Abstract
Long Covid results from the damage caused by SARS-CoV-2, involving the release of cytokines and the continuous activation of immune cells. This cross-sectional study investigates leukocyte and cytokine profiles in Long Covid patients in the Amazon, a region where such studies are limited. Blood samples were analysed for differential leukocyte counts and cytokine levels. We suggest elevated lymphocyte counts in hospitalised patients and those with severe COVID-19. Higher eosinophil counts were observed in patients with up to three months of Long Covid, and increased monocyte counts in those with up to six months. IL-2 levels were higher in patients with fewer symptoms and Long Covid duration of more than three months, whereas IL-10 may remain elevated for up to 12 months. We suggest positive correlations between neutrophils, monocytes, eosinophils, and lymphocytes with different cytokines (IFN-γ, IL-6, IL-4, IL-17a, IL-2). Women were associated with lower hospitalisation rates and longer durations of Long Covid; increased lymphocyte counts were linked to hospitalisation due to COVID-19, while higher monocyte counts were associated with Long Covid durations of up to six months. We suggest that Long Covid patients may exhibit alterations in inflammatory markers, indicating a persistently pro-inflammatory microenvironment that tends to diminish after 12 months of Long Covid.
Similar content being viewed by others
Introduction
According to The National Institute for Health and Care Excellence, Long Covid is characterized by persistent symptoms following the acute phase (≥ 4 weeks from) of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. This condition leads to increased morbidity, affecting a large segment of the population with prolonged symptoms of the disease1,2,3. Although there is no consensus on the exact causes, a multifactorial origin is considered along with direct and indirect damage (systemic inflammation) due to SARS-CoV-2, insufficient generation of antibodies, persistence of viral residues, and chronic inflammatory state, with the release of cytokines and continuous activation of immune system cells4,5,6.
Several studies have shown that biomarkers are essential tools for predicting the prognosis of patients with COVID-19, as they remain one of the main parameters for understanding the clinical relationship between patients and the maintenance of the syndrome5,7,8,9. These studies agree on the use of inflammatory biomarkers, such as white blood cells (WBC), lymphocytes (LYM), segmented neutrophils (SEG), eosinophils (EOS), basophils (BAS), monocytes (MON), and cytokines, along with clinical characteristics, to establish the role of the inflammatory microenvironment in Long Covid and to predict a risk model for the persistence of symptoms5,7,8,9.
Different alterations in cell counts and cytokine levels can be observed in different populations. Specifically in the Amazon region, the pattern of alterations may be even more distinct, considering that the population is constantly exposed to environmental factors that directly impact biodiversity and ecosystem structure, favouring the maintenance of endemic diseases. Additionally, socioeconomic factors, such as lack of access to basic sanitation, healthcare services, and adequate nutrition, and genetic factors, considering the genetic miscegenation of the Brazilian population, also play a relevant role. These factors can alter the clinical course and prognosis of the disease8,9,10. Although studies in the Amazon region have investigated anti- and pro-inflammatory cytokine levels7,11and immune cell counts in blood (e.g., eosinophils, lymphocytes, and monocytes)9,12, none have examined cytokine levels, immune cell counts, and clinical outcomes in Long Covid together.
Thus, this study aims to fill gaps in the knowledge, aiming not only to determine the profiles of clinical and laboratory variables in patients with Long Covid in the Amazon but also to explore the pathophysiological interactions that may underlie long-term inflammatory alterations, besides outlining the level of the individual’s baseline inflammatory response and how the immune response influences the continuity of symptoms in the post-acute phase of COVID-19. Therefore, this study aimed to investigate the profiles of pro-inflammatory and regulatory cytokines and white blood cell profile along with the clinical characteristics of patients with Long Covid.
Our study holds significant relevance in the context of Long Covid, as we characterised the leukocyte and cytokine profiles of patients concerning the severity of COVID-19, duration, and number of Long Covid symptoms. We started from the hypothesis that Long Covid patients may exhibit alterations in immune cell counts and the expression of different cytokines, which may change over time. Our findings suggest that individuals who experienced more severe symptoms during COVID-19 exhibited higher neutrophil and lymphocyte counts. Additionally, you noted that the presence of certain subsets of leukocytes and cytokines varies with the duration of Long Covid. However, there was no significant relationship between the number of symptoms and cell counts or cytokine expression. This information is vital for developing accessible tests that could help identify individuals with better or worse prognoses for Long Covid.
Results
Patient characteristics and clinical features
Among the 300 individuals in the study, 205 were female, with a mean age and SD of 48.9 ± 12.7. In all, 125 individuals reported having some comorbidity, with systemic arterial hypertension being the most prevalent, and 90 participants were hospitalised with COVID-19, with a mean hospitalisation time of 18 days (SD ± 16.4). The average number of symptoms in Long Covid was 3.1 (SD ± 2), with fatigue (n = 146), mental disorders (n = 108), and loss of smell and taste (n = 78) being the most common. The duration of Long Covid ranged from 35 to 985 days, with an average of 295.5 days (SD ± 178.5) (Table 1).
