Abstract
The global rise of multidrug-resistant pathogens, particularly Methicillin-resistant Staphylococcus aureus (MRSA), has become a critical public health concern, necessitating the urgent discovery of new antimicrobial agents. Griseorhodin C, a hydroxyquinone compound isolated from Streptomyces, has demonstrated significant inhibitory effects against MRSA. In this study, we employed a comprehensive approach combining transcriptome and metabolome analyses to investigate the underlying antimicrobial mechanism of Griseorhodin C. Our findings reveal that Griseorhodin C interferes with multiple bacterial metabolic pathways, including those essential for the biosynthesis and metabolism of amino acids, purine metabolism and energy metabolism, ultimately leading to bacterial growth inhibition and cell death. Notably, Griseorhodin C showed superior inhibitory effects compared to the clinical standard, vancomycin, both in vivo and vitro. These results highlight the potential of Griseorhodin C as a promising candidate for the development of new therapeutic strategies aimed at combating MRSA infections. The study underscores the importance of exploring natural products as sources of novel antibiotics in the ongoing fight against antimicrobial resistance.
Similar content being viewed by others
Introduction
The misuse of antibiotics promotes the development of multidrug-resistant strains, whereby bacteria develop defense mechanisms against antibiotics through frequent exposure, thereby reducing or eliminating their effectiveness. Currently, antimicrobial-resistant pathogens are among the most common causes of death worldwide, with at least 700,000 deaths attributed annually to antimicrobial resistance (AMR)1. Methicillin-resistant Staphylococcus aureus (MRSA) is a predominant pathogen of nosocomial and community-acquired infections globally, causing high mortality and infection rates, posing a significant public health threat. Common clinical manifestations include bacteremia, respiratory tract infections, skin and soft tissue infections, osteomyelitis, and septic arthritis et al.2. Vancomycin is the drug of choice for the clinical treatment of MRSA. With the emergence of vancomycin-insensitive S. aureus strains3, a new round of great challenges in the fight against MRSA has been posed. Finding new drugs effective against MRSA infection has become a focal point for researchers worldwide.
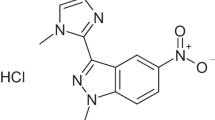
Actinomycetes have historically been rich sources of antibiotics such as vancomycin from Streptomyces orientalis, streptomycin from Streptomyces griseus, and erythromycin from Streptomyces erythreus4. In our previous studies, our research group isolated a series of antimicrobial and anticancer compounds from gut-derived actinomycetes of the Periplaneta americana. Among these, Griseorhodin C, a purple-red hydroxyquinone-type compound, was isolated from the Streptomyces strain WA23-2-1. Griseorhodin C has demonstrated significant anti-MRSA activity, suggesting its potential as a new antimicrobial agent. However, its precise mechanism of action remains unclear, underscoring the importance of understanding the molecular basis of antibacterial agents for developing effective antibiotics.
“Omics” technologies, including transcriptomics and metabolomics, are invaluable for comprehensively elucidating antimicrobial mechanisms5. Transcriptomics, for instance, detects gene expression at the cellular level under specific environmental conditions, reflecting differences induced by stresses such as antibiotic exposure. This technology has been employed to monitor bacterial gene expression in response to antimicrobial treatments6. For example, Osman Türkyılmaz et al. used transcriptomics and genomics to reveal transcriptional gene expression differences in Escherichia coli exposed to varying concentrations of ampicillin, laying the groundwork for understanding the molecular mechanisms of ampicillin resistance in E. coli7. Similarly, Shannon R. Coleman et al. studied how biofilm cells induce antimicrobial resistance in Pseudomonas aeruginosa by upregulating transcriptional regulators, two-component systems, sigma factors, virulence factors, and iron acquisition genes, while downregulating ribosomal genes8.
In addition to transcriptomics, metabolomics offers direct insights into cellular metabolic functions following drug exposure. For instance, linalool was found to inhibit the growth of Pseudomonas fragi by altering pathways involved in energy, amino acid, and nucleic acid metabolism9. Zhang et al. hypothesized, using metabolomics, that the antifungal activity of 1-nonanol, a major component of cereal volatiles, against Aspergillus flavus results from disruption of cell membrane integrity and mitochondrial function10. Furthermore, metabolomics studies have shown that the essential oil of Amomum villosum Lour disrupts amino acid metabolism and the tricarboxylic acid (TCA) cycle in MRSA, inhibiting ATP and ROS production. This disruption impairs cellular energy metabolism, ultimately resulting in cell death11.
However, transcriptomics typically only elucidates changes in gene expression levels post-transcription, without accurately revealing the ultimate alterations induced by drugs within organisms. In contrast, metabolomics provides a direct view of a series of changes in metabolites within organisms following drug stimulation. Therefore, combining transcriptomics and metabolomics enhances the persuasiveness of research findings and aids in achieving a more comprehensive understanding of antimicrobial mechanisms. exploring bacterial metabolic changes is crucial for understanding their pathogenic mechanisms and devising effective treatment strategies. Numerous studies now use integrated transcriptomic and metabolomic analyses to investigate antimicrobial mechanisms. For instance, Maytham Hussein et al. utilized these combined analyses to study the molecular mechanisms of ceftazidime/avibactam against multidrug-resistant Klebsiella pneumonia in urinary tract infection patients. They found that treatment with ceftazidime/avibactam disrupted the biogenesis of the cell envelope, bacterial membrane lipid metabolism, and central carbon metabolism of K. pneumonia, providing insights into the antimicrobial effects and resistance mechanisms of ceftazidime/avibactam12. Similarly, Ying et al. demonstrated that Lactobacillus salivarius bacteriocin XJS01 inhibited the growth of MDR S. aureus by significantly affecting its transcriptional and metabolic levels, involving functions related to metal uptake, cytotoxicity, amino acid and energy metabolism, bacterial stress response, and membrane transport13. This integrated approach is pivotal for deciphering bacterial metabolic responses and devising effective treatment strategies.
This study aims to explore the biosafety, antibacterial activity and mechanism of action of Griseorhodin C against MRSA. For the first time, integrated transcriptomic and metabolomic analyses were conducted on MRSA treated with Griseorhodin C, elucidating the molecular changes induced by this compound. Additionally, therapeutic effects of Griseorhodin C on MRSA-infected wounds were evaluated using a mouse model, providing comprehensive insights into its potential as a novel antibiotic.
