Abstract
This large community-based cohort study investigates the impact of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), specifically Liraglutide and Semaglutide, on the risk of developing psychiatric conditions such as depression, anxiety, and suicidal behaviors in patients with obesity. Utilizing post-marketing data, this research compares patients prescribed GLP-1 RAs (cases) with those not taking these medications (controls). The analysis spanned data from January 1, 2015, to December 31, 2023. To minimize selection bias, we employed 1:1 propensity score matching to account for demographic factors such as age, sex, race, and comorbidities. After matching, the study included 162,253 case and control patients. This study showed a significant association between GLP-1 RA treatment and an 98% increased risk of any psychiatric disorders. Notably, patients on GLP-1 RAs exhibited a 195% higher risk of major depression, a 108% increased risk for anxiety, and a 106% elevated risk for suicidal behavior. These findings underscore the critical need for physicians to thoroughly assess patient history before prescribing GLP-1 RAs and highlight the urgent requirement for further prospective clinical trials to fully understand the implications of GLP-1 RA use on mental health in the obese patient population.
Similar content being viewed by others
Introduction
The increasing global incidence of obesity is a major public health concern, escalating at a concerning rate and posing significant challenges to healthcare systems worldwide1. Obesity is a complex, multifactorial disorder, characterized not only by excessive fat accumulation but also by its association with a wide range of complications. These complications are extensive and diverse, encompassing metabolic disorders such as type 2 diabetes2, dyslipidemia3, cardiovascular diseases4 and an increased overall mortality risk5. Additionally, obesity contributes to joint and musculoskeletal disorders6, mental health challenges7, and a heightened risk of various cancers8. This broad spectrum of complications emphasizes the critical need for effective management strategies.
While bariatric surgery remains the most effective method for significant and sustained weight loss, its application is often limited by factors such as the availability of skilled surgeons, high costs, and the potential for postoperative complications9. These limitations necessitate exploring alternative therapeutic options. Pharmacological interventions, particularly Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RAs), including Liraglutide and Semaglutide have emerged as a significant treatment option. These agents, approved by the U.S. Food and Drug Administration (FDA) for obesity management, have shown efficacy not only in weight reduction but also in improving metabolic10,11,12,13,14,15 and cardiovascular outcomes16.
However, the role of GLP-1 RAs in psychiatric outcomes, particularly in depression or anxiety disorder, presents a mixed picture. While preclinical studies, primarily in animal models17,18, suggest a potential beneficial effect of GLP-1 RAs on mood and behavior, the clinical evidence in humans remains unclear19,20. It is important to note that patients with a history of major depression were actually excluded from the phase 3 randomized controlled trials (RCTs) of these medications10,11,12,13,14,15,21,22,23. This exclusion is significant considering that patients with obesity have a higher risk of developing depression24. As a result, there is currently no data available to assess the clinical implications of GLP-1 RA use in this patient population. This study aims to explore this gap by utilizing data from the large community-based database, TriNetX, to investigate the association between GLP-1 RA use and the risk of psychiatric diseases, including major depression, anxiety and suicidal behaviour in patients with obesity.
Results
This study enrolled 11,683,623 adults diagnosed with obesity (BMI ≥ 30) from January 2015 to December 2023, utilizing the TrinetX database (Fig. 1). It established two cohorts: 244,063 patients in the GLP-1 RA treatment group (Victoza, Saxenda, Ozempic, Wegovy) and 11,439,560 patients in the non-GLP-1 RA group. After the exclusion of 9,075 patients due to prior anti-obesity medication, and 72,731 patients for bipolar, schizophrenia, depressive disorders, anxiety and suicide ideation or attempts within 1 year before, the treatment cohort numbered 162,257 patients. From the non-GLP RA group, 2,332,778 were excluded, resulting in 9,103,212 patients. PSM with age, sex, race, and disease comorbidities was then conducted, yielding 162,253 matched patients for analysis.
