Abstract
To evaluate the prognosis and influencing factors of retinal vein occlusion (RVO) in patients with concomitant carotid artery disease receiving anti-vascular endothelial growth factor (VEGF) treatment. Patients diagnosed with RVO and receiving anti-VEGF treatment were included. Eye and clinical data were collected. The patients were divided into a group with concomitant carotid artery disease (Group A) and a group without concomitant carotid artery disease (Group B). The risk factors affecting the visual prognosis of RVO patients with concomitant carotid artery disease were analyzed. Among 177 eligible patients with RVO, 101 had concomitant carotid artery disease (Group A), while 76 did not (Group B). Group A had a significantly lower treatment effectiveness rate than Group B (P < 0.001). The age and platelet distribution width of Group A were significantly higher than Group B (P < 0.001). Multivariate logistic regression analysis showed that baseline best-corrected visual acuity (BCVA), disorganization of retinal inner layers (DRIL), external limiting membrane (ELM) disruption, and red blood cell distribution width (RDW) were significantly associated with the posttreatment visual prognosis of RVO patients with concomitant carotid artery disease(P < 0.05). RVO patients with concomitant carotid artery disease had a significantly lower treatment effectiveness rate than RVO patients without carotid artery disease. The poor baseline BCVA, DRIL, ELM disruption, and a greater RDW are risk factors for low anti-VEGF treatment efficacy among RVO patients with concomitant carotid artery disease.
Similar content being viewed by others
Introduction
Retinal vein occlusion (RVO) is a common retinal vascular disease, and its risk increases with age. Previous studies have shown that the prevalence of branch RVO (BRVO) among white individuals is 0.6–2%, while that of central RVO (CRVO) is 0.1–0.4%, with annual incidence rates of 0.12% and 0.03%, respectively1,2. RVO is a common cause of visual impairment and even loss of vision.
Previous studies have shown that vascular endothelial growth factor (VEGF) plays a significant role in the occurrence of RVO. Anti-VEGF therapy for macular edema caused by RVO is an important means of improving visual acuity3. However, some patients respond poorly to anti-VEGF treatment. Macular edema in some patients may not disappear, even after multiple intravitreal anti-VEGF injections. Moreover, even if it completely disappears, some patients still have poor visual prognoses. Therefore, paying attention to the visual prognosis and influencing factors of RVO patients receiving anti-VEGF treatment is vital for the development of RVO treatment strategies.
The exact pathogenesis of RVO is not yet fully understood, but it is known that arteriosclerosis is an important risk factor4. Changes in the carotid artery are considered an important indicator of the degree of systemic atherosclerosis4. However, research on the relationship between carotid artery disease and RVO is relatively limited. Studies using digital subtraction angiography and histopathological analyses have suggested a correlation between carotid artery lesions and the pathogenesis of ischemic RVO5,6. In a study of 57 RVO patients, Matsushima et al. found that the incidence of ischemic-type RVO was significantly higher in patients with concomitant carotid artery4disease than in those without this condition4. Moreover, in a study on prognostic factors for poor visual acuity in patients with CRVO, Nagasato et al. found a significant correlation between concomitant internal carotid artery disease and final visual impairment7. However, no study has systematically examined the visual prognosis and influencing factors of RVO patients with concomitant carotid artery disease.
In this study, a comprehensive investigation of the visual prognosis and influencing factors of RVO patients receiving standard anti-VEGF treatment was conducted. The visual prognoses and influencing factors of RVO patients with and without carotid artery lesions were evaluated. The results can provide a reference for the management of RVO patients.
Methods
Study design and patients
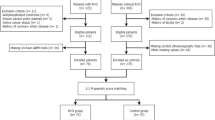
This study included patients diagnosed with RVO and receiving anti-VEGF treatment at the Sixth Affiliated Hospital of Guangzhou Medical University from June 2018 to June 2023. Patients with other diseases that might affect best-corrected visual acuity (BCVA) and those with unclear optical coherence tomography (OCT) images and/or missing clinical data were excluded. A total of 213 RVO patients receiving anti-VEGF treatment were evaluated for inclusion in this study. After excluding 36 patients with unclear OCT images and/or missing clinical data, 177 patients (177 eyes) were included in the analysis. The situation of the included patients is shown in Supplemental Fig. 1. The eligible patients were divided into patients with and without concomitant carotid artery disease and patients with good and poor responses to anti-VEGF treatment.