Materials and methods
Bacterial strains and cell strains, animals
The MRSA strain (ATCC 43300), Staphylococcus aureus, Bacillus subtilis, K. pneumoniae, and Staphylococcus epidermidis strains, were maintained at the Guangdong Provincial Key Laboratory of Pharmaceutical Bioactive Substances. Additionally, ten clinical isolates of MRSA (MRSA-244661, MRSA-244358, MRSA-244862,MRSA-244135, MRSA-244548, MRSA-244425, MRSA-244288, MRSA-244757, MRSA-244268, MRSA-244410) from the National Center for Respiratory Medicine at the First Affiliated Hospital of Guangzhou Medical University. These strains were cultured on LB agar (Huankai Microbial Technology Co., LTD, Guangdong, China). A single colony from the LB plate was inoculated into 5.0 mL of LB liquid medium (Huankai Microbial Technology Co., LTD, Guangdong, China) and shaken at 180 rpm for 12 h at 37 °C. The following day, 1.0 mL of the bacterial suspension was inoculated into 100 mL of LB liquid medium and incubated on a bacterial shaker (180 rpm, 37 °C) for 3 h to obtain MRSA cultures in the logarithmic growth phase (1.5 × 108 CFU/mL), which were used for subsequent experiments.
LO2 cells, HK-2 cells and HaCat cells were sourced from storage at -80 °C in the Guangdong Provincial Key Laboratory of Pharmaceutical Bioactive Substances. The cells were cultured in RPMI 1640 medium (Gibco, USA) at 37 °C with supplementation of 10% fetal bovine serum (Gemini, Australia) and 1% penicillin-streptomycin (Hyclone, USA) under 5% CO2.
SPF-grade male and female mice (18.0–22.0 g) were housed under controlled conditions approved by the the Animal Ethics Committee of Guangdong Pharmaceutical University (License Number: GDPULAC2024173). They were maintained in a standardized environment with temperatures ranging from 20 to 25 °C and humidity from 40 to 70%. Mice within the same experimental group were co-housed in individual ventilated cages with visual, auditory, and olfactory contact. They had ad libitum access to food and water and were subjected to a 12 h light-dark cycle to minimize potential confounding factors. All animal experiments were conducted in accordance with relevant institutional and governmental regulations regarding the use of experimental animals.
Minimum inhibitory concentration and minimum bactericidal concentration test
The MIC was determined using the broth dilution method in 96-well plates according to the guidelines of Huan Zhou et al.14. Bacteria were cultured to the logarithmic growth phase and the bacterial density was adjusted to 1 × 106 CFU/mL. After incubation at 37 °C for 18 h, the MIC was defined as the lowest drug concentration with no visible bacterial growth. To determine the MBC, 100.0 µL of culture from each well was inoculated onto MHA plates (Solarbio, Beijing, China) and incubated at 37 °C for 16–20 h. The MBC was defined as the lowest drug concentration with no visible bacterial growth on the MHA plates.
Time-kill assay
The MRSA bacterial solution was cultured to the logarithmic growth phase and adjusted to a concentration of 1 × 106 CFU/mL15. Different concentrations of Griseorhodin C (0, 1/2×MIC, MIC) were co-cultured with the bacterial solution in 24-well plates at 37 °C for 24 h. Samples were collected at 2 h intervals and observed at 570 nm in a microplate reader and data were recorded.
SYTOX green and PI assay
MRSA cells in the logarithmic growth phase were adjusted to a concentration of 1 × 106 CFU/mL and then treated with Griseorhodin C at low, medium, and high concentrations (1/2×, 1×, 2×MIC) for 12 h at 37 °C. Subsequently, the bacteria were stained using the SYTO 9 green and PI nucleic acid dye (Life Technologies, Inc. USA) for 30 min in the dark. The cell suspensions were washed twice with PBS (pH 7.5, 0.1 M). Finally, 5.0 µL of the bacterial suspension was observed under a fluorescence microscope (EVOS M5000, Invitrogen, USA).
Scanning electron microscopy (SEM)
MRSA cells in the logarithmic growth phase were treated with Griseorhodin C at a concentration of 2×MIC for 12 h at 37 °C. Untreated MRSA cells served as the blank control group. The cells were collected and washed three times with PBS, dehydrated using a graded ethanol series, and then fixed with 2.5% glutaraldehyde and 2% paraformaldehyde for 12 h. Finally, the air-dried bacterial cells were observed using scanning electron microscopy (FEI Company, USA).
Cytotoxicity assay
Cell viability assay using MTT
The cell viability was determined using the MTT kit according to the manufacturer’s instructions. LO2 cells, HK-2 cells and HaCat cells were digested and collected, and 1 × 105 cells/well were uniformly inoculated into a 96-well cell culture plate. The cell density reached approximately 80% after incubation for 24 h. After removing the medium, Griseorhodin C was added to each well at final concentrations of 100.0 µg/mL, 50.0 µg/mL, 25.0 µg/mL, 12.5 µg/mL, 6.25 µg/mL, 3.125 µg/mL, 1.5625 µg/mL, and 0.78125 µg/mL, respectively. Medium containing 10% FBS was used as the blank control group, and medium containing 0.5% DMSO was used as the negative control group. The cells were incubated at 37 °C with 5% CO2 for 24 h and 48 h. Then, 20.0 µL of MTT solution was added to each well and incubated for 4 h. The absorbance was measured at 490 nm using a microplate reader (BioTek Instruments, Inc. USA). The experiment was repeated three times. Cell viability (%) was calculated using the following formula:
Nonlinear regression and statistical analysis were performed using GraphPad Prism 8.02 (GraphPad Software, Inc., USA) to infer the maximum half-inhibitory concentration (IC50), the concentration of the compound that resulted in a 50% reduction in cell viability.
Haemolytic activity
The in vitro hemolysis assay was performed using rabbit red blood cells (RBCs) to assess the biocompatibility of Griseorhodin C in biological solutions. RBCs were exposed to Griseorhodin C at concentrations of 1/2×MIC, MIC, and 2×MIC for 1 h. After incubation, supernatants were collected for spectrophotometric analysis at 570 nm (BioTek Instruments, Inc. USA) and photographs of treated RBCs were taken to evaluate their integrity.
