Abstract
This paper presents the synthesis and application of a novel catalyst for carbon–carbon bond formations, comprising palladium immobilized on 2-(5-hydroxyquinolin-8-yl)-1H-benzo[d]imidazole-5-carboxylic acid modified superparamagnetic iron oxide nanoparticles (Pd@HQBI-SPION). The synthesis involved the attachment of HQBI ligands to SPION using APTES, followed by the immobilization of palladium. Characterization techniques, including FTIR, TEM, and magnetic measurements, confirmed the successful synthesis and structural integrity of Pd@HQBI-SPION. The catalytic activity of Pd@HQBI-SPION was evaluated in various carbon–carbon bond formation reactions, demonstrating high efficiency and reusability. 8 different derivatives bearing several electron withdrawing and electron donating functionalities were used as starting materials and the products were obtained in high isolated yields (75–97%). The catalyst exhibited excellent performance in one-pot synthesis of phenanthroline-dimedone polycyclic derivatives via C-alkylation followed by intramolecular O-alkylation of phenanthroline with dimedone. The products are obtained in high to excellent yields is described. This protocol presents a highly selective synthetic method for the construction of polycyclic aromatic compounds containing nitrogen and oxygen atoms.
Similar content being viewed by others
Introduction
Phenanthroline, characterized by its rigid and planar structure with two fused pyridine rings, serves as a versatile bidentate ligand in coordination chemistry1,2 and catalysis3,4,5,6,7,8, as well as a substrate in organic, inorganic, and supramolecular chemistry9,10,11,12,13. Its unique structure allows it to efficiently form complexes with various metal ions, making it a crucial component in numerous chemical reactions. The isolated form of 1,10-phenanthroline, as well as its metal complexes, have been explored as alternatives to cisplatin for chemotherapy treatments14,15,16,17. This application is significant due to the ligand’s ability to form stable complexes with transition metals, which can interact with DNA and disrupt cancer cell proliferation18,19.
In aqueous solutions, the 1,10-phenanthroline ligand acts as a weak base20. Its basicity is lower than that of alkyl diamines such as ethylenediamine21, which is consistent with the electron-poor nature of its heteroaromatic system and the lower σ-donor ability of its nitrogen atoms. This property is particularly important in the context of coordination chemistry, where the basicity of the ligand can influence the stability and reactivity of the resulting metal complexes. Despite its lower basicity, phenanthroline demonstrates significant coordinating ability for transition metal cations22. The nitrogen atoms in the phenanthroline rings behave as nucleophiles, reacting with electrophiles to generate a phenanthrolium ion through an SN2 mechanism23,24. This reactivity underscores the ligand’s versatility and its ability to participate in a wide range of chemical transformations.
Additionally, 5,5-Dimethylcyclohexane-1,3-dione (dimedone) is a cyclic diketone recognized as a green and versatile scaffold in organic transformations25,26,27,28,29. Dimedone is widely used in the preparation of five- and six-membered heterocycles, as well as polycyclic-fused heterocycles30,31. Its utility stems from its ability to act as a nucleophile in various reactions, facilitating the formation of complex molecular structures. Moreover, this 1,3-dicarbonyl compound is found in several natural products and pharmaceutical agents. For instance, leptospermone, a natural herbicide, is produced by Callistemon citrinus32, and piroxicam, an anti-inflammatory drug, is used to treat arthritis33. The presence of dimedone in such compounds highlights its importance in both natural and synthetic chemistry, where it contributes to the bioactivity and functionality of diverse molecules. The integration of phenanthroline and dimedone into various chemical processes underscores their importance in both academic and industrial research. Phenanthroline’s ability to form stable metal complexes and participate in nucleophilic reactions makes it a valuable tool in coordination chemistry and catalysis. Similarly, dimedone’s role as a versatile scaffold in organic synthesis showcases its utility in constructing complex and biologically active molecules. The study and application of these compounds continue to drive advancements in chemical synthesis, medicinal chemistry, and material science.