Laboratory findings of patients with Long Covid
Comparison of laboratory parameters and clinical features among the studied groups
Table 2 compares four groups: hospitalised and non-hospitalised COVID-19 patients; severe and mild/moderate COVID-19 patients; number of symptoms in Long Covid and time of Long Covid, in relation to differential leukocyte counts.
Our main findings suggest higher lymphocyte counts in 43.3% (P = 0.00) of patients hospitalised with COVID-19 compared to those who were not hospitalised, as well as elevated lymphocyte counts in 47% (P = 0.00) and elevated neutrophil counts in 33.8% (P = 0.03) of patients with severe COVID-19 compared to those with mild/moderate forms.
Additionally, eosinophil counts were higher in 33.3% of patients in the group with up to three months of Long Covid (P = 0.01), while monocyte counts were lower in 63.6% (P = 0.04) of patients in that same group compared to those with more than three months. Monocyte counts were higher in 19.5% (P = 0.02) of patients with up to six months of Long Covid compared to the group with more than six months.
Our findings suggest that lymphocyte counts may be higher in patients hospitalised with COVID-19, as well as higher neutrophil and lymphocyte counts in patients with severe COVID-19. Additionally, higher eosinophil counts and lower monocyte counts may be present in patients with a shorter duration of Long Covid. However, monocyte counts may be higher among patients who progress to Long Covid for up to six months.
Comparison of cytokines expression and clinical features among the studied groups
Table 3 compares four groups: hospitalised and non-hospitalised with COVID-19 patients; severe and mild/moderate COVID-19 patients; number of symptoms in Long Covid and duration of Long Covid, in relation to median cytokines levels.
Our findings suggest higher median levels of IL-2 in patients with up to six symptoms of Long Covid compared to those with more symptoms (8.28, IQR 1.80 vs. 7.33, IQR 1.38; P = 0.02) and higher median levels of IL-2 in patients with more than three months of Long Covid compared to those with up to three months (8.32, IQR 1.80 vs. 7.75, IQR 2.08; P = 0.04). However, higher median levels of IL-10 were observed in patients with up to 12 months of Long Covid compared to those with more than 12 months (9.2, IQR 2.78 vs. 8.44, IQR 3.05; P = 0.04).
These comparisons suggest that the pro-inflammatory cytokine IL-2 was higher in patients with fewer symptoms and a shorter time of Long Covid. On the other hand, the anti-inflammatory cytokine IL-10 was higher in patients with up to 12 months of Long Covid.
No significant differences in median cytokine levels were observed between hospitalised and severe COVID-19 patients.
Correlation between different leukocyte subsets and cytokine levels
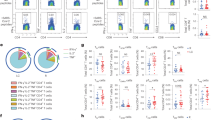
Figure 1 shows the correlation analysis between different leukocyte subsets and cytokine levels in 202 patients, according to the time of Long Covid.
Our significant findings suggest that segmented neutrophils exhibited a positive correlation with IFN-γ (r = 0.3788; P = 0.0469), IL-6 (r = 0.5619; P = 0.0019), and IL-4 (r = 0.5417; P = 0.0029). Monocytes showed a positive correlation with IFN-γ (r = 0.3941; P = 0.0380) and IL-17a (r = 0.3744; P = 0.0497). Eosinophils correlated positively with IL-6 (r = 0.4615; P = 0.0134) and IL-4 (r = 0.4063; P = 0.0319), while lymphocytes demonstrated a positive correlation with IL-2 (r = 0.3796; P = 0.0463) in patients with Long Covid for up to three months (Fig. 1a I-VIII).
Furthermore, these positive correlations were maintained between segmented neutrophils and IFN-γ (r = 0.1568; P = 0.0388) and IL-6 (r = 0.1794; P = 0.0188), as well as between monocytes and IFN-γ (r = 0.1704; P = 0.0246). A positive correlation was also observed between monocytes and IL-4 (r = 0.1493; P = 0.0492) and IL-6 (r = 0.2783; P = 0.0002) in patients with Long Covid for more than three months (Fig. 1b I-VIII). Only monocytes and IL-6 (r = 0.2540; P = 0.0020) showed a significant positive correlation in patients with Long Covid for over six months (Fig. 1c IV).
Our analyses suggest stronger positive correlations between immune cells and cytokine levels in the early stages of Long Covid, particularly among segmented neutrophils and IFN-γ, IL-6, and IL-4; monocytes and IFN-γ and IL-17a; eosinophils and IL-6 and IL-4; and lymphocytes and IL-2 in patients with a shorter duration of Long Covid. For those with Long Covid lasting more than three months, correlations were observed between segmented neutrophils and IFN-γ and IL-6, as well as between monocytes and IFN-γ, IL-4, and IL-6. Notably, only monocytes and IL-6 demonstrated a correlation after six months of Long Covid. Beyond 12 months, no significant correlations were found.
Spearman’s correlation coefficient (r) between different leukocyte counts and cytokines according to time of Long Covid [Spearman’s correlation: (a) I-V: leukocytes vs. cytokines in ≤3 months of Long Covid; (b) I-V: leukocytes vs. cytokines in >3 months of Long Covid; (c) I-V: leukocytes vs. cytokines in >6 months of Long Covid; (d) I-V: leukocytes vs. cytokines in >12 months of Long Covid. †Statistically significant].
