Abstract
To explore the performance of 99mTc-diethylene triaminepentaacetic acid (DTPA) SPECT/CT texture analysis in evaluating the activity of thyroid-associated ophthalmopathy (TAO) . This retrospective study examined 115 TAO patients from a single institution as an internal cohort and 58 TAO patients from another institution as an external validation set. Patients in the internal cohort were randomly divided into training (n = 81) and internal validation sets (n = 34). Radiomics signatures were constructed with the minimal redundancy maximal relevance and least absolute shrinkage and selection operator algorithms in training set. Multivariate logistic regression analysis was used to develop a clinical model and a combined clinical–radiomics model. Diagnostic performance of models was evaluated using receiver operating characteristic curve analysis, calibration curves and decision curve analysis. Compared with CT and SPECT radiomics models, Rad-scoreSPECT/CT demonstrated the best performance with areas under the receiver operating characteristic curve of 0.94 and 0.91 in the training and test sets, respectively. The combined clinical-radiomics model exhibited significantly better performance in evaluating TAO activity. Our results demonstrate the validity of a multimodal radiomic model of 99mTc-DTPA-SPECT/CT to assess TAO activity. The combined clinical-radiomics model exhibited significantly better diagnostic performance than the clinical model.
Similar content being viewed by others
Introduction
Thyroid-associated ophthalmopathy (TAO), also known as Graves’ ophthalmopathy (GO), is an autoimmune disease of the orbit1,2. Most cases occur in association with Graves’ disease3,4. TAO has an annual incidence of 42.2 per million and a female predominance5. The pathogenesis is complex and involves environmental, immune, genetic and other factors6,7,8,9. The disease is characterized by infiltration of inflammatory factors in the orbital connective tissue, which leads to orbital remodeling and structural changes in periocular tissues. The clinical manifestations of this may include exophthalmos, strabismus, eyelid retraction and compressive optic neuropathy. Facial appearance and visual function may be affected to varying degrees6,10,11.
The natural course of TAO consists of an active stage with obvious inflammatory reaction and an inactive stage with fibrosis and steatosis4,12. Treatment should be tailored according to the activity stage. The active stage responds better to hormone therapy, local radiotherapy and immunosuppressive agents, while the inactive stage is mainly treated with surgery1,12,13. At present, activity is determined using the clinical activity score (CAS)1,14. However, the CAS is mainly based on patient symptoms and physician judgment and experience, which are subjective. An objective and repeatable assessment method is needed.
Orbital 99mTc-diethylene triaminepentaacetic acid (DTPA) single photon emission computed tomography (SPECT)/computed tomography (CT) imaging can be used to assess TAO activity15,16,17,18,19. According to the principle of process-specificity, 99mTc-DTPA penetrates into the extracellular fluid through periorbital capillary walls, which have been destroyed by inflammation, and then combines with peptides in the extracellular fluid15. Inflammatory activity in the orbit is then visualized using SPECT/CT to evaluate the degree of activity. However, there is currently no consensus regarding imaging analysis and results determination.
Applying radiomics and deep learning to radiologic imaging has demonstrated potential in assisting with diagnosis, disease differentiation, assessing treatment response and predicting outcomes20,21,22. Zhao et al.23 demonstrated that the deep learning algorithms could automatically detect TAO activity by using 99mTc-DTPA SPECT/CT images. Texture analysis is an advanced image post-processing technology that analyses the gray level/pixel intensity of gray images to assess lesion heterogeneity24. It has been applied in the evaluation of several diseases, such as gastric carcinoma25, lung cancer26, breast cancer27 and Parkinson’s disease28. In this study, we aimed to explore whether texture analysis of 99mTc-DTPA SPECT/CT images can provide quantitative indicators of TAO activity. We also developed a practical radiomics model for distinguishing TAO activity and evaluated its performance.
Methods
Patients
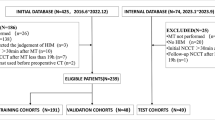
This retrospective study was performed in accordance with the Helsinki Declaration and approved by local Ethics Committee (approval no. UHCT22702). Adult patients (age ≥ 18 years) diagnosed with TAO who underwent orbital 99mTc-DTPA SPECT/CT between January 2021 and August 2022 in two Chinese medical centres were retrospectively analysed. We excluded those with the following characteristics: (1) acute systemic disease and electrolyte disorder; (2) pregnancy or lactation; (3) history of systemic or local hormone therapy, orbital surgery, immunosuppressive therapy, or radiation therapy; (4) other autoimmune, orbital or intracranial disease; (5) other disease that causes exophthalmos; and (6) < 6 months of follow-up. All subjects provided written informed consent. The study flowchart was summarized in Fig. 1.