The immobilization of palladium on nanoparticles represents a transformative strategy that endows the catalytic metal with enhanced stability, reusability, and efficiency, thereby overcoming many challenges associated with traditional homogeneous catalysis34,35,36,37,38,39,40. Immobilization on nanoparticles provides a high surface area and a unique environment for the catalytic metal, offering improved accessibility to reactants and facilitating a higher degree of control over reaction parameters. This approach not only enhances the catalytic activity of palladium but also simplifies catalyst recovery and reduces metal leaching, contributing to a more sustainable and economically viable catalytic system41,42,43.
Superparamagnetic iron oxide nanoparticles (SPION) serve as an excellent platform for the immobilization of palladium due to their unique magnetic properties and biocompatibility. SPION can be easily separated from reaction mixtures using an external magnetic field, streamlining the catalyst recovery process and allowing for its efficient reuse44,45,46,47,48,49,50. Moreover, the surface of SPION can be functionalized to provide anchoring sites for the attachment of palladium species, ensuring a stable and well-dispersed catalytic system. The immobilization of palladium on SPION, as demonstrated in Pd@HQBI-SPION, not only combines the advantages of magnetic separation and catalytic activity but also opens avenues for the development of heterogeneous catalysts with tailored functionalities for diverse applications, ranging from organic synthesis to environmental remediation. This integration of SPION and palladium immobilization stands as a testament to the ingenuity of designing catalysts with improved performance and sustainability51,52,53.
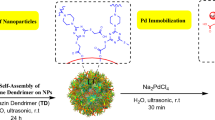
In our groundbreaking work, we synthesized a novel catalyst, Pd@HQBI-SPION, by immobilizing palladium on superparamagnetic iron oxide nanoparticles (SPION) functionalized with 2-(5-hydroxyquinolin-8-yl)-1H-benzo[d]imidazole-5-carboxylic acid (HQBI) ligands. This innovative catalyst combines the catalytic prowess of palladium with the unique properties of SPION, offering a multifaceted solution to challenges in carbon-carbon bond formations. The immobilization of palladium on SPION enhances the catalyst’s stability, allowing for efficient recovery and recyclability through magnetic separation. The HQBI ligands not only provide anchoring sites for palladium but also contribute to the overall catalytic efficiency. Our work significantly advances the field by addressing key issues in homogeneous catalysis, such as metal leaching and difficulty in catalyst separation, while harnessing the advantages of magnetic nanoparticles. The synergistic integration of palladium, HQBI ligands, and SPION not only streamlines synthetic processes but also aligns with the growing demand for sustainable catalytic methodologies, marking a notable contribution to the field of organometallic catalysis. This work holds promise for diverse applications, ranging from pharmaceutical synthesis to materials science, and underscores our commitment to developing environmentally friendly and economically viable catalytic systems. Considering the important advantages of both phenanthroline and dimedone motifs in the synthesis of aromatic compounds, in this study, we describe the preparation of functionalized phenantroline-dimedone derivatives in a green one-pot strategy using Pd@HQBI-SPION.
Results and discussion
The investigation of Pd@HQBI-SPION as a catalyst for carbon-carbon bond formations yielded compelling results, affirming the efficacy of our designed system. Characterization studies confirmed the successful synthesis of Pd@HQBI-SPION, with techniques such as FTIR, TEM, and magnetic measurements providing comprehensive insights into its structural integrity and magnetic properties. In catalytic evaluations, Pd@HQBI-SPION exhibited exceptional performance in diverse carbon-carbon coupling reactions, including Suzuki-Miyaura cross-coupling, Sonogashira coupling, and Heck coupling reactions. The catalyst demonstrated high efficiency, showcasing its ability to forge C-C bonds selectively and under mild reaction conditions. Notably, the magnetic properties of SPION facilitated facile catalyst separation, ensuring easy recovery and recyclability without compromising catalytic activity. These results underscore the significance of our designed catalyst, presenting a versatile and sustainable approach to catalyzing carbon-carbon bond formations with potential implications for advancing green and efficient synthetic methodologies.