Association between leukocyte abnormalities and clinical outcomes in the Long Covid group
Table 4 presents the multiple logistic regression analysis used to investigate the associations between three clinical outcomes: hospitalisation with COVID-19, time of Long Covid, and more than six symptoms in Long Covid, in relation to variables such as sex, age over 60 years, and higher counts of segmented neutrophils, eosinophils, basophils, monocytes, and lymphocytes.
Our analysis suggests that women were associated with a lower hospitalisation rate during COVID-19 (coefficient − 1.0322; P = 0.0002), but were more likely to experience Long Covid for over six months (coefficient 0.5515; P = 0.0488). Additionally, higher lymphocyte counts were linked to hospitalisation during COVID-19 (coefficient 0.7066; P = 0.0145), while higher monocyte counts were associated with Long Covid for up to six months (coefficient − 0.9091; P = 0.0204).
We suggest an association between female sex and reduced hospitalisation rates, alongside an increased duration of Long Covid beyond six months. Additionally, we found a association between higher lymphocyte counts and hospitalisation during COVID-19, as well as higher monocyte counts in patients with Long Covid for more than six months.
Discussion
The study aimed to explore and understand the underlying mechanisms that perpetuate Long Covid in the population of the Amazon Region and to identify the immunological processes that may be contributing to the persistent symptoms.
In this study, the majority of the patients were female and aged between 39 and 60 years. Approximately one-third were hospitalised during the acute phase of COVID-19, and the average duration of Long Covid was approximately 9 months. About 93.7% had six or fewer concomitant symptoms. Median lymphocyte counts were higher among patients hospitalised with COVID-19 compared to those not hospitalised, and both lymphocyte and segmented neutrophil counts were higher in patients with severe COVID-19. The number of symptoms during Long Covid did not appear to be related to changes in cell counts; however, median levels of the cytokine IL-2 were higher in individuals with fewer than six symptoms and more than three months of Long Covid.
Our results indicate a predominance of Long Covid among women, which may suggest a tendency for women to engage in self-care and utilise healthcare services, or a greater vulnerability due to biological differences in the immune system and hormonal factors, as suggested by Subramanian et al.14 and Calcaterra et al.15. In addition, although it is believed that adults and the elderly (40–60 years old) have a higher risk of developing Long Covid, which was the majority of our population, this study reinforces at the risk facing younger groups (18–39 years old), which makes up 25% of the individuals, who possibly go through a mild/moderate acute state and are to maintain the clinic even after recovery from COVID-192,7,16,17.
Furthermore, our results suggest that persistent laboratory anomalies, particularly elevated lymphocyte counts, may be present in 43.3% of our patients who were hospitalised with COVID-19. We also observed that during Long Covid, neutrophil and lymphocyte counts were elevated in 33.8% and 47% of patients who experienced severe COVID-19, respectively. Thus, we propose that those who are hospitalised and suffer from severe forms of COVID-19 are likely to progress to a more detrimental inflammatory process, which may result in persistent alterations in immune cell counts.
Some studies support the hypothesis of hyperinflammation among patients hospitalised with COVID-19, who typically exhibit leukocyte disorders (e.g., lymphopenia, neutrophilia, and monocytosis), as well as elevated levels pro-inflammatory cytokines (e.g., IL-6, IL-2, and IFN-γ) in peripheral blood5,7,9. This inflammatory process may be associated with multiple organ lesions, and as the infection resolves, immune cell counts and cytokine levels may or may not return to normal, leading to symptom regression7,18,19,20.
According to our results, which are similar to the findings of Galúcio et al.9, indicating that the average lymphocyte count was higher among hospitalised patients than among those not hospitalised for COVID-19 (2.5 ± 0.7 vs. 2.2 ± 1.0), we share the view that patients who were hospitalised for COVID-19 exhibited lymphopenia and had a compromised and ineffective adaptive immune response for viral clearance2,7,8,9. Thus, we infer that as lymphocytes are renewed, an inflammatory response may occur in Long Covid, resulting from an unresolved inflammatory process during the initial SARS-CoV-2 infection. This could contribute to increased lymphocyte counts and persistent symptoms in Long Covid, as reported by Glynne et al.5 and Yong21.
Furthermore, a study conducted by Berentschot et al.3 demonstrated an increase in the proportion of lymphocytes in Long Covid, particularly in the CD8 + T cell phenotype. Similarly, Shuwa et al.22 showed alterations in lymphocyte counts six months after hospital discharge. The authors suggest a persistent cytotoxic adaptive immune profile, indicating continued lymphocyte expression. Glynne P et al.5 also found changes in lymphocyte counts and lineages, adding that the alterations in lymphocytes in Long Covid may result from the persistence of viral antigens from SARS-CoV-2, indicating an unresolved infectious process during COVID-19.
