surgery when compared to HER2-low group, probably reflecting the higher proportion of pCR in HER2-0 group. Especially the Helena research and other previous studies, because of their retrospective design, treatment regimens were not standard and DD- regimens constitute a small part of the analyses. For example in the study by Jin et al. the pCR rates are similar in her2-0 and her2-low patients15 They put ER status of less than 10% into ER- group and the standard neoadjuvant regimens are TEC and EC which are not the current practice. In our study, the use of more standardized regimens is an important strength of it when compared to previous studies.
In the last decade there has been a surge in the anti-Her2 treatment options. As we have been trying to understand the characteristics of her2-low BC, a new definition for her2-0 emerged. “Her2 ultra low” is defined to select a group of patients who can be for antibody- drug conjugates against Her2. It is immunohistochemically characterized by faint/barely perceptible and incomplete staining in < 10% of tumor cells without amplification on FISH32. We have still limited data about its prognostic impact. In the following years, we will learn learn more about its properties in TNBC.
Although our study has numerous strengths, there were also a few limitations. While trying to get more sample size, we have collected data from 25 centers. Some of the patient records were missing. Because of that, TIL, androgen receptor, BRCA status and PD-L1 levels couldn’t be included in the analysis. In addition, pathology specimens were evaluated by different pathology centers. Unfortunately, we couldn’t get a Her2 retesting in one pathology center and a central laboratory revision. There is a well-defined discordance between institutions in terms Her2. This discordance has been reported to be higher in Her2-0 and Her2- low patients. So, the accuracy of Her2 testing may have confounded the results. The post-surgery IHC of Her2 also couldn’t be included in the analysis, because of the missing data. This may provide important data in terms of prognosis.
Conclusion
In our study, we analyzed the prognostic impact of Her2 status in 620 early triple negative breast cancer patients. Although there were pathological and clinical differences, the pCR, DFS and OS were similar between Her2-0 and Her2-low patients. The importance of Her2 status in neoadjuvant setting should be further studied.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to ethical issues but are available from the corresponding author on reasonable request.
Change history
18 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-80587-9
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249 (2021).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70 (1), 7–30 (2020).
Holliday, D. L. & Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 13 (4), 215 (2011).
Korde, L. A. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast Cancer: ASCO Guideline. J. Clin. Oncol. 39 (13), 1485–1505 (2021).
Domergue, C. et al. Impact of HER2 status on pathological response after Neoadjuvant Chemotherapy in Early Triple-negative breast Cancer. Cancers (Basel); 14(10), 2509 (2022).
Cherifi, F. et al. HELENA: HER2-Low as a prEdictive factor of response to neoadjuvant chemotherapy in eArly breast cancer. BMC Cancer. 22 (1), 1081 (2022).
Zhang, H., Katerji, H., Turner, B. M., Audeh, W. & Hicks, D. G. HER2-low breast cancers: incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod. Pathol. 35 (8), 1075–1082 (2022).
Tarantino, P. et al. HER2-Low breast Cancer: pathological and clinical Landscape. J. Clin. Oncol. 38 (17), 1951–1962 (2020).
Tarantino, P. et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann. Oncol. 34 (8), 645–659 (2023).
Schettini, F. et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 7 (1), 1 (2021).
Tang, Y. et al. The association between HER2-low expression and prognosis of breast cancer: a systematic review and meta-analysis. Ther. Adv. Med. Oncol. 15, 17588359231156669 (2023).
Qiao, W., Guo, W., Liu, Q., Guo, X. & Deng, M. Pathological complete response and prognosis after neoadjuvant chemotherapy in patients with HER2-low breast cancer. Ann. Diagn. Pathol. 64, 152125 (2023).
Kang, S. et al. Prognostic implications of HER2 changes after neoadjuvant chemotherapy in patients with HER2-zero and HER2-low breast cancer. Eur. J. Cancer. 191, 112956 (2023).
Li, J. J., Yu, Y. & Ge, J. HER2-low-positive and response to NACT and prognosis in HER2-negative non-metastatic BC. Breast Cancer. 30 (3), 364–378 (2023).
Jin, Y., Lan, A., Dai, Y., Jiang, L. & Liu, S. Comparison of the pCR rate and DFS among breast Cancer patients with different hormone receptor and HER2 statuses. Breast Cancer (Dove Med. Press). 15, 327–335 (2023).
Miglietta, F. et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer. 8 (1), 66 (2022).
Gourgou-Bourgade, S. et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (definition for the Assessment of Time-to-event endpoints in CANcer trials). Ann. Oncol. 26 (12), 2505–2506 (2015).
Denlinger, C. S. et al. Version 2.2017, NCCN Clinical Practice guidelines in Oncology. J. Natl. Compr. Canc Netw. 15 (9), 1140–1163 (2017).
Modi, S. et al. Trastuzumab Deruxtecan in previously treated HER2-Low advanced breast Cancer. N Engl. J. Med. 387 (1), 9–20 (2022).
Denkert, C. et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 22 (8), 1151–1161 (2021).
Tan, R. et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 20 (1), 105 (2022).
de Nonneville, A. et al. Pathological complete response rate and disease-free survival after neoadjuvant chemotherapy in patients with HER2-low and HER2-0 breast cancers. Eur. J. Cancer. 176, 181–188 (2022).
Wu, Q. et al. Analysis of clinicopathological characteristics and prognostic factors of early-stage human epidermal growth factor receptor 2 (HER2)-low breast cancer: compared with HER2-0 breast cancer. Cancer Med. 12 (19), 19560–19575 (2023).
Zhou, S. et al. Comparison of clinicopathological characteristics and response to neoadjuvant chemotherapy between HER2-low and HER2-zero breast cancer. Breast. 67, 1–7 (2023).
Won, H. S. et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean breast Cancer Society. Breast Cancer Res. 24 (1), 22 (2022).
Ergun, Y., Akagunduz, B., Karacin, C., Turker, S. & Ucar, G. The Effect of HER2-Low status on Pathological Complete Response and survival in Triple-negative breast Cancer: a systemic review and Meta-analysis. Clin. Breast Cancer. 23 (6), 567–575 (2023).
Mutai, R. et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 60, 62–69 (2021).
Ma, Y. et al. Prognostic value of the evolution of HER2-Low expression after Neoadjuvant Chemotherapy. Cancer Res. Treat. 55 (4), 1210–1221 (2023).
Spring, L. M., Bar, Y. & Isakoff, S. J. The evolving role of Neoadjuvant Therapy for operable breast Cancer. J. Natl. Compr. Canc Netw. 20 (6), 723–734 (2022).
de Moura Leite, L. et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res. Treat. 190 (1), 155–163 (2021).
Ilie, S. M. et al. Pathologic complete response and survival in HER2-low and HER2-zero early breast cancer treated with neoadjuvant chemotherapy. Breast Cancer 30(6), 997–1007 (2023).
Sajjadi, E., Venetis, K., Ivanova, M. & Fusco, N. Improving HER2 testing reproducibility in HER2-low breast cancer. Cancer Drug Resist. 5 (4), 882–888 (2022).
Author information
Authors and Affiliations
Contributions
All the authors contributed the study design and data collection process. NÖ and AA wrote the manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Esra Aşık which was incorrectly given as Esra Aydın.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Özyurt, N., Alkan, A., Gülbağcı, B. et al. The prognostic impact of Her2 status in early triple negative breast cancer: a Turkish Oncology Group (TOG) study. Sci Rep 14, 23556 (2024). https://doi.org/10.1038/s41598-024-75293-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75293-5