Table 1 presents the baseline characteristics of the GLP-1 and non-GLP-1 RA cohorts. Significant disparities were evident across all variables before matching. Post-PSM, a notable balance in these characteristics was obtained. The mean age in the matched cohorts was 52.4 years, with the population comprising 55.6% females and 61% Whites. The prevalence of comorbidities was as follows: 51.1% of patients with hypertension, 50% with diabetes mellitus, 5.2% with psychoactive substance use, and 0.5% experiencing problems related to housing and economic circumstances.
From 6 months to 5 years, Table 2 indicates a consistent increase in psychiatric outcomes among GLP-1 RA users. At 6 months, the incidence in the GLP-1 RA group was 9.36%, compared to 4.76% in the non-GLP-1 RA group. This trend continues at 1 year (15.29% vs. 7.63%), 3 years (29.62% vs. 16.45%), and 5 years (39.64% vs. 23.38%). The incidences of major depressive disorder, anxiety, and suicidal ideations or attempts, as well as their hazard ratios, are higher in the GLP-1 RA group, indicating an association with GLP-1 RA treatment over time. The hazard ratio (HR) for any psychiatric disease is 1.98 (95% CI 1.94–2.01). Specifically, the HR for major depressive disorder is 2.95 (95% CI 2.82–3.08), for anxiety 2.08 (95% CI 2.04–2.12), and for suicidal ideations or attempts 2.06 (95% CI 1.92–2.21), further demonstrating the significant association with GLP-1 RA treatment over time.
Table 3 presents a subgroup analysis of baseline demographics in psychiatric outcomes associated with the use of GLP-1 RA. Across all demographic subgroups, GLP-1 RA users consistently exhibited an elevated risk of psychiatric diseases. Female users had a 105% higher risk of any psychiatric disease (HR: 2.05, 95% CI 2.01–2.09) compared to non-users. Furthermore, individuals aged 18–49, 50–69, and ≥ 70 showed increased risks of 101%, 78%, and 71%, respectively, for any psychiatric disease when using GLP-1 RAs compared to non-users. Notably, the risk of major depressive disorder was the highest among female GLP-1 RA users, with a 216% elevated risk (HR: 3.16, 95% CI 2.98–3.34) compared to non-users, surpassing the risks of anxiety and suicidal ideations or attempts. Among racial groups, Black faced the highest risk of anxiety, with a 137% elevated risk (HR: 2.37, 95% CI 2.25–2.50) compared to non-users. These findings underscore the consistent relationship between psychiatric outcomes and GLP-1 RA usage, with a particular focus on the heightened risks for females, age, sex, and race.
The detailed subgroup analysis of different GLP-1 RA groups compared to non-GLP-1 RA users is demonstrated in Table 4. The HR for any psychiatric disease in the Victoza group was 1.65 (95% CI 1.59–1.72), while in the Saxenda group, it was 1.73 (95% CI 1.64–1.83). Notably, the Ozempic group showed an HR of 1.72 (95% CI 1.67–1.76), and the Wegovy group presented a significantly higher HR of 2.14 (95% CI 2.05–2.24). It is important to mention that the follow-up duration of Wegovy is only 3 years, whereas other GLP-1 RA medications were studied for 5 years. The most striking result is the suicidal ideations or attempts of the Ozempic group, which showed an approximately 2.4-fold increase in risk. These findings suggest varied psychiatric risks associated with different GLP-1 RA medications, with Wegovy demonstrating the highest risk for conditions such as major depressive disorder and anxiety.
Discussion
This study has provided evidence regarding the utilization of GLP-1 RAs, including medications such as liraglutide and semaglutide, at various dosages within real-world settings. It establishes an elevated risk of psychiatric consequences associated with these treatments, particularly in the domains of depression, anxiety, and suicidal outcomes.