In addition to the increase in lymphocyte counts, our results indicate an increase in neutrophil counts during Long Covid among those patients who experienced severe COVID-19. During COVID-19, the increase in neutrophils alongside the decrease in lymphocytes may suggest an imbalance between innate and adaptive immune responses, resulting in an uncontrolled inflammatory response that can lead to tissue damage and chronic inflammation9,18,20,23.
In our population, the abnormalities in lymphocyte and neutrophil counts among patients who progressed to more severe forms of COVID-19 may suggest a continuous attempt by the immune system to manage possible residual inflammation, resulting in persistent immune activation, as found in the study by Glynne et al.5 and suggested by Yong 21 This indicates a complex immune response where persistent inflammation and ongoing efforts to control it coexist. This imbalance may contribute to the persistence of symptoms in Long Covid. Overall, we hypothesise that hospitalisation and the severity of COVID-19 may implicate an unresolved inflammatory process, leading to a persistent inflammatory response with alterations in lymphocyte and neutrophil counts in Long Covid. This, in turn, may result in tissue damage, causing structural and functional changes, and contribute to the maintenance of symptoms.
Another important finding of this study is that changes in cell counts are independent of the number of symptoms in Long Covid; however, we found higher median levels of IL-2 in patients with fewer symptoms. In a study by Queiroz MAF, et al.7, median IL-2 levels were higher in individuals with mild Long Covid compared to those with severe forms (8.60, IQR 2.66 vs. 7.60, IQR 3.26). Espín et al.24 and Lai et al.2 also highlighted IL-2 as an important pro-inflammatory cytokine observed in Long Covid. According to these authors, IL-2 plays a crucial role in regulating the immune system and is primarily involved in the activation and proliferation of lymphocytes, which are essential for a robust immune response2,7,24.
Following this reasoning, although the study by Krishna et al.25 did not show a relationship between the number or duration of symptoms and IL-2 responses in Long Covid, we suggest that IL-2 may play a regulatory role in controlling a potential prolonged inflammatory response in Long Covid, leading to a more controlled and effective immune response, thereby reducing the likelihood of associated additional symptoms2,26. This may explain the lack of significance in the comparative analyses of immune cell count anomalies between groups with more or fewer symptoms.
Regarding the duration of Long Covid, median IL-2 levels were lower in patients with a shorter duration of Long Covid (7.75, IQR 2.08 vs. 8.32, IQR 1.80). We note that in this group alterations in eosinophil and monocyte counts were more prevalent, supporting our hypothesis that IL-2 regulates immune cell homeostasis and acts as a regulator of the immune response. Diminished levels of IL-2 may influence changes in immune cell counts in Long Covid, leading to a less controlled immune response.
Additionally, we can infer that the increase in eosinophil counts and the decrease in monocytes may be present in approximately 33.3% and 63.6% of patients with up to three months of Long Covid, respectively. An increase in eosinophils accompanied by a decrease in monocytes may lead to an altered immune response, with the potential for chronic inflammation. However, when the duration of Long Covid is extended to up to six months, this cellular pattern of monocytes may change, resulting in around 19.5% of patients exhibiting an increase in monocyte counts.
So far, several studies have identified persistent abnormalities in monocyte and eosinophil counts in Long Covid. Galúcio et al.9 found a higher average of eosinophils in patients within 90 days compared to those with more than 90 days of Long Covid (261.4 ± 85.6 vs. 214.9 ± 122.3), as well as a lower average of monocytes within 90 days compared to their control group (164.4 ± 76.3 vs. 215.5 ± 113.7). At up to 180 days, monocyte counts were higher (213.8 ± 142.8 vs. 208.1 ± 100).
Phetsouphanh et al.27 and Bohnacker et al.28 reported the highest frequencies of activated monocytes in Long Covid patients at three to five or eight months. Jukema et al.29 noted that eosinophil proportions were reduced during COVID-19 and increased in the early stages of Long Covid (23, IQR 10–34 vs. 76, IQR 30–375).
Regarding the changes in immune cell counts in patients with up to three months of Long Covid, a relationship with the temporal proximity to COVID-19 is suggested9,20,22,26,27. Specifically concerning eosinophils, studies have demonstrated a decrease in blood counts during COVID-19, which may be attributed to cellular infiltration into tissues and the inhibition of cell release from the bone marrow due to increased adrenal corticosteroid secretion during the infection26,30,31. Typically, eosinophil counts tend to rise in the early stages of recovery from COVID-1926,29and normalise over time28,30,31, which aligns with our findings, indicating a possible recovery or normalisation of eosinophils after three months of Long Covid.
However, asserting recovery precisely after three months of Long Covid is somewhat subjective, as it is suggested that some patients may have elevated monocyte counts for up to six months of Long Covid. According to some authors, monocytes tend to increase during the early stages of COVID-19, being associated with a hyper-inflammatory state, with a slow reversal during recovery, marking the end of the acute response6,23,26. However, other authors have reported a persistent functional reprogramming of monocytes, proportional to increased monocyte counts and symptom duration in Long Covid6,32. According to Phetsouphanh et al.27 and Bohnacker et al.28, this is an inflammatory hallmark of the monocyte/macrophage compartment, suggesting that SARS-CoV-2 exerts prolonged residual effects on the immune system that may lead to increased monocyte counts.