Transcriptomics and metabolomics sample preparation
MRSA cells were cultured to logarithmic growth phase. Subsequently, an equal volume of the MIC concentration of the compounds was added, and the cells were cultured overnight for 12 h at 37 °C, with untreated MRSA cells serving as the control group. After incubation, cells were harvested by centrifugation at 12,000 rpm. The supernatant was discarded, and the cell pellet was washed three times with PBS. The washed cells were immediately snap-frozen in liquid nitrogen and stored at -80 °C until further analysis. The experiments were independently repeated three times as three biological replicates for each group for transcriptomics analysis, and independently repeated six times as six biological replicates for each group for metabolomics analysis.
Total RNA isolation, library construction, sequencing, and bioinformatics analysis
Total RNA was extracted using a commercial kit from Guangdong MegaGene Biotechnology Co., Ltd. (Guangzhou, China), according to the manufacturer’s instructions. RNA degradation and contamination were assessed by electrophoresis on a 1% agarose gel. Additionally, RNA quantity was measured using the Qubit 3.0 Fluorometer (Thermo Fisher Scientific, MA, USA) and Nanodrop One spectrophotometer (Thermo Fisher Scientific, MA, USA). RNA integrity was evaluated using the Agilent 4200 TapeStation system (Agilent Technologies, Waldbronn, Germany) to ensure accuracy. Library construction was performed using the ALFA-SEQ RNA Library Prep Kit II following the manufacturer’s protocol.
Following the manufacturer’s protocol, the ALFA-SEQ RNA Library Prep Kit II was utilized for library construction. The procedure involved several key steps: (a) rRNA depletion from total RNA using a commercial ribosomal RNA removal kit (Guangdong MegaGene Biotechnology Co., Ltd.,China); (b) fragmentation of RNA using the RT Buffer within the ALFA-SEQ RNA Library Prep Kit II (Guangdong MegaGene Biotechnology Co., Ltd.,China); (c) synthesis of the first strand cDNA using random hexamer primers and reverse transcriptase, followed by second strand cDNA synthesis using DNA Polymerase I and RNase H; (d) end repair and 3’ adenylation; (e) adaptor ligation, size selection, and purification; (f) digestion of double-stranded DNA; (g) PCR amplification using Hi-Fi PCR Mix and primers; (h) purification of PCR products using magnetic beads, followed by assessment of library fragment sizes on the Qsep400 high-throughput nucleic acid and protein analysis system (Hangzhou Hua Zhu Biotech Co., Ltd., China) and quantification of library concentration using Qubit 4.0 (Thermo Fisher Scientific, Waltham, USA). Sequencing of the libraries was performed on the Illumina platform, generating paired-end reads of 150 bp. Raw data in fastq format were processed using fastp (v0.23.2) (https://github.com/OpenGene/fastp) to obtain clean data. Clean reads were mapped to the NCBI Rfam database, and rRNA sequences were removed using Bowtie2 (v2.4.5) (https://github.com/BenLangmead/bowtie2). Differential gene expression analysis between two conditions/groups was conducted using DESeq2 (v1.34.0) (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html), with genes having P value < 0.05 and |log2(fold change)| ≥ 1 considered differentially expressed. KEGG pathways were retrieved from the KEGG database (http://www.kegg.jp/kegg)16.
In addition, quantitative real-time PCR (RT-qPCR) was used to validate RNA-seq results. 7 DEGs belonging to different pathways were selected, namely hrt A, arg F, dab B, lex A, ald 1, nrd D, pur K. Specific primers used for qPCR analysis were designed using Beacon Designer 7 and their respective sequences are shown in Table 1. Details on qPCR experimental conditions and data analysis are presented in Supplemental Information.
Metabolome analysis
Samples prepared as described in “Transcriptomics and metabolomics sample preparation” section were transfer to EP tubes. An extraction solution containing isotopically labeled internal standards was added, followed by vortexing for 30 s and sonication. The samples were then incubated at -40 °C for 1 h and centrifuged for 15 min. The supernatants were collected into autosampler vials for analysis. An equal volume of supernatant from all samples was mixed to prepare a quality control (QC) sample for analysis.
Target compounds were chromatographically separated using a Waters ACQUITY UPLC BEH Amide column on a Vanquish UHPLC system (Thermo Fisher Scientific, USA.). The mobile phase consisted of water (Phase A) and acetonitrile (Phase B). The autosampler tray temperature was set at 4 °C with an injection volume of 2.0 µL. The Orbitrap Exploris 120 mass spectrometer acquired both first and second-stage mass spectrometry data under the control of Xcalibur software (Version 4.4, Thermo Fisher Scientific, USA.). Detailed instrument parameters were as follows: sheath gas flow rate of 50 Arb, auxiliary gas flow rate of 15 Arb, capillary temperature of 320 °C, and spray voltage of 3.8 kV (positive mode) or -3.4 kV (negative mode).
Effect of Griseorhodin C on mouse skin infection model
We utilized a mouse skin infection model to evaluate the in vivo anti-infective activity of Griseorhodin C. Following the methods described in Liang J17, the dorsal fur of mice was shaved, and they were randomly divided into four groups: blank group, model group, Griseorhodin C group, and positive group. Except for the blank group, the remaining three groups were subjected to scalding on both sides of their dorsal area using a scaldometer(YSL-5Q, Dongfang Huabo Technology Co., LTD, Beijing, China). The MRSA bacterial solution cultured to the logarithmic growth phase was diluted with sterile saline to a final concentration of 1.5-3 × 106 CFU/mL. A volume of 20.0 µL of the bacterial solution was evenly applied to the scalded wound surface. After the solution was fully absorbed, the wound was covered with sterile gauze. Upon successful model establishment, 20.0 µL of either Griseorhodin C or vancomycin (both at a concentration of 2.0 µg/mL) was applied to the scalded wound once daily at a fixed time. Throughout the study, researchers monitored the behavior patterns of the mice, including mental state, feeding, sleeping, and general activity levels. Visual observations of the infected wounds in each group were recorded, with changes in wound color, purulent discharge, ulceration, and scab formation documented photographically.
Statistical analysis
All experiments were performed at least three times, and the results are presented as the mean ± standard deviation (SD). Statistical analyses were carried out using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). Differences between or among groups were analyzed by one-way analysis of variance (ANOVA) and Student’s t-test. A P-value of less than 0.05 was considered statistically significant and is by *. * indicates P < 0.05, and ** indicates P < 0.01.
Ethics declarations
The study received approval from the Animal Ethics Committee of Guangdong Pharmaceutical University (License Number: GDPULAC2024173). All procedures conducted were in alignmentwith the guidelines provided by the Institute of Laboratory Animal Resources for the Care and Use of LaboratoryAnimals and in compliance with ARRIVE guidelines.