One hundred fifteen patients from Institution 1 were included as the internal cohort and 58 from the Institution 2 were included as the external validation set. Patients in the internal cohort were randomly divided into training and internal testing sets at a ratio of 7:3 (81 patients in the training set and 34 in the internal testing set); this cohort was used for model development and internal testing. The external validation set was used for external validation of the models.
Follow-up and treatment evaluation
Patient and clinical characteristics, laboratory testing results, treatment, and other data were retrospectively collected and organized through the electronic medical record and picture archiving and communication systems. Recorded data included age, gender, smoking history, history of hyperthyroidism, white blood cell count, neutrophil count, lymphocyte count, alanine aminotransferase, aspartate aminotransferase, free triiodothyronine, free thyroxine, thyroid-stimulating hormone, anti-thyroid peroxidase antibody, thyroid-stimulating hormone receptor antibody, and CAS.
Patients with hyperthyroidism and TAO were treated with anti-thyroid drugs to control thyroid function. For patients with moderate-to-severe active TAO, high-dose glucocorticoid pulse therapy was the main treatment; some patients received additional orbital radiotherapy. Certain patients with severe stable disease underwent orbital decompression surgery. The majority of patients with stable TAO didn’t receive any specific treatment and were regularly followed.
Patients underwent follow-up examinations once every 3 to 6 months. Routine blood testing and tests for thyroid function and thyroid-stimulating hormone receptor antibody were commonly performed during follow-up, as were determination of CAS and orbital 99mTc-DTPA SPECT/CT or orbital CT when necessary.
The treatment efficacy evaluation criteria were as follows: (1) ≥ 2 mm reduction in palpebral fissure; (2) ≥ 2 mm reduction of proptosis; (3) ≥ 2-point decrease in CAS ; (4) ≥ 3 mmHg decrease in intraocular pressure; (5) absence of primary gaze diplopia; and (6) ≥ 10° improvement in eye movement. If three or more criteria were met, the treatment was considered effective; otherwise, it was considered ineffective.
99mTc-DTPA SPECT/CT image acquisition and reconstruction
99mTc-DTPA (dose, 370–555 MBq) was injected via an elbow vein in each patient. After 30 to 45 min, SPECT/CT was performed using a Discovery NM/CT 670 Pro system (GE Healthcare, Milwaukee, WI, USA) equipped with a low-energy high-resolution collimator and dual-head detectors. The energy peak was set at 140 keV with a window width of 20% and magnification factor set to 1.00. The imaging matrix was 128 × 128 and the detectors rotated 360° at a rate of 3°/frame. Each frame was collected for 20 s. The CT acquisition parameters were as follows: tube voltage, 140 kV; tube current, 150–300 mA; slice thickness, 1.25 mm; and reconstruction layer thickness, 0.625 mm. Patients were placed in the supine position with their head in the machine’s headrest for the examinations. The bed height was adjusted so the entire head was within the imaging range of the detectors. The orbitoauricular line (the line connecting the external ear aperture and the lateral canthus) was used as the reference baseline. The position of the patient’s head was not moved during imaging.
The internal queue was processed using the Volumetrix MI program in the Xeleris 3.0 workstation and then motion correction, fusion correction, attenuation correction, scattering correction, and resolution recovery correction were performed. Next, the ordered subset expectation maximization algorithm was used for reconstruction (2 iterations, 10 subsets), followed by Hann filtering with a cut-off frequency of 0.9. The external queue also used the same algorithm for reconstruction.
99mTc-DTPA SPECT/CT image preprocessing
Prior to radiomics feature extraction, all original images were subjected to normalization processes utilizing MATLAB (version 2016a). Given that CT density is relative to technical parameters rather than an absolute value, the images were standardized and normalized. The standardization formula is as follows:
Here, µ represents the mean signal value within the images, x denotes the matrix, σ signifies the standard deviation, and NN is the number of voxels. The normalization equation is
Where xi indicates the signal of a voxel. In alignment with the standardized workflow of radiomics, image preprocessing is a prerequisite step before radiomics feature extraction can occur.