Specifically in patients with up to 12 months of Long Covid, a possible trend towards the normalisation of immune cell counts can be suggested, particularly due to the lack of significant alterations in cell counts in this group compared to those with more than 12 months. In this context, we infer that IL-10, as an anti-inflammatory cytokine, may play a role in the control and normalisation of immune cell counts in patients with up to 12 months of Long Covid, as the median was higher in this group (9.2, IQR 2.78).
Additionally, our correlation between cytokine levels and immune cell counts suggests a positive relationship for most of the cytokines assessed in the first three months of Long Covid. These findings support our hypothesis that the immune response in the early stages of Long Covid may be influenced by the temporal proximity to COVID-19, with cytokines playing a modulatory role in the dynamics of immune cells9,22,26. Our results suggest a complex inflammatory context during these stages of Long Covid, indicating a potential compensatory mechanism involving both anti-inflammatory cytokines (such as IL-4) and pro-inflammatory cytokines (such as IL-6, IL-2, IL-17 A, and IFN-γ).
From six months onwards, only IL-6 showed a positive correlation with monocytes, which may suggest a persistent activation of these monocytes and indicate a reprogramming of their function mediated by IL-6, sustaining a pro-inflammatory microenvironment in patients with Long Covid for over six months27,28. However, this pattern may not persist beyond 12 months.
A study conducted by Queiroz et al.7 assessed cytokine levels in patients with Long Covid and demonstrated results similar to ours, indicating higher levels of IL-17 and IL-2 (p < 0.05), suggesting a possible inflammatory molecular signature with a reduced anti-inflammatory response mediated by IL-4 and IL-10.
According to Phetsouphanh et al.27, patients with Long Covid exhibit highly activated innate immune cells (monocytes) and adaptive immune cells (lymphocytes), with elevated levels of IFN-γ and IL-6 for up to eight months. This suggests that Long Covid is associated with a combination of cytokines that promote the persistent activation of these cells, whether due to a residual inflammatory response or the persistence of viral antigens. Berentschot et al.3 reported that among the cytokines IFN-α, IFN-β, IFN-γ, IL-6, IL-7, IL-10, and IL-12, the IL-6 and IFN-γ showed the most significant increases in patients with Long Covid at three and six months. Furthermore, the expression of IL-6 was associated with greater monocyte activation.
From these findings, the immunological complexity associated with Long Covid becomes evident, highlighting the significant role of cytokines such as IL-6, IFN-γ, IL-2, and IL-17 A in potentially altering homeostasis and activating various immune cells3,7,27. One possibility for this pattern is that residual inflammation from COVID-19 may persist in certain individuals, gradually reducing after approximately 12 months.
In an effort to understand the immune response in Long Covid, we agree that the interactions between these cytokines and immune cells are interdependent. IL-6 stands out as a key cytokine for the activation of neutrophils, eosinophils, and monocytes, stimulating the production of other cytokines that perpetuate the inflammatory response. IL-2, in turn, may promote the activation of lymphocytes and increase the synthesis of IFN-γ, correlating with increased monocyte and neutrophil levels, thus creating a positive feedback loop that sustains the persistent inflammatory response. However, the rise in IL-4, correlated with increased lymphocytes and neutrophils, may indicate an attempt at regulation, particularly in the polarization of lymphocytes, leading to a less inflammatory response from neutrophils. This context reflects a complex balance between activation and regulation of the inflammatory response, where such interactions can contribute to a state of chronic inflammation, thereby perpetuating symptoms in Long Covid3,7,26,27,28,34.
Immune cell counts can indicate the state of the immune response, while cytokines modulate this response by either promoting or suppressing inflammation. Therefore, by correlating these two variables, it is possible to gain valuable insights into the immune response in Long Covid and identify immune response patterns that may be relevant for understanding the mechanisms and pathophysiology involved in the persistence of symptoms following COVID-192,25,26.
Despite the relevant results, our study has limitations given its cross-sectional design, which does not provide information on the long-term clinical and laboratory evolution, the lack of a control group to compare individuals without prolonged symptoms, and the limited use of inflammatory markers, making it difficult to provide detailed insights and guidance. In this sense, additional studies are needed to overcome the limitations of this study.
The Amazon region presents additional factors that may influence the clinical course and prognosis of diseases. This reality is shaped by environmental and socioeconomic factors that negatively impact infectious diseases. For example, around 30 million people (e.g., riverine communities, quilombolas, and urban dwellers) are considered vulnerable due to difficulties in accessing healthcare services, basic sanitation, and adequate nutrition35. Additionally, genetic ancestry profiles can determine the predisposition of certain population groups to disease development. For instance, Amerindian populations exhibit different immune responses compared to non-Amerindians, with a predominance of Th2-type immune responses, known to be inefficient against intracellular pathogens36. Therefore, it is essential that this population is studied to better understand the specific health needs and conditions of this region.