This was a retrospective study. This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Qingyuan Hospital of Guangzhou Medical University (IRB-202204-K15). The Ethics Committee waived informed consent. Prior to surgery, written informed consent was obtained from all patients who were recruited for this study.
Carotid ultrasound examination
Carotid ultrasound examinations of all patients were conducted by a doctor from the Ultrasound Department of Qingyuan Hospital affiliated with Guangzhou Medical University using diagnostic ultrasound equipment (Hitachi ARIETTA70, 10-MHz probe, Japan). A diagnosis of carotid artery disease was made on the basis of the identification of a plaque or stenosis. A plaque was defined as a localised protrusion in the intima media of the carotid artery, with a thickness of ≥ 2.1 mm4. The primary diagnostic and grading tools for internal carotid artery stenosis are the internal carotid artery peak systolic velocity (PSV) and the presence of plaque on grey-scale and/or colour Doppler images8. The degree of carotid artery stenosis was stratified into the following categories: normal (no stenosis), < 50% stenosis, 50-69% stenosis, ≥ 70% stenosis to near occlusion, near occlusion, and total occlusion8.
Anti-VEGF therapy
Antibiotic eye drops were used four times a day for three days before anti-VEGF injection. Proparacaine hydrochloride eye drops were used to induce surface anesthesia. The syringe needle was then inserted into the vitreous cavity aligned with the center of the affected eyeball 4.0 mm behind the scleral margin, and the anti-VEGF drug (ranibizumab or conbercept; 0.5 mg/0.05mL) was slowly injected. The anti-VEGF treatment followed a previous report according to which, after the initial treatment, patients should be followed up every four weeks to determine whether another injection was required. Patients with a central retinal thickness (CRT) of more than 250 μm or decreased BCVA should receive a new injection9. The same protocol was used for BRVO and CRVO injections. For patients with persistent and/or recurrent in edema, a combination intravitreal injection of dexamethasone was recommended10.
RVO determination and image grading
OCT images were obtained using a Cirrus HD-OCT scanner (Carl Zeiss Meditec, Inc., Dublin, CA, USA) and independently reviewed by two experts. The OCT image analysis was mainly based on the following criteria: baseline CRT, possible disorganization of retinal inner layers (DRIL), possible external limiting membrane (ELM) disruption, possible presence of intraretinal or subretinal fluid, and CRT at treatment end.
The CRT was defined as the average thickness of the central 1000 μm circle of the retina11. DRIL was defined as an alteration in the normal structural arrangement of the internal retinal layers, characterized by unrecognizable boundaries between ganglion cells, the inner plexiform layer, and the outer plexiform layer11. ELM disruption was defined as the presence of discontinuous signals or abnormal signal intensity in the ELM compared to the perimacular area.
Data collection
Detailed information on each RVO patient was collected. This information included two main aspects: the whole body and the eyes. Biological and systemic factors included gender, age, smoking history (yes/no), hypertension (yes/no), hyperlipidemia (yes/no), diabetes (yes/no), carotid artery disease (yes/no), coronary artery disease (yes/no), dialysis (yes/no), other systemic diseases, average platelet volume, platelet distribution width (PDW), hematocrit, red blood cell distribution width (RDW), ratio of granulocytes to lymphocytes, and ratio of platelets to lymphocytes. Ocular factors included eye type, RVO type (CRVO/BRVO), course of the disease, baseline BCVA, baseline CRT, DRIL (yes/no), ELM disruption (yes/no), presence of intraretinal or subretinal fluid (yes/no), frequency of anti-VEGF injections, BCVA at treatment end, CRT at treatment end, and presence of ocular complications (yes/no).