Our findings indicate that the risk of psychiatric outcomes varies across different demographic subgroups. For example, females had a higher risk of anxiety (2.19, 95% CI: 2.14–2.24) and suicidal ideations or attempts (2.53, 95% CI: 2.32–2.76) compared to males. Younger participants (18–49 years) exhibited a higher risk of suicidal ideations or attempts (3.01, 95% CI: 2.70–3.37), while older participants (≥ 70 years) had a lower overall risk of psychiatric diseases (1.71, 95% CI: 1.61–1.82). Additionally, racial differences were observed, with Black patients showing a higher risk of suicidal ideations or attempts (3.45, 95% CI: 2.86–4.17) compared to White and Asian patients. These findings underscore the importance of considering demographic factors when evaluating the safety and efficacy of GLP-1 RAs in clinical practice.
Moreover, our study provides a more in-depth exploration of the temporal aspect, revealing how this risk evolves and intensifies with prolonged exposure. This aligns with findings from the STEP-225 and STEP-6 studies25, which reported higher incidences of psychiatric events with higher doses of semaglutide compared to placebo. Intriguingly, the data portrays an escalating trajectory, suggesting that prolonged exposure to GLP-1 RAs may heighten the propensity for the development of psychiatric outcomes. The study also indicates a progressive trend, with Wegovy, recognized as the most potent GLP-1 RA, administered at the highest dose and renowned for its remarkable reduction in body weight. These findings emphasize the need for further research to understand the differential effects of these medications.
This finding holds significant importance, noting that phase 3 studies have typically excluded patients with a history of psychiatric disorders, resulting in a lack of reference data for understanding the long-term consequences of GLP-1 RAs use in patients with obesity. This cautious approach can be attributed to the historical context of Rimonabant, a previously promising anti-obesity agent withdrawn from the market in 2008 due to severe psychiatric side effects26.
The SCALE (Satiety and Clinical Adiposity—Liraglutide Evidence) study comprised a series of Phase 3 trials evaluating the efficacy of Liraglutide 3 mg in diverse populations, including general obese patients, as well as those with pre-diabetes, diabetes, and sleep apnea syndrome10,11,12,13,14,15. These studies uniformly showed that Liraglutide significantly reduced body weight when used as an adjunct therapy with diet and exercise. However, these studies excluded individuals with a history of major depression or psychiatric diseases and reported no significant differences in psychiatric outcomes between liraglutide and placebo groups. Our study, which included a larger and more diverse real-world population with a longer follow-up duration, demonstrated significant psychiatric risks. This difference highlights the importance of considering real-world settings and extended follow-up periods in evaluating the psychiatric safety of GLP-1 RAs.
Moreover, one phase 2 study conducted by Astrup et al.27 investigated the effects of varying doses of liraglutide, ranging from 1.2 to 3 mg, in comparison to placebo and orlistat, expanding our understanding of the relationship between liraglutide and psychiatric outcomes. This study was inclusive of participants who had a history of psychiatric diseases. The results revealed a slightly higher rate of psychiatric disorders, particularly insomnia, depressed mood, and nervousness, in those taking higher doses of liraglutide (2.4 mg and 3.0 mg). Serious psychiatric diseases like depression and anxiety were rare across all groups. Notably, two participants exited the study due to anxiety (placebo group) and food aversion (liraglutide 1.2 mg group). The study was later extended to a two-year follow-up period28. During this time, it was found that 17% of the participants had a history of psychiatric conditions, with insomnia being the most frequently reported issue. Although higher doses of liraglutide were associated with more psychiatric events, there was no consistent pattern in these occurrences.
In addition, Semaglutide, another long-acting GLP-1 RA, has demonstrated superior efficacy in lowering blood glucose levels and reducing body weight compared to placebo or Liraglutide. The SUSTAIN29,30,31,32,33,34,35 and SCALE studies10,11,12,13,14,15 have shown robust efficacy in lowering blood glucose levels in diabetic patients and inducing weight loss in obese patients over clinical trial periods ranging from 30 to 104 weeks. However, these studies excluded patients with a history of major depressive disorder and suicidal ideation, limiting the generalizability of their findings. Our study included these subgroups and found a higher risk of psychiatric outcomes with semaglutide use. The STEP-225 and STEP-6 studies25 supported these findings, showing higher incidences of psychiatric events with higher doses of semaglutide. This highlights the need for further research to establish the safety and efficacy of semaglutide in patients with a history of psychiatric conditions.