In a general analysis, we suggest that women were associated with lower hospitalisation rates for COVID-19 but experienced Long Covid lasting beyond six months. Additionally, higher lymphocyte counts were linked to hospitalisation for COVID-19, while patients with elevated monocyte counts were associated with prolonged duration of Long Covid beyond six months. Although there are multiple factors contributing to the onset of Long Covid, this cellular and clinical pattern serves as a basis for predicting the prognosis of COVID-19 and establishing targeted care, particularly in the Amazon region, where research into the immunological aspects of Long Covid is still in its early stages.
Despite indicating positive recovery, especially after 12 months of Long Covid, our results suggest that hospitalised patients with more severe COVID-19 may continue to exhibit alterations in lymphocyte and neutrophil counts. Furthermore, subsets of immune cells and cytokine levels may change when analysed according to the duration of Long Covid, particularly in the early stages. Thus, we infer that these patients may present a persistent pro-inflammatory microenvironment, particularly due to significant laboratory changes and correlations between various pro-inflammatory cytokines. These findings contribute to our knowledge of Long Covid and the involvement of the immune system. However, longitudinal studies are needed to evaluate the role of monocytes and eosinophils over time and clarify their temporal divergence in the context of Long Covid, given the potential evolution of monocytes and eosinophils. Additionally, it is important to increase the number of immunological markers and provide a better understanding of changes in the leukogram.
Methods
Methods and ethical approval
This observational, cross-sectional, descriptive analytical study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and was approved by the Ethics Committee for Research Involving Human Beings of the State University of Pará (opinion no. 4.252.664). Furthermore, this study was carried out in accordance with relevant guidelines/regulations and in strict compliance with the principles of the Declaration of Helsinki. All participants provided written informed consent.
Sampling and study groups
We included a total of 300 patients in the study with the aim of analysing leukocyte profiles and their clinical implications; of these, 202 had their cytokine levels quantified. The sample (n = 300) consisted of adults of both sexes (≥ 18 years), who voluntarily enrolled in the clinical follow-up programme for Long Covid at the State University of Pará (UEPA), located in the municipality of Belém, in the Brazilian Amazon region, between September 2020 and December 2022. Sampling was obtained by convenience. Informed consent was obtained from all participants involved in the study.
As a criterion for analytical inclusion, a diagnosis of Long Covid was considered based on the following aspects: (1) acute symptoms compatible with COVID-19 without attribution to other diseases, (2) COVID-19 confirmed by viral detection through quantitative reverse transcription polymerase chain reaction or positive result on antibody testing for anti-SARS-CoV-2 IgG/IgM, and (3) at least one symptom of Long Covid related to COVID-19 for at least 4 weeks after the onset of symptoms (e.g. loss of smell or taste, muscle pain, headache, post exertion malaise, chronic cough, brain fog, heart palpitations, chest pain, shortness of breath, fatigue, dizziness, and tremors). After this stage, laboratory tests were conducted.
Thirty-eight vaccinated patients, 41 patients with diabetes, and 1 patient with clinically relevant diseases that could complicate data interpretation were excluded from the initial population of 380 individuals. The remaining 300 study patients were divided into groups based on the criteria: ‘’hospitalisation with COVID-19 (reported by patients)’; ‘Severity in COVID-19’; ‘Time of Long Covid’ (time from symptom onset to the time of data collection); and ‘Number of symptoms in Long Covid’. This allowed for comparisons, correlations, and associations within the study population (Fig. 2).
Severity in the acute phase was defined according to the criteria established by the World Health Organization, which categorises the different clinical conditions of the disease as asymptomatic, mild/moderate, and severe13.
Flowchart of the process of recruiting and allocating study groups. [¹Characterisation considering WHO recommendations13].
Clinical assessment
Initially, a clinical evaluation was carried out at the respiratory disease’s outpatient clinic at Physiotherapy and Occupational Therapy Unit - UEPA/Specialized Rehabilitation Center. Individuals were interviewed to acquire demographic and clinical data, such as: name, sex, age, prolonged symptoms (≥ 4 weeks), duration of Long Covid symptoms, disease severity, and information on acute COVID-19 hospitalisation.
Leukocyte quantification
After clinical assessment, with patients fasting for at least 8 h, a minimum of 3 mL of blood was drawn via venipuncture into two Vacuette® tubes (Greiner Bio-One, Kremsmünster, Austria) containing the anticoagulant ethylenediaminetetraacetic acid (EDTA). Blood collection was performed in the morning between 7:30 and 9:00 a.m. The samples were then sent to the Clinical Analysis Laboratory/UEPA for quantitative analysis of leukocytes, segmented neutrophils (SEG), eosinophils (EOS), basophils (BAS), monocytes (MON), and lymphocytes (LYM).
The samples were analysed using a CELL-DYN Ruby automated haematology analyser (Abbott Laboratory, USA), which utilises multiangle polarised light scatter technology for the optical differentiation of leukocytes and laser flow cytometry for the quantitative analysis of leukocyte parameters, reported in absolute counts. The reference parameters followed the specifications provided by the manufacturer.