Statistical analysis
All data were statistically analyzed using Stata 15.0 software (Stata Corp., College Station, TX, USA). A t-test was used for RVO patients with concomitant carotid artery disease vs. patients without concomitant carotid artery disease and patients who responded well to anti-VEGF treatment vs. patients who did not respond well to treatment of normally distributed quantitative variables. Wilcoxon rank-sum test was used to compare qualitative and quantitative variables that did not show a normal distribution. The chi-squared test was used to compare categorical variables. Logistic regression was used to evaluate the factors affecting the efficacy of anti-VEGF therapy. Therapeutic efficacy was mainly assessed based on a comparison between pre- and post-treatment BCVA. For the purposes of statistical analysis, the BCVA was converted to the logarithm of the minimal angle of resolution (logMAR). Following a previous report, effective treatment was defined as a posttreatment BCVA increase of ≥ 2 lines compared to the baseline12. And the treatment effect was identified at 6 months after the beginning of anti-VEGF treatment9. P-values of < 0.05 were considered statistically significant.
Results
Table 1 shows the patients’ baseline characteristics. There were statistically significant differences (P < 0.05) in baseline BCVA and anti-VEGF injection frequency between the patients who responded well to treatment and those who did not. The patients who did not respond well to treatment had significantly higher proportions of DRIL, ELM disruption, concomitant carotid artery disease, and dialysis than those who did (P < 0.05). The hematocrit of the patients who did not respond well to treatment was significantly lower than that of the patients who did (P < 0.05).
Supplemental Table 1 presents the results of the multivariate logistic regression analysis of the factors related to the efficacy of RVO anti-VEGF treatment. Baseline BCVA (odds ratio [OR]: 26.96, 95% confidence interval [CI] : 6.68–108.78; P < 0.001), DRIL (OR: 12.51, 95% CI: 3.89–40.28; P < 0.001), ELM disruption (OR: 11.27, 95% CI: 3.61–35.25; P < 0.001), frequency of anti-VEGF injections (OR: 1.34, 95% CI: 1.06–1.69; P < 0.05), carotid artery lesions (OR: 5.75, 95% CI: 2.11–15.71; P < 0.05), and RDW (OR: 0.61, 95% CI: 0.41–0.90; P < 0.05) significantly correlated with the efficacy of RVO anti-VEGF treatment. Furthermore, patients were further divided into CRVO and BRVO subgroups, and ELM disruption (OR: 3.89, 95% CI: 1.20–12.57; P < 0.05) was detected significantly associated with the efficacy of anti-VEGF treatment in CRVO patients (Supplemental Table 2), while baseline BCVA (OR: 16.68, 95% CI : 2.83–98.17; P < 0.05), DRIL (OR: 39.98, 95% CI: 3.52–453.85; P < 0.05), and carotid artery lesions (OR: 6.02, 95% CI: 1.28–28.45; P < 0.05) were detected significantly associated with the efficacy of anti-VEGF treatment in BRVO patients (Supplemental Table 3).
Figure 1 displays an example of OCT images showing DRIL, ELM disruption, intraretinal fluid, and subretinal fluid. Figure 2 shows representative pre- and posttreatment OCT images of patients who responded well to treatment and patients who did not.
Representative OCT scans were taken before and after the anti-VEGF injection. (A) Pre-treatment representative OCT scans of patients who responded well to treatment, (B) Representative OCT scans of patients who responded well to treatment at 6 months after the beginning of anti-VEGF treatment, (C) Pre-treatment representative OCT scans of patients with ineffective treatment, (D) Representative OCT scans of patients with ineffective treatment at 6 months after the beginning of anti-VEGF treatment.
The 177 RVO patients were divided into a group with concomitant carotid artery disease (Group A) and a group without this condition (Group B). Group A consisted of 101 patients, while Group B had 76 patients. Table 2 shows a comparison of the clinical characteristics of the patients in Group A and Group B. The age and PDW of Group A were significantly higher than those of Group B (P < 0.001). Posttreatment BCVA was significantly poorer in Group A than in Group B (P < 0.001).