We conducted a detailed subgroup analysis comparing different GLP-1 RA groups to non-GLP-1 RA users. Our findings indicate that patients treated with semaglutide, particularly at higher doses such as Wegovy (2.4 mg), exhibited a higher incidence of psychiatric outcomes compared to non-GLP-1 RA users. In the STEP-2 study36, semaglutide 2.4 mg showed a 6.0% incidence of psychiatric events compared to 3.7% in the placebo group. Similarly, the STEP-6 study25 reported a 3% incidence of psychiatric events with semaglutide 2.4 mg, compared to 1% in the placebo group. Interestingly, the STEP-8 study22 revealed a higher incidence of psychiatric events with liraglutide 3 mg (15.0%) compared to semaglutide 2.4 mg (5.6%) and placebo (10.6%). These findings underscore the varying psychiatric risks associated with different GLP-1 RAs and the importance of individualized patient assessment in clinical practice.
When administering the lower dose of Semaglutide at 1 mg to treat patients with diabetes, the SUSTAIN study consistently demonstrated significant improvements in glucose control and body weight reduction29,30,31,32,33,34,35. However, all patients with a history of depression were excluded from these studies, and none of them reported the results of psychiatric outcomes. Our study found that patients using semaglutide 1 mg have a higher risk of depression, anxiety, and suicidal ideation when compared with non-semaglutide users, irrespective of age and sex differences. It is important to note that including patients with a known history of depression and/or anxiety in such studies could bias the results, as these patients are more prone to experience a new episode, which could be wrongfully attributed to GLP-1 RAs drugs. While a new episode could be triggered by GLP-1 RAs, this relationship needs to be established by future studies.
In real-world settings, the association between GLP-1 RAs and the risk of psychiatric outcomes has yielded mixed findings. Li et al. reported two cases of depression associated with the use of semaglutide19. The first case involves a 54-year-old man who, about one month after starting semaglutide, experienced a marked decline in energy, motivation, fatigue, difficulty concentrating, and mood disturbances. The close timing of these symptoms’ onset with the initiation of semaglutide, and their significant improvement within 11 days of discontinuing the medication, underscores a potential causal relationship between semaglutide and the rapid onset of depressive symptoms. In the second case, a 40-year-old woman with a history of depression reported worsening mood symptoms, including fatigue, cognitive slowing, and suicidal thoughts, approximately two months after beginning semaglutide for diabetes management. Her condition showed substantial improvement within two weeks of cessation of the medication. These cases collectively suggest a potential link between semaglutide use and the development or exacerbation of depressive symptoms, warranting closer monitoring in clinical practice.
In contrast, a recent study conducted by McIntyre et al. spanning from 2005 to October 2023 utilized the Food and Drug Administration Adverse Event Reporting System (FAERS) to investigate patterns of suicidality associated with GLP-1 RA usage. Their analysis revealed a disproportionate reporting of suicidal ideation and “depression/suicidal” linked to these medications. However, following the adjustment for potential confounding factors, they concluded that no causal link exists between GLP-1 RAs and suicidality37. Furthermore, in Taiwan, Tsai et al. utilized claims records extracted from the National Health Insurance Research Database (NHIRD) and suggested that patients with diabetes who underwent GLP-1 RA therapy experienced a significant reduction in the risk of anxiety20.