Cytokine quantification
Plasma samples containing EDTA as an anticoagulant were sent to the Virology Laboratory of the Federal University of Pará and used to determine cytokine levels, which were quantified by flow cytometry using the Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Kit (BD Biosciences, San Diego, CA, USA) and BD FACSCanto II flow cytometer.
The CBA BD assay consists of using capture beads of known sizes and fluorescence. Each bead was conjugated with a specific antibody for the detection of seven specific cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and IL-17). These beads were incubated with the investigated samples, allowing the cytokines present in the samples to bind to the capture antibodies on the beads. After incubation, a detection antibody conjugated to phycoerythrin (PE) was added. This antibody binds to the cytokines captured on the beads, providing a fluorescent signal, which was detected by the flow cytometer. Varying the fluorescence intensity of the beads allows the identification and quantification of each cytokine. According to the standardization of the kit, the concentration of each cytokine was expressed in picograms per millilitre (pg/mL). Cytokine concentrations were compared between the different groups evaluated in the study to verify the existence of patterns that indicate the differences between them.
Statistical analysis
The collected data were tabulated in an Excel® spreadsheet (Microsoft Corporation, Redmond, WA, USA) and analysed using GraphPad Prism® software version 8.4.3 (GraphPad Software, San Diego, CA, USA). The D’Agostino–Pearson test was carried out to determine the normality of the data distribution, using the mean and standard deviation (SD) for normal data or the median and interquartile range (IQR) for non-normal data. For comparisons between groups, the Mann–Whitney and chi-square tests were used to compare non-parametric variables.
Multiple logistic regression analysis was conducted to check the predictors and associations between the different study variables. In addition, Spearman’s coefficient was determined to assess the correlations between white blood cell counts and cytokines according to the duration of Long Covid. Statistical significance was set at p < 0.05.
Supplementary Information
Supplementary data are available at IJE online. The following supporting information can be downloaded from: Table S1: Reference values adopted in laboratory tests.
Data availability
The data supporting the findings of this study are available upon request to the corresponding author (PDLdL or LFMF). The data are not publicly available due to containing information that could compromise the privacy of research participants.E-mail: PDLdL ([email protected]); LFMF ([email protected]).
References
Carvalho-Schneider, C. et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 27(2), 258–263 (2021).
Lai, C. C. et al. Long COVID: an inevitable sequela of SARS-CoV-2 infection. J. Microbiol. Immunol. Infect. 56(1), 1–9 (2023).
Berentschot, J. C. et al. Immunological profiling in long COVID: Overall low grade inflammation and T-lymphocyte senescence and increased monocyte activation correlating with increasing fatigue severity. Front Immunol. 14, 1254899 (2023).
Ribeiro, B. M. S. S. & Carvalho, E. F. D. Post Covid-19 syndrome: Main alterations in the sensory system. Health Environ. Mag. 14(2) (2022).
Glynne, P. et al. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig Med. 70 (1), 61–67 (2022).
Klein, J. et al. Distinguishing features of long COVID identified through immune profiling. Nature. 623 (7985), 139–148 (2023).
Queiroz, M. A. F. et al. Cytokine profiles associated with Acute COVID-19 and long COVID-19 syndrome. Front. Cell. Infect. Microbiol. 12, 922422 (2022).
de Lima, I. C. et al. Liver function in patients with Long-Term Coronavirus Disease 2019 of up to 20 months: a cross-sectional study. Int. J. Environ. Res. Public Health. 20(7), 5281 (2023).
Galúcio, V. C. A. et al. Evaluation of the hematological patterns from up to 985 days of long COVID: A cross-sectional study. Viruses. 15 (4), 879 (2023).
Nascimento, R. A. et al. Effect of long COVID-19 syndrome on health-related quality of life: A cross-sectional study. Front. Psychol. 18 (2024).
Queiroz, M. A. F. et al. Severe COVID-19 and long COVID are associated with high expression of STING, cGAS and IFN-α. Sci. Rep. 14, 4974 (2024).
Galúcio, V. C. A. et al. Laboratory profiling of patients with long COVID in the Brazilian Amazon region: A cross-sectional study. J. Med. Virol. 96(8) (2024).
WHO Global Clinical Platform for Clinical Characterization of COVID-19: Statistical Analysis Plan. Disponível em (2021). https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical-Analytic-plan-2021
Subramanian, A. et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 28(8) (2022).
Calcaterra, V. et al. Long COVID in children, adults, and vulnerable populations: A comprehensive overview for an integrated approach. Diseases. 12(5), 95 (2024).
Mohiuddin, C. et al. Clinical characteristics and the long-term post-recovery manifestations of the COVID-19 patients a prospective multicenter cross-sectional study. Front. Med. 8, 663670 (2021).
Schultheiß, C. et al. From online data collection to identification of disease mechanisms: The IL-1ß, IL-6 and TNF-α cytokine triad is associated with post-acute sequelae of COVID-19 in a digital research cohort. Lancet, 15 (2021).
Liu, J. et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 55, 102763 (2020).
Rowe, S. L. et al. Associations between COVID-19 and hospitalisation with respiratory and non-respiratory conditions: A record linkage study. Med. J. Australia. 218 (1), 33–39 (2023).