Table 3 shows a comparison of the treatment outcomes of RVO patients stratified according to the presence or absence of carotid artery lesions. Among the 177 patients, 65 showed significantly improved vision after anti-VEGF treatment, with a treatment effectiveness rate of 36.72%. Treatment efficacy was significantly lower among the patients with concomitant carotid artery disease than among those without this condition (P < 0.001). Furthermore, age-matched group studies were conducted. Our results revealed that the efficacy of treatment was significantly inferior in patients with carotid artery disease compared to those without carotid artery disease both in over 60 years old group and in under 60 years old group (P < 0.05). For over 60 years old group, the treatment effect was significantly inferior in patients with carotid artery disease than that in patients without carotid artery disease (P < 0.001).
Table 4 presents the results of the multivariate logistic regression analysis of the factors related to the efficacy of anti-VEGF treatment for the RVO patients with concomitant carotid artery disease. Baseline BCVA (OR: 27.24, 95% CI: 4.33–171.50; P < 0.001), DRIL (OR: 10.06, 95% CI: 2.14–47.18; P < 0.05), ELM disruption (OR: 18.91, 95% CI: 3.77–94.85; P < 0.001), and RDW (OR: 0.41, 95% CI: 0.18–0.93; P < 0.05) significantly correlated with the efficacy of anti-VEGF therapy in this group.
Discussion
Approximately 35% of the RVO patients in this study showed significantly improved vision after anti-VEGF treatment. The posttreatment visual acuity of the patients with concomitant carotid artery disease improved by less than 20%, and their treatment effectiveness rate was significantly lower than that of the patients without concomitant carotid artery disease. Poor baseline BCVA, DRIL, ELM disruption, and a greater RDW were risk factors for poor anti-VEGF treatment efficacy among patients with concomitant carotid artery disease. The age and PDW of the patients with concomitant carotid artery disease were significantly higher than those of the patients without this condition.
The RVO patients with concomitant carotid artery disease in this study had a significantly lower anti-VEGF therapy effectiveness rate than the patients without carotid artery disease. This is consistent with Nagasato et al.’s study, which found that concomitant internal carotid artery disease significantly correlated with final visual acuity in patients with CRVO, suggesting that it was a prognostic factor for poor vision7. Previous studies have suggested a correlation between the presence of carotid artery lesions and the pathogenesis of ischemic RVO5,6. Using fluorescein angiography, Matsushima et al. also showed that the incidence of ischemic-type RVO among patients with concomitant carotid artery disease was significantly higher than among patients without this condition. Among the CRVO cases, no patient with concomitant carotid artery disease had a final decimal visual acuity of ≥ 0.8, which was significantly lower than that of the patients without carotid artery disease4.
The ophthalmic artery is a branch of the internal carotid artery, and carotid artery stenosis is associated with ischemic diseases of the eye13. Karamia et al. conducted clipping or suturing ligation of bilateral common carotid arteries in an experimental rat model of retinal ischemia and found that the retinal oxygen transport and oxygen metabolism rates of the experimental group were significantly lower than those of the control group14. Drakopoulos et al. found a local decrease in the linear density of the retinal superficial capillary plexus in patients with carotid artery stenosis15. Kang et al. showed that symptomatic patients with carotid artery stenosis had a significantly lower average subchondral choroidal thickness compared with the control group eyes and that the choroidal and lumen areas were significantly smaller than those of the unaffected contralateral eyes16. Therefore, we speculate that changes in the retinal vascular system caused by carotid artery disease may accelerate the deterioration of retinal microcirculation, ultimately leading to the disruption of the automatic regulatory system of retinal blood vessels, thereby affecting the efficacy of anti-VEGF treatment for RVO patients.
In this study, multivariate logistic regression showed that poor baseline BCVA, DRIL, ELM disruption, and a greater RDW were risk factors for poor anti-VEGF treatment efficacy among RVO patients with concomitant carotid artery disease. Previous studies have also found a significant correlation between baseline BCVA and the prognosis of RVO patients7,17. Baseline BCVA is an important predictor of posttreatment visual acuity improvement in RVO patients. Janusckowski et al. conducted a multicenter study of 316 eyes treated with intravitreal bevacizumab injections for macular edema caused by CRVO, with a follow-up period of 24–48 weeks. Multiple regression analysis confirmed that baseline BCVA was an important prognostic factor for visual improvement18. Similarly, in a study of prognostic factors for extremely poor vision in CRVO patients, Nagasato et al. found that patients with extremely poor vision had significantly worse baseline BCVA than controls7. In a retrospective study, Lloyd et al. showed that better baseline BCVA was associated with faster visual recovery19. Good baseline vision in patients with retinal vascular diseases may indicate lower disease severity and a lower likelihood of irreversible damage to photoreceptors or other retinal cells20, and thus a higher likelihood of visual improvement after treatment.