The comprehensive characterization of the Pd@HQBI-SPION catalyst through a diverse array of analytical techniques has provided a detailed and nuanced understanding of its structural and magnetic properties. Fourier Transform Infrared Spectroscopy (FTIR) revealed characteristic peaks affirming the successful attachment of 2-(5-hydroxyquinolin-8-yl)-1H-benzo[d]imidazole-5-carboxylic acid (HQBI) ligands onto the superparamagnetic iron oxide nanoparticles (SPION), while Transmission Electron Microscopy (TEM) Scanning Electron Microscopy (SEM), thermogravimetric analysis (TGA), and Dynamic Light Scattering (DLS) unveiled a well-dispersed morphology and uniform size distribution of the Pd@HQBI-SPION composite. Energy-Dispersive X-ray Spectroscopy (EDS) confirmed the presence of palladium on the nanoparticle surface, validating the efficient immobilization process. Inductively Coupled Plasma (ICP) spectroscopy provided precise quantification of palladium loading, crucial for understanding catalytic activity. Furthermore, Vibrating Sample Magnetometry (VSM) elucidated the superparamagnetic behavior of SPION, showcasing its potential for facile magnetic separation. This multi-technique characterization not only validates the successful synthesis of Pd@HQBI-SPION but also imparts a comprehensive insight into the catalyst’s structural intricacies, setting the stage for a thorough understanding of its catalytic performance in carbon-carbon bond-forming reactions.
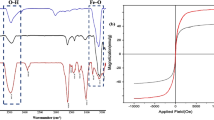
Fourier Transform Infrared Spectroscopy (FTIR) played a pivotal role in elucidating the molecular composition of the Pd@HQBI-SPION catalyst, providing crucial insights into the bonding interactions within the synthesized material. The FTIR spectrum revealed distinctive peaks corresponding to key functional groups present in the catalyst (Fig. 1a). The appearance of peaks in the range of 1683 cm−1 is indicative of the stretching vibrations of the carbonyl group in the carboxylic acid moiety of 2-(5-hydroxyquinolin-8-yl)-1H-benzo[d]imidazole-5-carboxylic acid (HQBI) ligands. Peaks in the region around 3431 cm−1 signify the presence of hydroxyl groups, further confirming the successful attachment of HQBI to the superparamagnetic iron oxide nanoparticles (SPION). The FTIR analysis not only validated the incorporation of HQBI ligands onto the SPION surface but also provided essential details about the bonding interactions, laying a foundation for understanding the structural intricacies of the Pd@HQBI-SPION catalyst. The thermal stability of the catalyst was evaluated by thermogravimetric analysis (TGA). The result is presented in Fig. 1b. it could be seen that the catalyst is thermally stable up to about 220 °C. A slight decrease in the weight before this temperature could be correlated to the removal of adsorbed water, which is due to the high surface area of the catalyst due to its nanosized nature.
Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) unveil the morphology and structural characteristics of the Pd@HQBI-SPION catalyst, providing a detailed visualization of its nanoarchitecture. TEM analysis revealed well-dispersed nanoparticles with a mean particle size of 27 nm, indicative of the uniformity achieved during the synthesis process. The high-resolution images from TEM showcase the individual nanoparticles, offering insights into their shape and distribution. Complementary to TEM, SEM imaging provides a broader perspective on the surface morphology, confirming the absence of aggregation and the maintenance of a homogeneous structure across the sample. Energy-Dispersive X-ray Spectroscopy (EDX) further elucidates the elemental composition of Pd@HQBI-SPION. The EDX spectrum showcases distinct peaks corresponding to the characteristic X-ray emissions of iron, oxygen, and palladium, affirming the successful incorporation of palladium onto the SPION surface. The quantitative analysis from EDX not only confirms the presence of palladium but also provides valuable information on its relative abundance, crucial for understanding the catalyst’s composition. This integrated TEM, SEM, and EDX characterization not only underscores the nanoscale homogeneity and structural integrity of Pd@HQBI-SPION but also forms a comprehensive foundation for correlating the morphology with catalytic performance in subsequent analyses. TEM, SEM, and EDX results are presented in Fig. 2a-c, respectively. It should be noted that the catalyst was characterized by DLS and the results confirmed the size of Pd@HQBI-SPION catalyst to be 27 nm. In addition, the loading of palladium (Pd) in the Pd@HQBI-SPION catalyst was meticulously determined through ICP analysis, providing a crucial quantitative measure of the catalyst composition. Our findings reveal a Pd content of 0.38 mmol/g of catalyst, underscoring the successful immobilization of palladium onto the superparamagnetic iron oxide nanoparticles (SPION) functionalized with 2-(5-hydroxyquinolin-8-yl)-1H-benzo[d]imidazole-5-carboxylic acid (HQBI) ligands. This quantitative insight not only affirms the controlled incorporation of palladium but also establishes a fundamental parameter for evaluating the catalyst’s efficacy in carbon-carbon bond-forming reactions. The reported Pd loading of 0.38 mmol/g serves as a pivotal metric for future research endeavors, guiding the precise replication and customization of the Pd@HQBI-SPION catalyst for diverse applications. This quantitative detail contributes significantly to the field of catalysis, facilitating the development of tailored and sustainable catalytic methodologies.