99mTc-DTPA SPECT/CT image quantization
Image quantization refers to the transformation of image grayscale values into a discrete set of grayscale values. Before radiomics feature extraction, the image was quantized with a fixed bin width of 5, a choice based on the example settings of Pyradiomics (https://github.com/AIMHarvard/pyradiomics/tree/master/examples/exampleSettings). According to the protocol, the ideal range for the number of bins is between 16 and 128 bins. A suitable bin width can be determined by extracting a feature known as the first-order range, ensuring it falls within this bin range. The outcomes of this range are detailed in Supplementary file. An absolute discretization was carried out with a fixed bin size (binsize = 5), assigning new bins to pixel intensities for each BS grayscale level starting at 0. The formula is as follows:
Here, I(x)I(x) signifies the intensity of a voxel; BS (binsize) indicates the bin size, and IBS(x)IBS(x) represents the discretized grayscale level of voxel xx (29). All images were then converted into Gaussian and wavelet forms. Wavelet filtering results in eight decompositions at each level. The wavelet filter settings are as follows: (1) start_level [0]: an integer representing the base level of wavelet from which the first set of decompositions for signature calculation is derived. (2) level [1]: an integer for the number of wavelet decomposition levels used for signature calculation. (3) wavelet [“coif1”]: a string indicating the type of wavelet decomposition. It is an enumerated value validated against possible values present in pyWavelet.wavelist. The current possible values are for pywavelet version 0.4.0. The Gaussian image is obtained by convolving the image with the Laplacian of a Gaussian kernel. The Gaussian kernel is calculated using the following formula:
Region-of-interest segmentation, image pre-processing and feature extractions
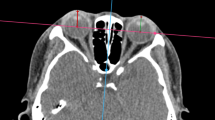
The maximum cross-sectional area of the superior, inferior, medial, and lateral rectus on both eyes was selected as the region of interest(ROIs), which were segmented on the CT images through the open source 3D-Slicer software version 5.0.2 (https://download.slicer.org/) (Fig. 2). The ROIs of both the internal and external queues were delineated manually by two nuclear medicine physicians with 2–3 years’ experience who were blinded to subject information. All ROIs were confirmed by another nuclear medicine physician who had over 8 years of experience.
Radiomics workflow. The term ‘LASSO’ stands for Least Absolute Shrinkage and Selection Operator, a widely used method for regression analysis when dealing with high-dimensional data. LASSO regression selects significant and distinguishable features to construct the radiomics signature, effectively simplifying the model and reducing the risk of overfitting.
To accurately map the delineated ROIs to SPECT, MATLAB software version 2016a (MathWorks, Natick, MA, USA) was used to achieve complete spatial matching of slice thickness, number of slices, and inter-slice spacing between the CT and SPECT images in order to ensure accuracy. Before extracting the texture features of the ROIs, all images were standardized, normalized, and discretized according to the Image Biomarker Standardization Initiative. Subsequently, the CT and SPECT images were subjected to Gaussian and wavelet transformations. Then, the voxels were reconstructed to a size of 1 × 1 × 1 mm3. Finally, three sets of images were obtained: original images, Gaussian-transformed images, and wavelet-transformed images.
According to the feature guidelines of the Image Biomarker Standardization Initiative, the pyradiomics feature package (github.com/Radiomics/pyradiomics) was used for image feature extraction in Anaconda Prompt software version 4.2.0 (https://www.anaconda.com/download). Six types of texture features were extracted based on the original, Gaussian-transformed, and wavelet-transformed images: first-order, shape, gray-level co-occurrence matrix, gray-level run-length matrix, gray-level size-zone matrix and gray-level distance-zone matrix.