DRIL refers to an alteration in the normal structural arrangement of the internal retinal layers. The ELM is a small band attached between photoreceptors and retinal cells that reflects the functional characteristics of photoreceptors. To a certain extent, DRIL and ELM disruption indicate worse baseline visual acuity and, consequently, a poorer prognosis21,22.
RDW reflects variations in red blood cell volume, which increases under conditions of insufficient red blood cell generation or increased red blood cell destruction. In line with Ozkok et al.’s and Pinna et al.’s findings23,24, we found a significant correlation between RDW and the visual prognosis of RVO patients with concomitant carotid artery disease. Ozkok et al. were the first to demonstrate a correlation between RDW and visual outcomes in RVO patients23. The authors found that RVO patients had a significantly greater RDW than controls and that the greater the RDW, the lower the baseline and final visual acuity was23. Similarly, in a retrospective case–control study, Pinna et al. showed that RVO patients had significantly higher RDW values than controls24. The mechanism underlying the association between RDW and clinical outcomes in RVO patients is currently not clear, but it is believed that oxidative stress, chronic inflammation, and endothelial dysfunction may play a role23.
In our study, the RVO patients with concomitant carotid artery disease had a significantly greater PDW and were significantly older than the patients without carotid artery disease. These findings are consistent with previous studies in which the PDW of RVO patients was significantly greater than that of controls25,26. Yilmaz et al. were the first to confirm the association between PDW and RVO. In that study, patients with RVO had a significantly greater PDW than controls, and a greater PDW was associated with an 8.68 times higher risk of developing RVO27. Subsequently, Beyazyildiz et al. also showed that the PDW of BRVO patients was significantly higher than that of healthy controls25. A recent systematic review and meta-analysis concerning the relationship between the platelet index and RVO also found significantly higher PDW values in RVO patients than in controls26. PDW is an indicator of platelet size changes and is considered a more specific platelet activation marker than the mean platelet volume. A greater PDW may indicate impaired platelet deformability and may be associated with microvascular resistance, which may increase the risk of vascular complications28. Jindal et al. showed that patients with diabetes, especially those with microvascular complications, had a significantly greater PDW compared to the control subjects29. Moreover, Li et al. found that the average PDW of diabetic retinopathy patients with secondary neovascular glaucoma was significantly greater than that of patients with diabetes and that a greater PDW and mean platelet volume were associated with secondary neovascular glaucoma in diabetic retinopathy30. Therefore, we speculate that in RVO patients with concomitant carotid artery disease, an increase in PDW may increase retinal ischemia and even neovascularization complications, ultimately leading to poor visual outcomes. Elderly patients’ poorer responses to anti-VEGF treatment may be related to age-related changes in the vitreous structure, such as changes in collagen fibrils and hyaluronic acid components, which affect the solubility and diffusion rate of anti-VEGF drugs and increase their elimination rate31,32. Moreover, a decrease in retinal blood flow in elderly individuals may accelerate the release of VEGF, which may be one of the reasons for the poorer response of elderly patients to anti-VEGF treatment20.
A limitation of our study is that due to its retrospective design, long-term follow-up of RVO patients with concomitant carotid artery disease after anti-VEGF treatment was not possible. Moreover, all data were sourced from a single center, and although we performed multivariate logistic regression analyses of factors that might affect the efficacy of anti-VEGF treatment for RVO patients with concomitant carotid artery disease, the results may have been influenced by confounding factors. Therefore, large-scale multicenter studies are needed.