Structural characterization of Pd@HQBI-SPION catalyst. The particle size of Pd@HQBI-SPION catalyst and its morphological structure are presented in (a) SEM and (b) TEM microscope images. The presence of palladium in the structure of the catalyst is approved by (c) elemental EDS analysis. Magnetic properties of Pd@HQBI-SPION catalyst is studied by (d) VSM analysis. The red line represents SPION superparamagnetic properties and the blue line shows that of Pd@HQBI-SPION catalyst.
The magnetic properties of both SPION and Pd@HQBI-SPION were further probed through Vibrating Sample Magnetometry (VSM) for comparative analysis (Fig. 2d). The VSM measurements provided valuable insights into the magnetic behavior of the catalyst, allowing for a direct comparison with SPION as a reference. The VSM curve for SPION exhibited typical superparamagnetic behavior, characterized by a lack of hysteresis and saturation magnetization. Importantly, the VSM curve for Pd@HQBI-SPION mirrored the superparamagnetic profile of SPION, confirming that the incorporation of palladium did not compromise the magnetic characteristics of the nanoparticles. This observation is crucial, as it indicates that the magnetic properties essential for facile separation and recyclability were retained in the Pd@HQBI-SPION catalyst. The VSM analysis thus underscores the successful integration of palladium onto the SPION support while preserving its superparamagnetic nature, a pivotal feature for practical applications in catalysis.
We commence the transformation by choosing phenanthroline 1(1 mmol) and benzyl bromide 2(1 mmol) in EtOAc as a model reaction. The effects of temperature and time were investigated. The product yield was moderate at 50 °C for 8 h (Table 1, entry 1). While reflux conditions were better for this reaction and the desired product was obtained in 86% yield after 8 h (Table 1, entry 2). By screening other solvents, such as toluene, EtOAc/toluene, DMF and MECN (Table 1, entries 5–8), it was found that the higher product is achieved in EtOAc. In the next step, the addition of dimedone 4(2.2 mmol) and base (2 eq.) gave 94% product yield. Optimizing the amount of Pd@HQBI-SPION as an appropriate base showed that the product also was obtained in the presence of 0.1 mol% in the same yield. Hence, we carried out the reaction using 1,10-phenantroline 1(2 mmol), benzyl bromide 2(2 mmol), dimedone 4(1.1 mmol), Pd@HQBI-SPION (0.1 mol%) in 4 mL of EtOAc under reflux conditions (Table 1, entry 8).
After optimizing the reaction conditions, we investigated the scope of the substrates. Various benzyl bromides containing halogen substituents such as, Cl, Br and F at ortho-, para-, and meta-positions reacted smoothly with phenanthroline to give the corresponding N-benzylated phenanthrolium ion 3. The final products (5a–h) were obtained in high to excellent yields. Benzyl bromide bearing strong electron-withdrawing NO2 group (2h) was also compatible in this reaction and resulted in the desired product in 75% yield. Unfortunately, benzyl iodide and benzyl chloride did not work in this method. The results are presented in Table 2.