Feature selection and radiomics model establishment
Texture features of the training set were used to construct radiomics labels. First, the minimal redundancy maximal relevance algorithm (mRMR) was used to remove redundant and irrelevant features. Then, the least absolute shrinkage and selection operator algorithm was used to build radiomics signature based on the minimum binomial deviation that could distinguish TAO activity were selected (Fig. 2). In the 99mTc-DTPA SPECT/CT images, the Logλ(0.0342) identified 20 features (Fig. 2). These relevant radiomics signatures were wavelet_LLL_firstorder_ 90Percentile. despect, wavelet_ LLL_ glrlm_GrayLevelNonUniformity.ct, wavelet_LLL_glrlm_ GrayLevel NonUniformity.ct, wavelet_LLL_glcm_Imc2.ct, log_sigma_3_0_mm_3D_ glcm_Idmn.ct, wavelet_LLL_firstorder_Maximum.despect, wavelet_LHL_glrlm_ LongRunEmphasis.despect, wavelet_LLL_firstorder_Skewness.ct, wavelet_LLL_ glszm_ZoneVariance.ct, wavelet_HHH_glszm_GrayLevelNonUniformity Normalized. despect, wavelet_LLL_glcm_InverseVariance.despect, original_glcm_ Imc2.ct, wavelet_HHH_glszm_LowGrayLevelZoneEmphasis.ct, log_sigma_3_0_ mm_ 3D_ glszm_SmallAreaLowGrayLevelEmphasis.despect, log_sigma_2_0_mm_ 3D_ glszm_SmallAreaLowGrayLevelEmphasis.ct, wavelet_LLL_glcm_Idmn.despect, original_glrlm_ShortRunLowGrayLevelEmphasis.despect, original_glcm_ Cluster Prominence.ct, wavelet_HHL_glszm_SmallAreaLowGrayLevelEmphasis.ct,
Wavelet_HLL_glcm_Imc1.ct (Supplementary file). Rad-score was established by combining the corresponding weights of texture, and the training set patient data were subjected to 10-fold cross-validation. Since this study included both CT and SPECT images, three Rad-scores were constructed, including Rad-scoreCT, Rad-scoreSPECT, and Rad-scoreSPECT/CT .
Establishment of clinical models and combined clinical-radiomics models
First, clinical characteristics were selected to built clinical model to analysis, active and inactive disease in the training and internal testing sets. Then, the multivariate logistic regression model was used to built clinical model based the minimum. Akaike information criterion (AIC) were used to analyse the clinical features of the training set. Patient age and CAS were ultimately included in the clinical model construction. In addition, a combined model based Rad-score and retained clinical parameters were built using a multivariate logistic regression model.
Model effectiveness evaluation
All constructed models were first validated in the training and internal validation sets. The performance of all radiomics models for determining TAO activity was evaluated by the area under the receiver operating characteristic (ROC) curve (AUC). By stepwise comparison of the statistical differences between the ROC curves of each model, the optimal radiomics model was ultimately selected. The Hosmer–Lemeshow test and calibration curve were used to validate the fitting degree between the predicted values and the true values of the model. Decision curve analysis was used to evaluate the clinical benefits among three models. Finally, the clinical utility of the model for the assessment of TAO activity was validated using the AUC in the external validation set.
Statistical analysis
Statistical analyses were performed using R software 3.6.4 (www.Rproject.org) and the following statistical packages: tidyverse, caret, pROC, DMWR, rmda, ggpubr, mRMRe, DescTOOLs and irr. Medcalc software (www.medcalc.org) was used to analyse the ROC curves. The differences between ROC curves of the different models were compared using the Delong test. Line graphs were plotted using Prism software (GraphPad, San Diego, CA, USA).
Results
Patient characteristics
Among the 115 patients with TAO in the internal cohort, disease activity was classified as active in 54 patients (47%) and inactive in 61 (53%). Characteristics of patients in the training and test sets were summarized in supplementary files (Table S1). In the training set, CAS and eyelid swelling significantly differed between the patients with active and inactive TAO. No clinical variables significantly differed between the active and inactive TAO groups in the internal test set. As shown in Table 1, among the 58 patients with TAO in the external validation set, disease activity was classified as active in 25 patients (43%) and inactive in 33 (57%).
Diagnostic performance of radiomics signatures
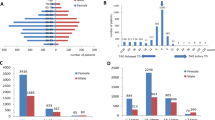
This study involved two types of single imaging modes. We extracted six texture features from CT and 14 from SPECT (Fig. 2) to construct Rad-scoreCT and Rad-scoreSPECT. All the radiomics signatures demonstrated significant differences between active and inactive TAO patients in both the training and test sets (Fig. 3a,b). ROC analysis showed that Rad-scoreSPECT had better diagnostic performance than Rad-scoreCT; the AUC for Rad-scoreSPECT in the training and test sets was 0.90 (95% confidence interval [CI], 0.83–0.97) and 0.88 (95% CI, 0.77-1.00), respectively (Fig. 3c,d; Table 2).