A strength of this study is that it is the first to compare the efficacy of anti-VEGF treatment as well as clinical characteristics between RVO patients with and without carotid artery disease. Moreover, we comprehensively evaluated ocular and systemic factors that might affect the visual prognosis of patients with concomitant carotid artery disease.
In summary, our findings indicate that some RVO patients respond poorly to anti-VEGF treatment, even after multiple intravitreal injections. Furthermore, the prognosis of patients with concomitant carotid artery disease is worse than that of patients without this condition. Poor baseline BCVA, DRIL, ELM disruption, and a greater RDW are risk factors for a poor response to anti-VEGF treatment among RVO patients with concomitant carotid artery disease. Moreover, patients with concomitant carotid artery disease exhibit a significantly greater PDW than patients without this condition. Ophthalmologists should pay attention not only to the eye examination results of RVO patients but also to changes in systemic indicators. A detailed evaluation of eye and systemic biomarkers may facilitate risk assessment and patient management decision-making.
Data availability
Data is provided within the manuscript or supplementary information files. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cugati, S., Wang, J. J., Rochtchina, E. & Mitchell, P. Ten-year incidence of retinal vein occlusion in an older population: The Blue mountains Eye Study. Arch. Ophthalmol. 124, 726–732 (2006).
Mitchell, P., Smith, W. & Chang, A. Prevalence and associations of retinal vein occlusion in Australia. The Blue mountains Eye Study. Arch. Ophthalmol. 114, 1243–1247 (1996).
Regnier, S. A., Larsen, M., Bezlyak, V. & Allen, F. Comparative efficacy and safety of approved treatments for macular oedema secondary to branch retinal vein occlusion: A network meta-analysis. BMJ Open. 5, e007527 (2015).
Matsushima, C. et al. Relationship between retinal vein occlusion and carotid artery lesions. Retina. 27, 1038–1043 (2007).
Brown, G. C., Shah, H. G., Magargal, L. E. & Savino, P. J. Central retinal vein obstruction and carotid artery disease. Ophthalmology. 91, 1627–1633 (1984).
Green, W. R., Chan, C. C., Hutchins, G. M. & Terry, J. M. Central retinal vein occlusion: A prospective histopathologic study of 29 eyes in 28 cases. Trans. Am. Ophthalmol. Soc. 79, 371–422 (1981).
Nagasato, D. et al. Factors associated with extremely poor visual outcomes in patients with central retinal vein occlusion. Sci. Rep. 10, 19667 (2020).
Grant, E. G. et al. Carotid artery stenosis: Gray-scale and doppler US diagnosis–society of radiologists in ultrasound consensus conference. Radiology. 229, 340–346 (2003).
Liu, X. et al. A retrospective study assessing the factors associated with visual outcome in retinal vein occlusion patients after anti-VEGF therapy. PeerJ. 9, e12599 (2021).
Vilela, M. A. Use of anti-VEGF drugs in retinal vein occlusions. Curr. Drug Targets. 21, 1181–1193 (2020).
Midena, E. et al. The disorganization of retinal inner layers is correlated to muller cells impairment in diabetic macular edema: An imaging and omics study. Int. J. Mol. Sci. 24 (2023).
Rayess, N. et al. Baseline choroidal thickness as a predictor for treatment outcomes in central retinal vein occlusion. Am. J. Ophthalmol. 171, 47–52 (2016).
Lee, D. et al. Ocular Ischemic syndrome and its related experimental models. Int. J. Molecular Sci. , 23 (2022).
Karamian, P. et al. Alterations in retinal oxygen delivery, metabolism, and extraction fraction during bilateral common carotid artery occlusion in rats. Invest. Ophthalmol. Vis. Sci. 60, 3247–3253 (2019).
Drakopoulos, M. et al. Swept-source optical coherence tomography angiography metrics of retinal ischaemic perivascular lesions in patients being evaluated for carotid artery stenosis and controls. BMJ Open. Ophthalmol.; 8. (2023).
Kang, H. M., Choi, J. H., Koh, H. J. & Lee, S. C. Significant changes of the choroid in patients with ocular ischemic syndrome and symptomatic carotid artery stenosis. PLoS One. 14, e0224210 (2019).