Moreover, Wang et al. concluded that Semaglutide is not associated with an increased risk of suicidal ideation in the real-world settings38. However, their study has different methodological with our study. The initial patient demographics included individuals with histories of major depression, mood disorders, and anxiety. In a study targeting suicide attempts as the endpoint, a control group with a higher prevalence of depression and suicidal ideation might lead to outcome bias, as evidenced by the increased incidence of suicidal ideation within the control group. Furthermore, the study’s sole focus on suicidal ideation as the primary outcome is less suitable for retrospective studies, where patients more commonly present with major depression or anxiety, potentially accompanied by suicidal ideation, rather than with suicidal ideation alone. Nevertheless, in our study, the risk of suicidal ideation and attempts increased after 1 year of follow-up.
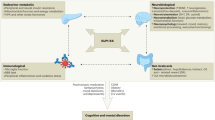
Several mechanisms may contribute to how GLP-1 RAs reduce body weight. GLP-1 RAs promotes weight loss primarily by decreasing appetite and caloric intake, rather than by increasing energy expenditure. It enhances glycemic control and modulates appetite, which collectively contribute to reduced food intake39. Furthermore, another study highlighted GLP-1 RAs’ direct impact on the brain, especially the hypothalamus40. This effect leads to changes in neuropeptide levels, crucial for the regulation of appetite and energy balance41.
The hypothesis regarding the impact of GLP-1 RAs on depression suggests a complex interaction within the dopaminergic system. It proposes that stimulation of GLP-1 receptors enhances the activity of dopaminergic neurons in the ventral tegmental area (VTA) through a presynaptic mechanism42. Simultaneously, there is an increase in the expression of the dopamine transporter on neuronal cells within the limbic system (LS) and striatum, leading to more efficient reuptake and reduced availability of free dopamine in these areas. Considering dopamine’s pivotal role in the brain’s reward system, such alterations could lead to an imbalance in dopaminergic neurotransmission43,44. This imbalance is hypothesized to contribute to dysfunctions in the reward system, potentially manifesting as anhedonia and other depressive symptoms45, a clinical observation that aligns with the psychiatric side effects noted in GLP-1 RAs therapy.
It is imperative to deepen our comprehension of the intricate relationship between GLP-1 RAs and psychiatric outcomes, particularly given the substantial association between obesity and psychiatric diseases. To address this vital question, the next step should involve undertaking further prospective clinical trials. These trials should be thoughtfully designed to encompass diverse patient populations, investigate varying doses of GLP-1 RAs, and incorporate extended follow-up periods. Such an approach is poised to provide more definitive and robust insights into the influence of these medications on psychiatric outcomes in individuals contending with obesity and related psychiatric conditions.
Our study possesses both strengths and limitations. Among its strengths is its status as the largest study to date that focuses on the association between GLP-1 RA usage and the risk of psychiatric outcomes in real-world scenarios. Additionally, our research was conducted over an extended period, facilitating a thorough and comprehensive evaluation. We also meticulously investigated how different doses of Liraglutide and Semaglutide influence the risk of psychiatric outcomes. Another significant strength is our study’s adherence to strict methodology, including stringent inclusion and exclusion criteria, which ensures the robustness and reliability of our findings.
However, our study is not without its limitations. As a retrospective cohort analysis, it is inherently subject to biases such as selection bias. We heavily relied on diagnostic and medication codes for data collection, which may introduce some potential inaccuracies. Additionally, excluding patients with a diagnosis of any psychiatric diseases within one year before or one month after the index date may not be sufficient to avoid bias. Furthermore, the side effects of the drugs could have a significant impact on the quality of life and contribute to the occurrence of depressive episodes. The possible interaction of these medications with other drugs the patients were taking could also influence the outcomes. Another limitation is the lack of data on the evolution of BMI during the follow-up period, which could affect the interpretation of our results. Lastly, we lacked information about patient compliance with prescribed treatments, which could have influenced our results.