Results
MIC and MBC assay
Using the broth microdilution method, the antibacterial activity of Griseorhodin C against MRSA (ATCC 433000) was determined, revealing MIC and MBC values of 1.0 µg/mL and 2.0 µg/mL, respectively. Under the same experimental conditions, vancomycin MIC value of 1.0 µg/mL. The antibacterial activity of Griseorhodin C against 10 clinical isolates of MRSA and Gram-positive bacteria are shown in Table 2.
Time-kill assay
The time-kill kinetics assay was employed to assess the bacteriostatic effect of Griseorhodin C against MRSA (ATCC 433000) at different concentrations. The results indicated that the compound at 1/2×MIC exhibited some inhibition of MRSA growth compared to the control group (Fig. 1A). Moreover, Griseorhodin C at MIC demonstrated stronger bacteriostatic activity against MRSA than the clinically positive control drug, vancomycin.
SYTOX green and PI assay
As shown in the Fig. 1C, with the increasing concentration of Griseorhodin C, the amount of red fluorescence also increases, while the corresponding green fluorescence representing viable cells decreases. This indicates that Griseorhodin C exhibits bactericidal effects against MRSA (ATCC 433000).
Scanning electron microscopy (SEM)
Morphological changes of MRSA (ATCC 433000) treated with Griseorhodin C were observed using SEM (Fig. 1B). In the control group, bacterial cells exhibited a round and spherical shape with a smooth surface and intact morphology. In contrast, treatment with Griseorhodin C at 2×MIC concentration induced noticeable morphological alterations: the cell walls appeared wrinkled, the surfaces showed signs of damage, and cellular integrity was compromised. The results indicate that Griseorhodin C exerts a significant inhibitory effect on MRSA, leading to observable changes in bacterial morphology that may ultimately result in cell death.
(A) The time-kill curves of Griseorhodin C at 1/2 × MIC and 1 × MIC against MRSA. (B) Changes in the ultrastructure of MRSA cells were observed after treatment with 2×MIC concentrations of Griseorhodin C for 12 h using SEM. Scale bar: 2 μm and 1 μm. (C) CLSM image of LIVE/DEAD stained MRSA on a cell slide: green (viable cells) and red (dead cells). Scale bar: 125 μm.
Mammalian cytotoxicity assay
Succinate dehydrogenase (SDH) within mitochondria of living cells reduces MTT to formazan, a water-insoluble blue-purple crystal, while this reaction is absent in dead cells. To assess the impact of Griseorhodin C on cell viability, LO2 cells, HK-2 cells and HaCat cells were exposed to varying concentrations of the compound for 48 h. Cell viability was assessed via MTT assay, and cellular morphology was examined microscopically.
The inhibitory effect of Griseorhodin C on LO2 cells, HK-2 cells and HaCat cells was indicated by IC50 values, which were 156.2 µg/mL, 86.32 µg/mL and 59.27 µg/mL, respectively (see Fig. 2A). The effect of Griseorhodin C on the cell morphology of the three cells was observed under the microscope. We found that at the concentration of 50 ~ 100 µg/mL, luteolin C inhibited the activity of the three cells, but the cell morphology remained normal and the arrangement was regular, and no fragmentation or cell death was observed, as depicted in Fig. 2B, C and D.
Effects of Griseorhdin C on Cells IC50 and cells morphology. (A) IC50 Values. (B) Morphological observation of LO2 cells. (C) Morphological observation of HeCat cells. (D) Morphological observation of HK-2 cells. (a: 0.0 µg/mL Griseorhdin C; b: DMSO control group; c: 50.0 µg/mL Griseorhdin C; d: 100.0 µg/mL Griseorhdin C), Scale bar: 50 μm.
Haemolytic activity
Rabbit erythrocytes were exposed to varying concentrations of Griseorhodin C for 1 h, followed by centrifugation to collect erythrocyte supernatants. Spectrophotometric analysis was performed, and photographs were taken of the supernatants. As shown in the Fig. 3, the supernatants from erythrocyte suspensions treated with 1/2 MIC, MIC, and 2×MIC concentrations of Griseorhodin C were clear, similar to the negative control group (PBS), while those in the positive control group appeared reddish and turbid. Hemolysis rates were measured at 0.70%, 1.77%, and 2.13%, respectively, for the three concentrations tested, indicating that Griseorhodin C does not cause red blood cell rupture at low, medium, and high concentrations. A hemolysis rate below 5% is considered safe in biomedical applications. This finding suggests that Griseorhodin C exhibits excellent blood compatibility under physiological conditions, with no evidence of red blood cell hemolysis.
Transcriptomics analysis
The impact of Griseorhodin C on MRSA (ATCC 433000) was investigated at the gene expression level using RNA-Seq. Volcano plots (Fig. 4B) and heat maps (Fig. 4C) illustrated the identification of 415 differentially expressed genes (DEGs) between Griseorhodin C-treated MRSA cells and the untreated control group (criteria: |log2 fold change| ≥ 1 and P < 0.05). Among these, 182 DEGs were up-regulated, while 233 DEGs were down-regulated. Principal component analysis (PCA) (Fig. 4A) revealed distinct clustering of gene expression patterns between the control and MIC-treated groups, underscoring Griseorhodin C’s molecular impact on MRSA.
To elucidate the functional implications of these DEGs, KEGG pathway enrichment analysis was conducted (P < 0.05). As depicted in Fig. 4D, these DEGs primarily participate in pathways associated with carbohydrate metabolism, ABC transporters, purine metabolism, glycine, serine, and threonine metabolism, arginine biosynthesis, glycolysis/gluconeogenesis, and nitrogen metabolism.
Screening of DEGs in MRSA induced by Griseorhodin C treatment. (A) PCA plots of DEGs. (B) Volcano plots. (C) Heatmaps of the DEGs between Griseorhodin C-treated samples (MIC 1–MIC 3) and controls (C1–C3). (D) KEGG pathways. Statistically significant DEGs were defined with an adjusted P-value (P-adj) < 0.05 and absolute log2FoldChange ≥ 1 as the cutoff threshold.
Validation of DEGs by Q-PCR
To validate the accuracy of the RNA-Seq data, a subset of 7 genes was randomly selected, and their expression levels were assessed using Q-PCR (see Supplemental Fig. S1). The results demonstrated a significant correlation between the gene expression levels measured by qPCR and those obtained from RNA-Seq, confirming the robustness and reliability of the transcriptomic data.