Yaksi, N., Teker, A. G. & Imre, A. Long COVID in hospitalised COVID-19 patients: A retrospective cohort study. Iran. J. Public. Health. 51 (1), 88–95 (2022).
Yong, S. J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. (Lond). 53 (10), 737–754 (2021).
Shuwa, H. A. et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Medicine 2(6), 720–735 (2021).
Lucas, C. A. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 584 (7821), 463–469 (2020).
Espín, E. et al. Cellular and molecular biomarkers of long COVID: a scoping review. EbioMedicine (2023).
Krishna, B. A. et al. Spontaneous, persistent, T cell-dependent IFN-γ release in patients who progress to Long Covid. Sci. Adv., 10(8) (2024).
Rodriguez, L. et al. Systems-Level Immunomonitoring from Acute to Recovery phase of severe COVID-19. Cell Rep. Med. 1(5), 100078 (2020).
Phetsouphanh, C., Darley, D. R. & Wilson, D. B. Immune dysfunction persists for 8 months after initial mild to moderate SARS-CoV-2 infection. Nat. Immunol. 23, 210–216 (2022).
Bohnacker, S. et al. Correction to: mild COVID-19 imprints a long-term inflammatory eicosanoid and chemokine memory in monocyte-derived macrophages. Mucosal Immunol. 15 (4), 798 (2022).
Jukema, B. N. et al. Neutrophil and eosinophil responses remain abnormal for several months in primary care patients with COVID-19 disease. Front. Allergy (2022).
Pereira-Roche, N. et al. Hematological alterations in patients recovered from SARS-CoV-2 infection in Havana, Cuba. MEDICC Rev. 24 (2), 7–14 (2022).
Zein, J. G. et al. Eosinophilia is Associated with Improved COVID-19 outcomes in inhaled corticosteroid treated patients. J. Allergy Clin. Immunol. Pract. 10 (3), 742–750 (2022).
Brauns, E. et al. Functional reprogramming of monocytes in patients with acute and convalescent severe COVID-19. JCI Insight (2022).
Barry, J. C. et al. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci. Rep. 6, 21244 (2016).
Kappelmann, N., Dantzer, R. & Khandaker, G. M. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology. 131, 105295 (2021).
Codeço, C. T. et al. Epidemiology, biodiversity, and technological trajectories in the Brazilian Amazon: From Malaria to COVID-19. Front. Public. Health. 9, 647754 (2021).
Suarez-Kurtz, G. et al. Pharmacogenomic implications of population admixture: Brazil as a model case. Pharmacogenomics. 15 (2), 209–219 (2014).
Acknowledgements
The authors would like to acknowledge the support of the Federal University of Pará for this publication through PROPESP/UFPA (PAPQ - Qualified Publication Support Program).
Funding
This research was funded by the Amazon Research Foundation (FAPESPA), grant no. 005/2020 and 006/2020; The National Council for Scientific and Technological Development (CNPQ #401235/2020-3); the Secretariat for Science, Technology and Higher, Professional and Technological Education (SECTET), grant no. 09/2021; the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES), grant no. “Financial code 001, notice no. 13/2020”; and the National Council for Scientific and Technological Development - Brazil, grant no. “INCT: 406360/2022-7”.
Author information
Authors and Affiliations
Contributions
Conceptualisation, E.C.R.C., P.D.L.d.L. and L.F.M.F.; methodology, E.C.R.C., P.D.L.d.L. and M.H.C.R.; software, E.C.R.C. and D.C.d.M.; validation, P.D.L.d.L., M.H.C.R. and L.F.M.F.; formal analysis and investigation, E.C.R.C., D.C.d.M., I.C.d.L. M.A.F.Q., I.B.C., V.C.A.G., A.C.R.V., M.C.B., A.R.d.S. and J.A.S.Q.; resources, P.D.L.d.L., J.A.S.Q. and L.F.M.F.; data curation, E.C.R.C., L.F.M.F., P.D.L.d.L. and M.H.C.R.; writing - preparation of original draft, E.C.R.C., J.A.S.Q., M.H.C.R., D.C.d.M., I.C.d.L., V.C.A.G., M.A.F.Q., I.B.C., J.R.d.S., M.C.B., A.R.d.S., A.C.R.V., L.F.M.F. and P.D.L.d.L.; writing - proofreading and editing, E.C.R.C., J.A.S.Q., M.H.C.R., J.R.d.S., V.C.A.G., D.C.d.M., I.C.d.L., M.A.F.Q., I.B.C., M.C.B., A.R.d.S., A.C.R.V., L.F.M.F. and P.D.L.d.L.; visualisation, supervision and project administration P.D.L.d.L., L.F.M.F., A.C.R.V. and J.A.S.Q.; acquisition of funding, J.A.S.Q. and L.F.M.F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chaves, E.C.R., Quaresma, J.A.S., Rodrigues, M.H.C. et al. Altered leukocyte pattern and inflammatory markers in unvaccinated long covid patients: a cross-sectional study. Sci Rep 14, 28617 (2024). https://doi.org/10.1038/s41598-024-75920-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75920-1