In conclusion, our study conducted within real-world clinical settings revealed a noteworthy association between the use of GLP-1 RAs, including Liraglutide and Semaglutide, and a heightened risk of psychiatric outcomes, encompassing depression, anxiety, and suicidal behaviour. This finding underscores the importance of clinical physicians being aware of and conducting comprehensive patient history assessments before prescribing these medications. Such practices are essential to ensure patient safety and well-being while navigating the complexities of obesity management and its potential psychiatric implications.
Methods
Study population
This study was conducted as a retrospective cohort analysis, employing data sourced from the TriNetX network, a collaborative platform in the United States encompassing 66 healthcare organizations. The TriNetX database provided a comprehensive resource, encompassing complete diagnostic codes, medications prescribed, and procedures undertaken, facilitating a thorough analysis.
Patient data utilized in this study were fully de-identified to ensure privacy and confidentiality. The de-identification process involved the removal or modification of personal identifiers in the data, such as names, addresses, and social security numbers. This procedure ensured that individual patients could not be directly or indirectly identified, safeguarding patient privacy in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations. Because of the retrospective nature of the study and the use of de-identified data, this study received an exemption waiver for approval and informed consent from the Institutional Review Board (IRB) of Chung Shan Medical University Hospital, identified by the reference number CS1-24047. All methods were carried out in accordance with relevant guidelines and regulations.
This study enrolled adults aged 18 years or older who were diagnosed with obesity, defined either through diagnostic codes E66.0 and E66.9 or a Body Mass Index (BMI) of 30 or above, and who had ≥ 2 medical encounters related to this diagnosis in the database. The analysis spanned data from January 1, 2015, to December 31, 2023. The intervention group consisted of patients who were prescribed GLP-1 RA medications, including Victoza (Liraglutide 1.8 mg), Saxenda (Liraglutide 3 mg), Ozempic (Semaglutide 1 mg), and Wegovy (Semaglutide 2.4 mg). The index date for this group was defined as the first date any of these GLP-1 medications were prescribed.
In contrast, the control group comprised patients with obesity who did not receive a GLP-1 prescription during the study timeframe. The index date for this group corresponded to the initial date of their obesity diagnosis.
The study’s exclusion criteria included the following: first, any use of weight management medications, such as orlistat, bupropion, naltrexone, topiramate, phentermine, liraglutide, or semaglutide before the index date; second, any diagnosis of bipolar, schizophrenia, depressive disorders, anxiety, suicidal behavior or attempts within one year before or one month after the index date. These criteria were established to isolate the impact of GLP-1 prescriptions on study outcomes while controlling for potential confounding factors, with a specific focus on the risk of depression, anxiety, and suicide. Additionally, data on age, sex, and race were collected for all participants. These criteria and collected variables were established to isolate the impact of GLP-1 prescriptions on study outcomes while controlling for potential confounding factors, with a specific focus on the risk of depression, anxiety, and suicide.
This study encompassed a comprehensive set of variables, including those related to socioeconomic factors and key comorbidities. Socioeconomic factors were represented by problems associated with housing and economic circumstances (Z59). Comorbidities included hypertensive diseases (I10-I16), Type 2 diabetes mellitus (E11), chronic lower respiratory diseases (J40-J47), neoplasms (C00-D49), psychoactive substance use (F10-F19), ischemic heart diseases (I20-I25), chronic kidney disease (N18), and cerebrovascular diseases (I60-I69). The inclusion of these variables played a crucial role in controlling confounding factors within our study.
In this retrospective cohort study, the TriNetX platform inherently manages missing data and patient follow-up based on the practices of contributing healthcare organizations. As such, the completeness and follow-up of data are dependent on the source data quality and consistency. Missing data for key variables were handled using available case analysis, which included only the participants with complete data for those variables in the analysis. This method was deemed appropriate given the nature of the dataset and the retrospective design of the study. Patients lost to follow-up were managed using the available data up to the point of their last recorded visit. The primary analysis included all patients with at least one follow-up visit after the index date.