Metabolomic analysis
LC-MS was used to detect the dynamic changes of MRSA (ATCC 433000) cell metabolites after compound treatment. The statistical method of least orthogonal partial least squares-discriminant analysis was chosen to analyze the expression of metabolites between the compound treated group and the untreated group. As shown in the OPLS-DA (Fig. 5A), the two subgroups were clearly separated and there was a good aggregation between 6 samples in each group, indicating that the experiment had a good reproducibility. This demonstrated that the compounds significantly altered MRSA cell metabolism. Based on Volcano Plots (Fig. 5B) and statistical analysis, a total of 278 significant differential metabolites (DMs) were screened according to the P-value of Student’s t-test (≤0.05) and the Variable Importance in the Projection (VIP value ≥ 1) and fold change (|log2 (fold change)| ≥ 0).
To further understand the pathways of DMs, enrichment analysis based on KEGG pathways (see Supplemental Fig. S2) revealed their involvement primarily in arginine biosynthesis, aminoacyl-tRNA biosynthesis, alanine, aspartate, and glutamate metabolism, as well as metabolism of D-Glutamine and D-glutamate metabolism. These findings are partially consistent with earlier transcriptomic findings.Through a comprehensive analysis of the pathways associated with differential metabolites, including enrichment and topology analyses, we identified that the arginine biosynthesis pathway is the key pathway most strongly correlated with the differences in metabolites (Fig. 5C).
Integrated transcriptomics and metabolomics analysis
Integrated analysis of selected significant DEGs (Table 3) and DMs (Table 4) from transcriptomics and metabolomics revealed key pathways jointly impacted by both approaches, enriched primarily in amino acid synthesis and metabolism, as well as purine metabolism (Fig. 6). Notably, carbamate kinase (ArcC), involved in the arginine degradation pathway, was significantly downregulated at the gene level. Concurrently, metabolomics analysis showed a marked upregulation of arginine and a corresponding downregulation of its metabolite ATP. Additionally, amino acids including glutamate, glutamine, aspartate, and alanine exhibited downregulation due to compound exposure. In the purine metabolism pathway, among the 15 detected DEGs, 10 were significantly upregulated, with purC, purN, purL, purQ, and purM identified as pivotal regulators in purine biosynthesis. Consistently, metabolomic analysis indicated a significant reduction in nucleotide levels among the metabolites.
Mouse skin infected model effect of Griseordin C on mouse skin infection model
On the 1st day of modeling, all mouse skin wounds appeared round and pale, with neat edges, mild edema and sclerosis. By the 3rd day, the wounds became circular or oval-shaped, exhibiting redness and hardening of the purulent surface. By the 5th day, the wounds began to shrink and form brownish-black scabs with slight concavity. By the 9th day, the wounds had significantly shrunk and hardened, deepening in color to brown, with separation of scabs at some edges and formation of fresh red granulation tissue. On the 13th day, the scabs were completely fallen off. The drug-treated groups exhibited fully recovered skin with macular tissue, whereas the model group still had residual small wounds (Fig. 7A). Using Image J, wound area was quantified to calculate the wound healing rate for each group using the formula: Wound healing rate = (Original wound area - Unhealed wound area) / Original wound area × 100%. On the 13th day, the model group showed a wound healing rate of 81.94%; the Griseorhdin C group exhibited a wound healing rate of 96.58%; and the Vancomycin group showed a wound healing rate of 95.72% (Fig. 7B). These results indicate that Griseorhodin C effectively treats MRSA-infected burn wounds in mice.
The HE staining results were show in Fig. 7C, the skin epidermis of the blank control group exhibited an intact structure with a clear stratum corneum, orderly arranged cells, and intact appendages such as hair follicles and sebaceous glands. Adipocytes and myofibers were neatly arranged, dense, uniform in size, with normal nuclear morphology, and no significant pathological changes were observed. Untreated mouse skin showed massive infiltration of inflammatory cells and necrosis of epidermis and hair follicles. Skin from drug-treated mice also exhibited inflammatory cell infiltration, albeit to a lesser extent compared to the model group, with regeneration observed in the epidermis and clear hair follicle structures. The Griseorhodin C-treated group showed fewer inflammatory cells, lower thickness, and better structural integrity of hair follicles and other structures compared to the Vancomycin-treated group, indicating a superior wound healing effect of Griseorhodin C over Vancomycin.
Efficacy of Gresiordin C in mouse skin wound infection model. (A) Daily treatment with Griseorhodin C (2.0 µg/ml) or vancomycin (2.0 µg/ml) was administered to wounds in the treatment group, while the model group received an equal volume of solvent. Representative photographs of wounds were taken daily for 13 consecutive days. (B) Representative histological sections of skin wounds stained with hematoxylin and eosin on day 13 after infection were captured using an optical microscope (n = 6). (C) Healing rate of infected wounds in mice.
Discussion
In our preliminary study, we isolated 159 actinomycete strains from the intestinal tract of Periplaneta americana. Among these, the fermentation broth of strain WA23-2-1 exhibited significant anti-MRSA activity. We conducted large-scale fermentation of this strain and ultimately isolated a purple antimicrobial compound, Griseorhodin C. Griseorhodin C is a compound from the rubromycin family. Rubromycins are generally characterized by a hexacyclic framework composed of a bis-benzannulated [5,6]-spiroketal core flanked with a highly oxygenated naphthazarin motif and an isocoumarin unit18. Due to the significant antimicrobial19, anti-cancer, or enzyme inhibition properties20 of rubromycin compounds, they have attracted great attention from researchers. K. Eckardt et al. first isolated and identified Griseorhodin C from S. californicus JA 2640. Griseorhodin C exhibited inhibitory activity against B. subtilis, S. aureus, P. notatum, and human KB carcinoma cells21. Given these properties, we aimed to explore Griseorhodin C’s potential as an antibacterial agent, particularly against MRSA.