Liu, J. C. et al. Outcomes in patients with retinal vein occlusion with good baseline visual acuity. Eye (Lond). 37, 3203–3208 (2023).
Januschowski, K. et al. Predictive factors for functional improvement following intravitreal bevacizumab injections after central retinal vein occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 255, 457–462 (2017).
Lloyd Clark, W. et al. Baseline characteristics associated with early visual acuity gains after ranibizumab treatment for retinal vein occlusion. BMC Ophthalmol. 19, 11 (2019).
Yin, S., Cui, Y., Jiao, W. & Zhao, B. Potential prognostic indicators for patients with retinal vein occlusion. Front. Med. 9, 839082 (2022).
Khokhlova, D. Y., Drozdova, E. A., Kurysheva, N. I. & Loskutov, I. A. Optical coherence tomographic patterns in patients with retinal vein occlusion and macular edema treated by ranibizumab: A predictive and personalized approach. EPMA J. 12, 57–66 (2021).
Chan, E. W. et al. Disorganization of retinal inner layers and ellipsoid zone disruption predict visual outcomes in central retinal vein occlusion. Ophthalmol. Retina. 3, 83–92 (2019).
Ozkok, A., Nesmith, B. L. W. & Schaal, S. Association of red cell distribution width values with vision potential in retinal vein occlusion. Ophthalmol. Retina. 2, 582–586 (2018).
Pinna, A. et al. Mean platelet volume, red cell distribution width, and complete blood cell count indices in retinal vein occlusions. Ophthalmic Epidemiol. 28, 39–47 (2021).
Beyazyildiz, E. et al. Branch retinal vein occlusion associated with platelet activation. Turk. J. Med. Sci. 49, 283–287 (2019).
Liu, Z., Perry, L. A., & Edwards, T. L. Association between platelet indices and retinal vein occlusion: A systematic review and Meta-analysis. Retina. 41, 238–248 (2021).
Yilmaz, T. & Yilmaz, A. Altered platelet morphological parameters in patients with retinal vein occlusion. Eur. Rev. Med. Pharmacol. Sci. 20, 1934–1939 (2016).
Yaylali, Y. T. et al. Increased red blood cell deformability and decreased aggregation as potential adaptive mechanisms in the slow coronary flow phenomenon. Coron. Artery Dis. 24, 11–15 (2013).
Jindal, S. et al. Platelet indices in diabetes mellitus: Indicators of diabetic microvascular complications. Hematology. 16, 86–89 (2011).
Li, S., Cao, W. & Sun, X. Role of platelet parameters on neovascular Glaucoma: A retrospective case-control study in China. PLoS One. 11, e0166893 (2016).
Gisladottir, S., Loftsson, T. & Stefansson, E. Diffusion characteristics of vitreous humour and saline solution follow the Stokes Einstein equation. Graefes Arch. Clin. Exp. Ophthalmol. 247, 1677–1684 (2009).
Schindler, R. H., Chandler, D., Thresher, R. & Machemer, R. The clearance of intravitreal triamcinolone acetonide. Am. J. Ophthalmol. 93, 415–417 (1982).
Funding
This study was supported by the National Natural Science Foundation of China (81900841), and the Qingyuan City’s Self Funded Science and Technology Plan Project in the Field of Social Development (211029104560243).
Author information
Authors and Affiliations
Contributions
TY, YL, GJ and JL designed the study; TY, RH, RY and CZ collected the data; FZ and TY analysed and interpreted the data; TY and YL drafted the manuscript; TY, YL, GJ, JL and FZ revised the manuscript. JL and GJ are responsible for the overall content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study adheres to the tenets of the Declaration of Helsinki and was approved by the ethics committee of the Affiliated Qingyuan Hospital of Guangzhou Medical University (IRB-202204-K15). Written informed consent was obtained from the patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, T., Lu, Y., Zeng, F. et al. Prognosis and factors related to anti-VEGF therapy in patients with retinal vein occlusion and concomitant carotid artery disease. Sci Rep 14, 24634 (2024). https://doi.org/10.1038/s41598-024-75604-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75604-w