The radiomics signatures from (a) CT and (b) SPECT showed significant differences (P < 0.05) between patients with active and inactive thyroid-associated ophthalmopathy (TAO) in the training and test cohorts (Label 0: inactive TAO, Label 1: active TAO). The receiver operating characteristic curves of three types of radiomics models in the (c) training set and (d) test set showed that Rad-scoreSPECT/CT performed best (the area under the curve in the training and test sets was 0. 94 and 0.91, respectively). Nomogram based on clinical characteristics and Rad-score of SPECT/CT (e). The receiver operating characteristic curves of the Rad-scoreSPECT/CT, clinical model and clinical–radiomics combined model in the (f) training set and (g) test set.
To further explore the value of multimodal radiomics, we fused CT and SPECT images and constructed the Rad-scoreSPECT/CT using CT and SPECT texture features extracted from the fused images. Rad-scoreSPECT/CT showed the best diagnostic performance: the AUC in the training and test sets was 0.94 (95% CI, 0.89–0.99) and 0.91 (95% CI, 0.81-1.00), respectively (Fig. 3c,d; Table 2).
Diagnostic performance of clinical and combined clinical–radiomics models
The clinical model was constructed apart from the above radiomics models using multiple logistic regression analysis and the minimum AIC principle and was based on eyelid swelling. For optimization, we also constructed a clinical-radiomics combined model comprising eyelid swelling and Rad-scoreSPECT/CT. The nomogram based on clinical factors and Rad-score is shown in Fig. 3e. The model equation was Nomoscore = Intercept × 0.0543062543361371 + swelling of eyelids × 0.525538208148291 + Rad-scoreSPECT/CT × 2.19743383205928.
Performance data for the clinical, Rad-scoreSPECT/CT and combined clinical–radiomics models were shown in Fig. 3f,g and Table 3. The AUC for the combined model was 0.94 (95% CI, 0.89-1.00) in the training set and 0.91 (95% CI, 0.81-1.00) in the test set. These values significantly differed from those for the clinical model (training test AUC, 0.63 [95% CI, 0.53–0.72]; test set AUC, 0.57 [95% CI, 0.44–0.70]). However, they did not significantly differ compared with the Rad-scoreSPECT/CT AUC values. The Hosmer–Lemeshow test demonstrated no significant difference between the combined model results and the actual data in evaluating TAO activity (training set P = 0.91; test set P = 0.65). Calibration testing showed the combined model was effective for assessing TAO activity and consistent with that of the statistically optimal model (Fig. 4a,b). Decision curve analysis showed that the clinical benefit of the combined model was higher when the thresholds of the training and test sets were between zero and 1 (Fig. 4c,d).
External validation set
In the external validation set (n = 58), the AUC for the Rad-scoreSPECT/CT, clinical, and combined clinical–radiomics models was 0.81, 0.61 and 0.82, respectively (supplementary file Fig. S1).
Discussion
TAO is closely related to autoimmune thyroid disease29,30 and its state of activity is crucial for determining the appropriate treatment1,31. Current clinical methods for evaluating disease activity are subjective and may be inaccurate. In particular, the CAS appears to be unreliable for diagnosing active inflammation as indicated by 99mTc-DTPA SPECT/CT32. This study aimed to examine radiomics models as objective and repeatable means of evaluating TAO activity and to determine an optimal radiomics signature based on 99mTc-DTPA SPECT/CT that provides structural and functional information.
Orbital 99mTc-DTPA SPECT/CT imaging is an emerging method to detect TAO activity, since increased accumulation of the radiotracer (99mTc-DTPA) in the extraocular muscle visualized by SPECT/CT directly reflect the activity of inflammation in TAO patients15. However, evaluation criteria have not been standardized15,17,33. Based on 99mTc-DTPA SPECT/CT images, we first established a radiomics model based on single CT and SPECT images to differentiate activity. Surprisingly, the diagnostic performance of Rad-scoreSPECT was significantly better than that of Rad-scoreCT in both the training and internal test sets (Table 3), which may be related to the fact that functional metabolic changes often precede the structural changes.To further improve diagnostic efficiency, we constructed a new radiomics model based on fused CT and SPECT images (Rad-scoreSPECT/CT). This model exhibited improvements in all measures of performance compared to Rad-scoreSPECT and Rad-scoreCT (Table 3), which suggests that multimodal imaging obtains more diagnostic information via synergy.