Primary outcome
The primary outcome of this study is the first occurrence of any psychiatric diseases. This includes depressive disorders (F32-33), anxiety (F40-F48), and various aspects of suicidality. Specifically, suicidality is detailed as suicidal ideations (R45.851), suicide attempts (T14.91), and intentional self-harm (X71-X83). To enhance the reliability of our findings, we analyzed the primary outcome starting one month after the index date. This approach ensures that potential psychiatric conditions are more accurately attributed to the period following the intervention or diagnosis, increasing the validity of our study’s conclusions.
Statistical analysis
In this study, propensity score matching (PSM) was utilized to ensure comparability between the intervention and control groups, with particular attention to key variables such as age, sex, race, and disease comorbidities. The effectiveness of the matching was evaluated using the Standardized Mean Difference (SMD), with an SMD value of less than 0.1 indicating a negligible difference between the groups, thus affirming the efficacy of the matching process.
The hazard ratio (HR) was employed for the comparative risk analysis between cases (patients prescribed GLP-1 medications) and controls (patients not prescribed GLP-1 medications). All calculations and statistical analyses were performed within the TriNetX platform. This robust platform facilitated the comprehensive analysis of the study data, ensuring accurate and reliable statistical computations. A p-value of less than 0.05 was set as the threshold for clinical significance.
Data availability
Data availability statement: This population-based study obtained data from the TrinetX platform (accessible at https://trinetx.com/), for which third-party restrictions apply to the availability of this data. The data were used under license for this study with restrictions that do not allow for data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX ([email protected]), but costs might be incurred, and a data-sharing agreement would be necessary.
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390(10113), 2627–2642 (2017).
Bjerregaard, L. G. et al. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl. J. Med. 378, 1302–1312 (2018).
Neeland, I. J., Poirier, P. & Després, J. P. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for Management. Circulation 137, 1391–1406 (2018).
Twig, G. et al. Body-Mass Index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl. J. Med. 374, 2430–2440 (2016).
De Berrington, A. et al. Body-mass index and mortality among 1.46 million white adults. N Engl. J. Med. 363, 2211–2219 (2010).
Collins, K. H. et al. Obesity, metabolic syndrome, and musculoskeletal disease: common inflammatory pathways suggest a central role for loss of muscle Integrity. Front. Physiol. 9, 112 (2018).
Ebbeling, C. B., Pawlak, D. B. & Ludwig, D. S. Childhood obesity: public-health crisis, common sense cure. Lancet 360, 473–482 (2002).
Reeves, G. K. et al. Cancer incidence and mortality in relation to body mass index in the million women study: cohort study. BMJ 335, 1134 (2007).
Arterburn, D. E., Telem, D. A., Kushner, R. F. & Courcoulas, A. P. Benefits and risks of bariatric surgery in adults: a review. JAMA 324, 879 (2020).
Wadden, T. A. et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity 28, 529–536 (2020).
Le Roux, C. W. et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 389, 1399–1409 (2017).
Blackman, A. et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int. J. Obes. (Lond) 40(8), 1310–1319. https://doi.org/10.1038/ijo.2016.52 (2016).
Pi-Sunyer, X. et al. A Randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl. J. Med. 373, 11–22 (2015).
Davies, M. J. et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 314, 687 (2015).
Wadden, T. A. et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int. J. Obes. 39(1), 187 (2013).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl. J. Med. 389, 2221–2232 (2023).
Seo, M. K. et al. Effects of liraglutide on depressive behavior in a mouse depression model and cognition in the probe trial of Morris water maze test. J. Affect. Disord. 324, 8–15 (2023).
Palleria, C. et al. Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav. Brain Res. 321, 157–169 (2017).
Li, J. R., Cao, J., Wei, J. & Geng, W. Case Report: Semaglutide-associated depression: a report of two cases. Front. Psychiatry 14, 1238353 (2023).