In our antibacterial activity study, we were pleasantly surprised to find that Griseorhodin C exhibits the same inhibitory effect on MRSA and S. aureus, with a MIC of 1 µg/mL. The MIC range for 10 clinical strains of MRSA was found to be between 0.5 and 2 µg/mL. Griseorhodin C exhibits antibacterial activity against MRSA similar to that of 8-Methoxygriseorhodin C, which has a MIC of 0.78 µg/mL against MRSA22. In Bailey W. Miller et al.’s study, simultaneous treatment of 7,8-dideoxygriseorhodin C with oxacillin resulted in an approximately tenfold decrease in MIC with a combination index of < 0.5, indicating synergistic anti-MRSA activity23, however, in our preliminary study, Griseorhodin C and oxacillin did not exhibit a synergistic bactericidal effect against MRSA (FIC = 0.75, the results are not presented in this article). Under the same experimental conditions, we compared the antibacterial activity of vancomycin and griseorhodin C against MRSA (ATCC 433000) and we were surprised to find that the antibacterial activities of Vancomycin and Griseorhodin C against MRSA had the same MIC value. To further investigate its antibacterial effects, we conducted time-kill curve assays using varying concentrations of Griseorhodin C against MRSA (ATCC 433000). The results demonstrated a strong concentration-dependent inhibitory effect of Griseorhodin C on MRSA. Interestingly, in the time-kill curve experiments, Griseorhodin C demonstrated superior inhibitory effects compared to vancomycin. Furthermore, in subsequent mouse skin burn models, Griseorhodin C also showed enhanced recovery outcomes. These findings suggest that Griseorhodin C may be a more effective treatment option for MRSA infections.
Cytotoxicity is a critical factor in determining the safety of biomedical materials24. Therefore, we evaluated the biosafety of Griseorhodin C through mammalian cytotoxicity assays and hemolysis tests. Griseorhodin compounds exhibited moderate cytotoxicity at micromolar concentrations to tumor cells20,21, however, our results indicated that Griseorhodin C does not inhibit the activity of LO2, HK-2, and HaCat cells within a certain concentration range and does not cause red blood cell rupture or hemolysis under physiological conditions, demonstrating good blood compatibility. This may be related to the specific inhibition of the telomerase enzyme by Griseorhodin C. Telomerase is highly expressed in 80–90% of cancer cells while almost completely absent in neighboring healthy cells25. Additionally, we investigated the potential therapeutic effect of Griseorhodin C on a mouse model of MRSA infection in scalded wounds. MRSA is thought to be the most common cause of shallow incision surgical site infections (SSIs), which involve only the skin or subcutaneous tissue of the incision. These infections impede wound healing, significantly affect keratin formation, and delay angiogenesis26. Our study demonstrated that Griseorhodin C has a favorable bactericidal effect and biosafety, suggesting that it is a promising candidate for the development of new antibiotics against MRSA-associated infections.
Although the antibacterial activity of rubromycins against MRSA has been reported in the literature, the antibacterial mechanism of rubromycins has not been detailed. In structure–activity relationship study, the naphthazarin moiety and the free methylene at C-3′ were regarded as critical for antimicrobial activity27, and while the intact [5,6]-spiroketal and isocoumarin units were essential for telomerase inhibitory activity. Nevertheless, it is becoming evident from various studies that bactericidal activity is often multifaceted. For instance, research into the inactivation mechanism of Escherichia coli by graphene quantum dots (GQDs) has revealed that GQDs induce E. coli inactivation through a combination of effects, including disruption of biofilm formation and disturbances in sulfur and nitrogen metabolism28. Similarly, Maomao Ma and colleagues discovered that the compound monocaprin controls the growth of MRSA pathogens in pork by causing osmotic imbalance, inhibiting amino acid metabolism, disrupting energy metabolism, and impairing aerobic respiration. Additionally, the X33 antimicrobial oligopeptide (X33 AMOP) inhibits the growth of Penicillium digitatum by affecting cell integrity, genetic information processing, oxidative stress tolerance, and energy metabolism29.
To more comprehensive elucidate the mechanism by which Griseorhodin C inhibits MRSA, we performed comprehensive transcriptomic and metabolomic analyses. Our data revealed significant changes in the expression of genes and metabolites associated with amino acid biosynthesis, metabolism, and purine metabolism. Specifically, we identified 415 DEGs and 278 DEMs. Combining metabolomic and transcriptomic analyses, we found that Griseorhodin C may exert its antibacterial effects by influencing amino acid and purine metabolic pathways. Findings from other studies have shown that β-lactam, aminoglycoside, and quinolone antibiotics induce significant alterations in metabolic pathways related to pyrimidine metabolism, amino acid metabolism, and purine metabolism in both Methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA strains30. These observations are consistent with our data, suggesting that Griseorhodin C may similarly influence these metabolic pathways in MRSA.
Arginine is an essential amino acid in humans and serves as a nitrogen, carbon, and energy source for many bacteria, enhancing their survival in adverse environments. Arginine degradation can occur through the arginase pathways and arginine deiminase (ADI) pathways, with the latter being commonly found in bacteria31. The arginine deiminase (ADI) pathway is crucial for arginine degradation metabolism in microbiota, facilitating microorganism survival and resistance to host attacks in diverse harsh conditions32. Within bacteria, arginine can be metabolically converted to ATP, NH4+, and CO2 via the ADI pathway, which typically serves as an important energy source and plays a significant role in protecting bacterial cells from acidic environments33. Research by Sattaporn Weawsiangsang et al. demonstrated that hydroquinone inhibits the growth of multidrug-resistant Pseudomonas aeruginosa by suppressing the arginine deiminase (ADI) pathway34. Recent studies have shown that disrupting the ADI pathway can restore the biofilm of Streptococcus pyogenes to a level sensitive to antibiotics, thereby providing a new therapeutic target for clinical antibiofilm therapy35. The ADI pathway consists of arginine/ornithine antiporter (arcD), arginine deiminase (arcA), ornithine transcarbamylase (arcB), and carbamate kinase (arcC). Carbamate kinase (CK) catalyzes the final step of the ADI pathway, converting carbamoyl phosphate into ATP, NH4+, and CO236. After treatment of MRSA with Griseorhodin C, transcriptomic analysis revealed significant downregulation of CK, while metabolomic analysis showed increased expression of arginine and decreased expression of ATP. Therefore, we hypothesize that the compound can inhibit the ADI pathway in MRSA cells, reducing the production of ATP and NH4+, thereby affecting the energy supply required for bacterial growth. This leads to an excessively acidic intracellular environment, impacting cell vitality, rendering the bacteria acid-intolerant, and resulting in cell death. Moreover, ammonia is crucial for the synthesis of glutamate and glutamine within cells; its depletion impedes glutamate biosynthesis.Metabolomic consequence indicated significant downregulation of metabolites in the pathways of alanine, aspartate, and glutamate, which are vital for stress tolerance in bacteria against acidity, metals, and oxidative stress37. Studies suggest that glutamate enhance the acid tolerance of Lactic Acid Bacteria (LAB), enabling survival in highly acidic environments38. During the freeze-drying process of Lactobacillus plantarum LIP-1, the addition of aspartic acid significantly reduces damage to the bacterial cell wall, cell membrane, and DNA, indicating the important role of aspartic acid in maintaining bacterial survival39. These results suggest that Griseorhodin C may exert its bactericidal effect on MRSA by disrupting the ADI pathway, thereby impairing arginine synthesis and metabolism. This disruption leads to compromised energy metabolism and reduced environmental tolerance in MRSA, ultimately resulting in bacterial cell death.