99mTc-DTPA SPECT/CT is mainly based on visual analysis and quantitative measurement of orbital tissue uptake values to determine TAO activity. Suzumowski et al.15 reported that the sensitivity and specificity of pure 99mTc-DTPA SPECT/CT for diagnosis of TAO activity was 93% and 89%, respectively. And Jiang et al.34 found that the thickness and uptake ratio of each extraocular muscle in active TAO were higher than those in the inactive TAO(P<0.01). Based on the fact that the texture analysis can observe internal heterogeneity through image gray and/or pixel intensity post-processing, the diagnostic specificity of our radiomics model was relatively increased (95%), and the sensitivity was 87%, which may reduce the influence of nasopharyngeal 99mTc-DTPA uptake in clinic. Compared to MRI orbital imaging, our radiomics model utilized both anatomic and functional features, and the RadscoreSPECT/CT showed better performance than MRI in the training(AUC, 0.940 vs. 0.820) and test group (AUC, 0.910 vs. o.751)23.
We also constructed a combined clinical–radiomics model by combining the optimal Rad-scoreSPECT/CT with clinical variables. The diagnostic performance and clinical utility of the purely clinical model were not satisfactory, possibly because the relevant clinical features are subjective and non-specific. Performance did not significantly differ between the combined clinical–radiomics model and Rad-scoreSPECT/CT. The combined model also demonstrated good diagnostic efficacy in the external validation set (AUC, 0.82; 95% CI, 0.71–0.93). Therefore, it appears important to incorporate clinical characteristics with imaging texture signatures, since the imaging might be a supplement for the clinical parameters.
Our study has several limitations. First, diagnosis of TAO activity was not based on pathologic examination as a gold standard. We assessed activity strictly based on clinical follow-up results to guarantee accurate diagnosis. Second, the internal validation set had a small sample size and the CAS in most patients was low. A more stable radiomics model and more reliable results would have been obtained with a larger sample. Therefore, we enrolled patients from a second institution as the external validation set to verify the accuracy of the established radiomics model. Third, this was a retrospective study in which the clinical characteristics were limited and nonspecific, so bias may have been present. Finally, manual ROI mapping is highly operator-dependent. Expanding the amount of data and depicting ROIs automatically are our goals in the next research phase.
In radiomics research, a key challenge is ensuring that radiomic features are not only statistically significant but also clinically relevant. These features are sensitive to variations in image acquisition and reconstruction, affecting the reproducibility of results and posing a barrier to clinical translation. Additionally, the “black box” nature of some radiomic analyses, especially those involving deep learning, complicates model interpretability and integration into clinical practice. To fully realize the potential of radiomics in precision medicine, it is crucial to develop standardized imaging protocols, improve model transparency, and establish robust correlations with pathological outcomes.
In summary, while radiomics holds significant promise for advancing precision medicine, it is crucial to address existing limitations to fully realize its potential. Future efforts should focus on developing standardized protocols, improving model interpretability, and establishing robust correlations with pathological outcomes. By overcoming these challenges, radiomics can be more effectively integrated into clinical practice, enhancing its impact on patient care.
Conclusion
A multimodal radiomics model based on 99mTc-DTPA SPECT/CT can provide structural and functional information simultaneously and be applied to evaluate TAO activity. Performance of the multimodal model was superior to that of single CT and SPECT radiomics models. Additionally, the combined clinical–radiomics model exhibited significantly better diagnostic performance than the clinical model. Our image-based radiomics model can be used as a supplement to clinical variables to better diagnose TAO activity.
Data availability
The data supporting the conclusions of this article are included in the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TAO:
-
Thyroid-associated ophthalmopathy
- CAS:
-
Clinical activity score
- DTPA:
-
Diethylene triaminepentaacetic acid
- SPECT/CT:
-
Single photon emission computed tomography/computed tomography
- ROIs:
-
Regions of interest ROIs
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the receiver operating characteristic curve
References
Bartalena, L. et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 185, G43–G67. https://doi.org/10.1530/EJE-21-0479 (2021).
Hiromatsu, Y., Wall, J. R., Kahaly, G. J. & Kakizaki, H. Graves’ Orbitopathy. Int. J. Endocrinol. 2015, 634234. https://doi.org/10.1155/2015/634234 (2015).
Smith, T. J. & Hegedus, L. Graves’ disease. N. Engl. J. Med. 375, 1552–1565. https://doi.org/10.1056/NEJMra1510030 (2016).