Tsai, W. H. et al. Corrigendum: Decreased risk of anxiety in Diabetic patients receiving Glucagon-Like Peptide-1 receptor agonist: a Nationwide, Population-based Cohort Study. Front. Pharmacol. 13, 886343 (2022).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl. J. Med. 384, 989–1002 (2021).
Rubino, D. M. et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 327, 138 (2022).
Wadden, T. A. et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 Randomized Clinical Trial. JAMA. 325, 1403 (2021).
Pereira-Miranda, E., Costa, P. R. F., Queiroz, V. A. O., Pereira-Santos, M. & Santana, M. L. P. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J. Am. Coll. Nutr. 36, 223–233 (2017).
Kadowaki, T. et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 10, 193–206 (2022).
Christensen, R., Kristensen, P. K., Bartels, E. M., Bliddal, H. & Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713 (2007).
Astrup, A. et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374, 1606–1616 (2009).
Astrup, A. et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. (Lond). 36 (6), 843–854 (2012).
Sorli, C. et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 5, 251–260 (2017).
Ahrén, B. et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 5, 341–354 (2017).
Ahmann, A. J. et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care 41, 258–266 (2018).
Aroda, V. R. et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 5, 355–366 (2017).
Rodbard, H. W. et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J. Clin. Endocrinol. Metab. 103, 2291–2301 (2018).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl. J. Med. 375, 1834–1844 (2016).
Pratley, R. E. et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 6, 275–286 (2018).
Davies, M. et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 397, 971–984 (2021).
McIntyre, R. S., Mansur, R. B., Rosenblat, J. D. & Kwan, A. T. H. The association between glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: reports to the Food and Drug Administration adverse event reporting System (FAERS). Expert Opin. Drug Saf. 23, 47–55 (2024).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Van Can, J. et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 38, 784–793 (2014).
Knudsen, L. B., Secher, A., Hecksher-Sørensen, J. & Pyke, C. Long‐acting glucagon‐like peptide‐1 receptor agonists have direct access to and effects on pro‐opiomelanocortin/cocaine‐ and amphetamine‐stimulated transcript neurons in the mouse hypothalamus. J. Diabetes Investig. 7, 56–63 (2016).
Gabery, S. et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 5, e133429 (2020).
Mietlicki-Baase, E. G. et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. -Endocrinol Metab. 305, E1367–E1374 (2013).
Jensen, M. E. et al. Glucagon-like peptide-1 receptor regulation of basal dopamine transporter activity is species-dependent. Neurochem Int. 138, 104772 (2020).
Reddy, I. A. et al. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl Psychiatry 6, e809–e809 (2016).
Westbrook, A. et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science 367, 1362–1366 (2020).
Acknowledgements
Special thanks for all study team for their dedication and support in this study.
Funding
This work was supported by grants from the Chung Shan Medical University Hospital (CSH-2020-C-016).
Author information
Authors and Affiliations
Contributions
Edy Kornelius: Conceptualization, Methodology, Writing – Original DraftJing-Yang Huang: Data curation, Investigation, Software, Visualization, Data AnalysisShi-Chang Lo: Conceptualization, MethodologyChien-Ning Huang: Resources, Supervision, Data analysisYi-Sun Yang: Methodology, Data analysis, Writing – Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kornelius, E., Huang, JY., Lo, SC. et al. The risk of depression, anxiety, and suicidal behavior in patients with obesity on glucagon like peptide-1 receptor agonist therapy. Sci Rep 14, 24433 (2024). https://doi.org/10.1038/s41598-024-75965-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75965-2
Keywords
This article is cited by
-
What is the Role of Primary Prevention of Obesity in an Age of Effective Pharmaceuticals?
Current Obesity Reports (2025)
-
The impact of glucagon-like peptide-1 (GLP-1) agonists in the treatment of eating disorders: a systematic review and meta-analysis
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2025)
-
Psychiatric adverse events linked to glucagon-like peptide 1 analogues: a disproportionality analysis in American, Canadian and Australian adverse event databases
International Journal of Clinical Pharmacy (2025)