Purines play a central role in cellular physiology, serving as essential components in energy synthesis, cellular signal transduction, and nucleotide biosynthesis40, making them indispensable for bacterial survival. In this study. RNA-Seq analysis revealed that out of 15 DEGs involved in purine metabolism, 10 genes were significantly upregulated. Among these, purC, purN, purL, purQ, and purM are regulators of purine biosynthesis, suggesting an upregulation of purine synthesis in MRSA cells under the influence of Griseorhdin C. Additionally, energy carriers adenosine triphosphate (ATP) and guanosine triphosphate (GTP) are derived from their respective nucleotide adenosine and guanosine33,41. However, in our metabolomics analysis, we observed a decrease in purine synthesis products such as dGTP, ADP, UMP, ATP, CDP, UDP, NAD, and CTP by factors of 1.69, 0.52, 0.58, 1.69, 1.14, 1.46, and 1.28, respectively. This suggests that exposure to the stress conditions induced by the compound leads to DNA damage in MRSA cells, resulting in a decrease in nucleotides. Several studies have shown that the expression levels of purine synthesis related genes are significantly correlated with drug resistance. For example, Emmanuel Mongodin et al. demonstrated that serial passaging of MRSA strains with vancomycin in vitro led to increased resistance compared to the parental strain42. They observed significant upregulation of purine biosynthesis regulatory genes in these adapted strains. They hypothesized that this phenomenon occurs because resistant strains require more energy to sustain survival, thus enhancing purine synthesis pathways to compensate for energy deficiencies. This supports the viewpoint that purine biosynthesis is associated with bacterial survival under stressful conditions. Here, we speculate that due to the compound’s action, it exerts strong stimuli on MRSA cells, leading to nucleotide and energy disruption in MRSA, prompting bacteria to enhance purine synthesis pathways to withstand drug-induced damage and ensure survival. Moreover, studies by Yee R. et al. show that genes (purB and purM) involved in purine biosynthesis are important for antibiotic and stress tolerance in S. aureus43. These findings collectively underscore the importance of purine metabolism in bacterial adaptation to stress and resistance mechanisms, highlighting potential targets for therapeutic interventions against resistant pathogens. In conclusion, Griseorhodin C can induce bacterial DNA damage, thereby inhibiting bacterial growth.
Conclusion
In conclusion, Griseorhodin C exerts its antibacterial effects through a multifaceted mechanism involving metabolic disruption and DNA damage. Its potent activity and favorable biosafety profile position Griseorhodin C as a promising new antibiotic candidate for combating MRSA infections. Future research and clinical trials are needed to further explore its potential and application in medical practice.
Data availability
The datasets generated during and/or analysed during the current study are available. Transcriptomics data have been deposited in the SRA database with dataset identifiers: PRJNA1151237. The remaining data are presented in supplementary materials. The raw data supporting the conclusions of this article will be made available by the authors (Jingyang Liao) on request.
References
Mancuso, G., Midiri, A., Gerace, E. & Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens. 10. https://doi.org/10.3390/pathogens10101310 (2021).
Chalmers, S. J. & Wylam, M. E. Methicillin-Resistant Staphylococcus Aureus (MRSA) Protocols Cutting-Edge Technologies and Advancements Third Edition. Methods in Molecular Biology http://www.springer.com/series/7651 (2019).
Bateman, R. M. et al. 36th International symposium on intensive care and emergency medicine. Crit. Care. 20, 94 (2016).
Parra, J. et al. Antibiotics from rare actinomycetes, beyond the genus Streptomyces. Curr. Opin. Microbiol. 76. https://doi.org/10.1016/j.mib.2023.102385 (2023).
Francine, P. Systems biology: New insight into antibiotic resistance. Microorganisms. 10. https://doi.org/10.3390/microorganisms10122362 (2022).
Chernov, V. M., Chernova, O. A., Mouzykantov, A. A., Lopukhov, L. L. & Aminov, R. I. Omics of antimicrobials and antimicrobial resistance. Expert Opin. Drug Discov. 14, 455–468. https://doi.org/10.1080/17460441.2019.1588880 (2019).
Türkyılmaz, O. & Darcan, C. Resistance mechanism of Escherichia coli strains with different ampicillin resistance levels. Appl. Microbiol. Biotechnol. 108, 1–14 (2024).
Coleman, S. R., Blimkie, T., Falsafi, R. & Hancock, R. E. W. Multidrug adaptive resistance of Pseudomonas aeruginosa swarming cells. https://doi.org/10.1128/AAC (2020).
Li, Y., He, R., Chen, H., Chen, D. & Chen, W. Respiratory depression as antibacterial mechanism of linalool against Pseudomonas fragi based on metabolomics. Int. J. Mol. Sci. 23, (2022).
Zhang, S. B. et al. Antifungal mechanism of 1-nonanol against Aspergillus flavus growth revealed by metabolomic analyses. Appl. Microbiol. Biotechnol. 105, 7871–7888 (2021).
Tang, C. et al. Exploring antimicrobial mechanism of essential oil of Amomum villosum Lour through metabolomics based on gas chromatography-mass spectrometry in methicillin-resistant Staphylococcus aureus. Microbiol. Res. 242, (2021).
Hussein, M. et al. Integrated transcriptomic and metabolomic mapping reveals the mechanism of action of Ceftazidime/Avibactam against pan-drug-resistant Klebsiella pneumoniae. ACS Infect. Dis. 9, 2409–2422 (2023).
Ying, J. P. et al. Combined analysis of transcriptomics and metabolomics provide insights into the antibacterial mechanism of bacteriocin XJS01 against multidrug-resistant Staphylococcus aureus. Sci. Total Environ. 917, (2024).
Zhou, H., Chen, L., Ouyang, K., Zhang, Q. & Wang, W. Antibacterial activity and mechanism of flavonoids from Chimonanthus salicifolius S. Y. Hu. and its transcriptome analysis against Staphylococcus aureus. Front. Microbiol. 13, (2023).