Smith, T. J. & Janssen, J. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr. Rev. 40, 236–267. https://doi.org/10.1210/er.2018-00066 (2019).
Bartalena, L. et al. The 2016 European thyroid association/european group on graves’ orbitopathy guidelines for the management of graves’ orbitopathy. Eur. Thyroid J. 5, 9–26. https://doi.org/10.1159/000443828 (2016).
Cao, J., Su, Y., Chen, Z., Ma, C. & Xiong, W. The risk factors for Graves’ ophthalmopathy. Graefes Arch. Clin. Exp. Ophthalmol. 260, 1043–1054. https://doi.org/10.1007/s00417-021-05456-x (2022).
Douglas, R. S. & Gupta, S. The pathophysiology of thyroid eye disease: Implications for immunotherapy. Curr. Opin. Ophthalmol. 22, 385–390. https://doi.org/10.1097/ICU.0b013e3283499446 (2011).
Prabhakar, B. S., Bahn, R. S. & Smith, T. J. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr. Rev. 24, 802–835. https://doi.org/10.1210/er.2002-0020 (2003).
Stan, M. N. & Bahn, R. S. Risk factors for development or deterioration of Graves’ ophthalmopathy. Thyroid 20, 777–783. https://doi.org/10.1089/thy.2010.1634 (2010).
Douglas, R. S. et al. Teprotumumab for the treatment of active thyroid eye disease. N. Engl. J. Med. 382, 341–352. https://doi.org/10.1056/NEJMoa1910434 (2020).
Khalilzadeh, O., Noshad, S., Rashidi, A. & Amirzargar, A. Graves’ ophthalmopathy: A review of immunogenetics. Curr. Genom. 12, 564–575. https://doi.org/10.2174/138920211798120844 (2011).
Bartalena, L. et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur. J. Endocrinol. 158, 273–285. https://doi.org/10.1530/EJE-07-0666 (2008).
Bartalena, L. et al. Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid 18, 333–346. https://doi.org/10.1089/thy.2007.0315 (2008).
Mourits, M. P. et al. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: A novel approach. Br. J. Ophthalmol. 73, 639–644. https://doi.org/10.1136/bjo.73.8.639 (1989).
Szumowski, P. et al. Efficacy of (99m)Tc-DTPA SPECT/CT in diagnosing orbitopathy in graves’ disease. BMC Endocr. Disord. 19, 10. https://doi.org/10.1186/s12902-019-0340-0 (2019).
Galuska, L., Barna, S. K., Varga, J., Garai, I. & Nagy, E. V. The role of 99mTc-DTPA retrobulbar SPECT in staging and follow-up of Graves’ orbitopathy. Nucl. Med. Rev. Central Eastern Eur. 21, 54–58. https://doi.org/10.5603/NMR.a2018.0005 (2018).
Galuska, L. et al. SPECT using 99mTc-DTPA for the assessment of disease activity in Graves’ ophthalmopathy: A comparison with the results from MRI. Nucl. Med. Commun. 23, 1211–1216. https://doi.org/10.1097/00006231-200212000-00010 (2002).
Alevizaki-Harhalaki, M., Alevizaki, C., Georgiou, E., Proukauis, C. & Ikkos, D. Increased Tc-99m DTPA uptake in active Graves’ ophthalmopathy and pretibial myxoedema. J. Nucl. Med.: Off. Publ. Soc. Nucl. Med. 24, 174–176 (1983).
Galuska, L. et al. Active retrobulbar inflammation in Graves’ ophthalmopathy visualized by Tc-99m DTPA SPECT. Clin. Nucl. Med. 28, 515–516. https://doi.org/10.1097/01.Rlu.0000067520.87766.B6 (2003).
Pan, W. et al. Development and validation of a clinical-image model for quantitatively distinguishing uncertain lipid-poor adrenal adenomas from nonadenomas. Front. Oncol. 12, 902991. https://doi.org/10.3389/fonc.2022.902991 (2022).
Colombi, D. et al. Computed tomography texture analysis of carotid plaque as predictor of unfavorable outcome after carotid artery stenting: A preliminary study. diagnostics (Basel, Switzerland) 11, https://doi.org/10.3390/diagnostics11122214 (2021).
Fan, X. et al. Texture analysis of (18)F-FDG PET/CT for differential diagnosis spinal metastases. Front. Med. 7, 605746. https://doi.org/10.3389/fmed.2020.605746 (2020).
Yao, N. et al. Deep learning-based diagnosis of disease activity in patients with Graves’ orbitopathy using orbital SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 50, 3666–3674. https://doi.org/10.1007/s00259-023-06312-2 (2023).
Castellano, G., Bonilha, L., Li, L. M. & Cendes, F. Texture analysis of medical images. Clin. Radiol. 59, 1061–1069. https://doi.org/10.1016/j.crad.2004.07.008 (2004).
Giganti, F. et al. Gastric cancer: Texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur. Radiol. 27, 1831–1839. https://doi.org/10.1007/s00330-016-4540-y (2017).
Agazzi, G. M. et al. CT texture analysis for prediction of EGFR mutational status and ALK rearrangement in patients with non-small cell lung cancer. La Radiologia Medica 126, 786–794. https://doi.org/10.1007/s11547-020-01323-7 (2021).
Song, S. E. et al. Prediction of inflammatory breast cancer survival outcomes using computed tomography-based texture analysis. Front. Bioeng. Biotechnol. 9, 695305. https://doi.org/10.3389/fbioe.2021.695305 (2021).
Hu, X. et al. Multivariate radiomics models based on (18)F-FDG hybrid PET/MRI for distinguishing between Parkinson’s disease and multiple system atrophy. Eur. J. Nucl. Med. Mol. Imaging 48, 3469–3481. https://doi.org/10.1007/s00259-021-05325-z (2021).
Bahn, R. S. Graves’ ophthalmopathy. N. Engl. J. Med. 362, 726–738. https://doi.org/10.1056/NEJMra0905750 (2010).
Ferrari, S. M. et al. The association of other autoimmune diseases in patients with Graves’ disease (with or without ophthalmopathy): Review of the literature and report of a large series. Autoimmun. Rev. 18, 287–292. https://doi.org/10.1016/j.autrev.2018.10.001 (2019).
González-García, A. & Sales-Sanz, M. Treatment of Graves’ ophthalmopathy. Med. Clinica 156, 180–186. https://doi.org/10.1016/j.medcli.2020.07.031 (2021).
Liu, D. et al. 99mTc-DTPA SPECT/CT provided guide on triamcinolone therapy in Graves’ ophthalmopathy patients. Int. Ophthalmol. 40, 553–561. https://doi.org/10.1007/s10792-019-01213-6 (2020).
Ujhelyi, B. et al. Retrobulbar 99mTc-diethylenetriamine-pentaacetic-acid uptake may predict the effectiveness of immunosuppressive therapy in Graves’ ophthalmopathy. Thyroid : Off. J. Am. Thyroid Associat. 19, 375–380. https://doi.org/10.1089/thy.2008.0298 (2009).
Jiang, C. et al. Monitoring and predicting treatment response of extraocular muscles in grave’s orbitopathy by (99m)Tc-DTPA SPECT/CT. Front. Med. (Lausanne) 8, 791131. https://doi.org/10.3389/fmed.2021.791131 (2021).
Acknowledgements
We thank the patients participated in this study and Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Funding
This research was supported by 2020 Thyroid Research Program of Young and Middle-aged Physicians (China Health Promotion Foundation), 2022 Thyroid Research Program of Young and Middle-aged Physicians (BJHPA-2022-JZHXZHQNYJ-LCH-03, Beijing Health Promotion Foundation), Research Foundation of Hubei Key Laboratory of Molecular Imaging (2020FZYX016) and Hubei Provincial Natural Science Foundation Project (2024AFB633).
Author information
Authors and Affiliations
Contributions
M.Z., W.C. and X.X. conceived and designed the study devised and supervised the project. Y.L. and M.L. wrote the manuscript. H.C., W.L. and M.H. analyzed the image data. Y.L. and M.L. segmented the images. F.H., L.M., and S.H. contributed to the follow-up of the patients.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and patient consent statement
This retrospective study was performed in accordance with the Helsinki Declaration and approved by Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was waived due to the retrospective nature of the study and the use of anonymized data by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Li, M., Chen, H. et al. Diagnostic precision in thyroid-associated ophthalmopathy using multi-center radiomics with 99mTc-DTPA SPECT/CT. Sci Rep 14, 25810 (2024). https://doi.org/10.1038/s41598-024-76018-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76018-4