Wu, J. et al. Longistylin A, a natural stilbene isolated from the leaves of Cajanus cajan, exhibits significant anti-MRSA activity. Int. J. Antimicrob. Agents. 55, (2020).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28http://www.genome.ad.jp/kegg/ (2000).
Liang, J. et al. Study on skin infection model of Staphylococcus aureus based on analytic hierarchy process and Delphi method. Heliyon. 9, (2023).
Atkinson, D. J. & Brimble, M. A. Isolation, biological activity, biosynthesis and synthetic studies towards the rubromycin family of natural products. Nat. Prod. Rep. 32, 811–840. https://doi.org/10.1039/c4np00153b (2015).
Eckardt, K. et al. The structure of the antibiotic griseorhodin C. J. Antibiot. 970–973 (1978).
Yunt, Z. et al. Cleavage of four carbon-carbon bonds during biosynthesis of the griseorhodin a spiroketal pharmacophore. J. Am. Chem. Soc. 131, 2297–2305 (2009).
Stroshane, R. M. et al. Isolation and structure elucidation of a novel griseorhodin. J. Antibiot. 197–204 (1979).
Yang, J. X. et al. 8-Methoxygriseorhodin C, a new member of griseorhodin antibiotic. J. Antibiot. 1277–1279 (1991).
Panzone G. et al. Isolation and structure elucidation of 7,8-dideoxy-6-oxo-griseorhodin C produced by Actinoplanes ianthinogenes. J. Antibiot. 665–670 (1997).
Zhao, D. et al. Screening of chitosan derivatives-carbon dots based on antibacterial activity and application in anti-Staphylococcus aureus biofilm. Int. J. Nanomed. 17, 937–952 (2022).
Ueno, T. et al. Inhibition of human telomerase by rubromycins: Implication of spiroketal system of the compounds as an active moiety. Biochemistry 39, 5995–6002 (2000).
Simonetti, O. et al. New Evidence and Insights on Dalbavancin and Wound Healing in a Mouse Model of Skin Infection. https://journals.asm.org/journal/aac (2020).
Lackner, G. et al. Biosynthesis of pentangular polyphenols: Deductions from the benastatin and griseorhodin pathways. J. Am. Chem. Soc. 129, 9306–9312 (2007).
Fang, Y. et al. Transcriptomic and metabolomic investigation of molecular inactivation mechanisms in Escherichia coli triggered by graphene quantum dots. Chemosphere. 311, (2023).
Lin, S., Wang, Y., Lu, Q., Zhang, B. & Wu, X. Combined transcriptome and metabolome analyses reveal the potential mechanism for the inhibition of Penicillium digitatum by X33 antimicrobial oligopeptide. Bioresour. Bioprocess. 8, (2021).
Schelli, K., Zhong, F. & Zhu, J. Comparative metabolomics revealing Staphylococcus aureus metabolic response to different antibiotics. Microb. Biotechnol. 10, 1764–1774 (2017).
Cunin, R., Glansdorff, N., Pierard, A. & Stalon, V. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50https://journals.asm.org/journal/mr (1986).
Xiong, L. et al. Arginine metabolism in bacterial pathogenesis and cancer therapy. Int. J. Mol. Sci. 17https://doi.org/10.3390/ijms17030363 (2016).
Casiano-Colon, A. & Marquis, R. E. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl. Environ. Microbiol. https://journals.asm.org/journal/aem (1988).
Weawsiangsang, S. et al. Hydroquinine inhibits the growth of multidrug-resistant Pseudomonas aeruginosa via the suppression of the arginine deiminase pathway genes. Int. J. Mol. Sci. 24, (2023).
Freiberg, J. A. et al. The arginine deiminase pathway impacts antibiotic tolerance during biofilm-mediated Streptococcus pyogenes infections. https://doi.org/10.1128/mBio (2020)
Pols, T., Singh, S., Deelman-Driessen, C., Gaastra, B. F. & Poolman, B. Enzymology of the pathway for ATP production by arginine breakdown. FEBS J. 288, 293–309 (2021).
Djoko, K. Y. et al. Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc. Natl. Acad. Sci. U S A 114, 6818–6823 (2017).
Qiao, Y. et al. Metabolic profiles of cysteine, methionine, glutamate, glutamine, arginine, aspartate, asparagine, alanine and glutathione in Streptococcus thermophilus during pH-controlled batch fermentations. Sci. Rep. 8, (2018).
Chen, Z. et al. The effect of aspartic acid on the freeze-drying survival rate of Lactobacillus plantarum LIP-1 and its inherent mechanism. LWT 155, (2022).
Wu, H. liang et al. Targeting nucleotide metabolism: a promising approach to enhance cancer immunotherapy. J. Hematol. Oncol. 15https://doi.org/10.1186/s13045-022-01263-x (2022).
Giuliani, A. L., Sarti, A. C. & Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 205 16–24. https://doi.org/10.1016/j.imlet.2018.11.006 (2019).
Kilstrup, M., Hammer, K., Jensen, P. R. & Martinussen, J. Nucleotide metabolism and its control in lactic acid bacteria. FEMS Microbiol. Rev. 29 555–590. https://doi.org/10.1016/j.femsre.2005.04.006 (2005).
Mongodin, E. et al. Microarray transcription analysis of clinical Staphylococcus aureus isolates resistant to vancomycin. J. Bacteriol. 185, 4638–4643 (2003).
Yee, R., Cui, P., Shi, W., Feng, J. & Zhang, Y. Genetic screen reveals the role of purine metabolism in Staphylococcus aureus persistence to rifampicin. Antibiotics 4, 627–642 (2015).
Funding
This work was funded by the Guangzhou Science and technology planning project (202201010357), and the Guangdong Medical Science and Technology Research Foundation (A2024478;B2023440).
Author information
Authors and Affiliations
Contributions
W. L, X J designed and planned the research. J. L wrote the main manuscript text. J .T, X .L finished transcriptomics and metabolomics . D.H, L .W finished Figs. 1, 2, 3 and 4 and Z. C, L.G finished Figs. 5, 6 and 7,software, L .Z, writing review and editing, X .J, supervision, W .L, X. J. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liao, J., Tan, J., Li, X. et al. Combined transcriptome and metabolome analysis revealed the antimicrobial mechanism of Griseorhodin C against Methicillin-resistant Staphylococcus aureus. Sci Rep 14, 30242 (2024). https://doi.org/10.1038/s41598-024-76212-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76212-4