A possible mechanism for the synthesis of phenanthroline-dimedone products is proposed. In the first step, phenanthroline 1 reacted with benzyl bromide 2 to form iminium ion 3via an SN2 mechanism. On the other hand, dimedone 4 was converted into A, or A′ in the presence of a base. The nucleophilic attack of A on the carbon atom of iminium moiety of 3 led to the formation of intermediate Bvia C-alkylation. Intermediate C was formed after the hydrogen abstraction from the base. Intramolecular nucleophilic cyclization in the presence of the base delivered the desired product 5via O-alkylation.
The recyclability of the Pd@HQBI-SPION catalyst emerges as a key feature, underscoring its practical utility and environmental sustainability in the synthesis of phenanthroline-dimedone derivatives. After each catalytic cycle, the catalyst was easily recovered through a simple magnetic separation process owing to the superparamagnetic properties of the iron oxide nanoparticles (SPION). The recovered Pd@HQBI-SPION catalyst exhibited consistent catalytic activity over multiple cycles without a significant loss of performance, showcasing its robustness and stability. The magnetic separation approach not only facilitated the efficient recovery of the catalyst but also minimized the generation of waste, aligning with the principles of green and sustainable chemistry. This recyclability feature enhances the economic feasibility of the catalytic system, offering a cost-effective and environmentally friendly alternative for carbon-carbon bond formations. The demonstrated ability to retain catalytic activity through successive cycles positions Pd@HQBI-SPION as a promising and practical catalyst with potential applications in large-scale synthetic processes. For further characterization of the catalyst after recovery, the recovered catalyst was characterized by ICP analysis and the palladium content was measured as 0.36 mmol/g, which is very close to the initial value. These results confirm the stability of Pd@HQBI-SPION catalyst under the reaction conditions. For studying the stability of the catalyst, after the separation of the catalyst, the reaction mixture was characterized by ICP, and no palladium was detected in the residue. This observation confirms the stable bonding of palladium to the catalyst. The recovery results up to 10 runs and the SEM image of the recovered catalyst are presented in Fig. 3a and b, respectively.
Conclusion
In conclusion, the development and characterization of the Pd@HQBI-SPION catalyst represent a significant advancement in the field of organometallic catalysis, particularly in carbon-carbon bond-forming reactions. The comprehensive synthesis of the catalyst, involving the immobilization of palladium on superparamagnetic iron oxide nanoparticles (SPION) functionalized with 2-(5-hydroxyquinolin-8-yl)-1H-benzo[d]imidazole-5-carboxylic acid (HQBI) ligands, demonstrated structural integrity and successful incorporation of palladium. The thorough characterization through various techniques, including FTIR, TEM, SEM, EDS, ICP, TGA, and VSM, provided detailed insights into the catalyst’s composition, morphology, and magnetic properties. The catalytic performance of Pd@HQBI-SPION was particularly notable in the synthesis of novel phenanthroline-dimedone derivatives. The catalyst exhibited a broad substrate scope and generality, accommodating a diverse range of products. The recyclability of Pd@HQBI-SPION, facilitated by its superparamagnetic nature, emerged as a practical and environmentally sustainable feature. The catalyst displayed consistent performance over multiple cycles, demonstrating its potential for large-scale applications.
Experimental
Synthesis of SPION nanoparticles
Superparamagnetic iron oxide nanoparticles (SPION) were synthesized via a modified co-precipitation method. In a typical synthesis, FeCl3·6H2O (13.5 g) and FeCl2·4H2O (5.2 g) were dissolved in 100 mL of deionized water under a nitrogen atmosphere to prevent oxidation. Ammonium hydroxide (10 mL) was added dropwise to the solution with vigorous stirring at room temperature, resulting in the formation of a black precipitate, indicative of Fe3O4 nanoparticle formation. The reaction mixture was stirred for an additional hour to ensure complete precipitation. The resulting nanoparticles were magnetically separated and washed several times with deionized water and ethanol until the pH of the washings was neutral. The purified SPION were dried under vacuum at 60 °C overnight.
Synthesis of HQBI-APTES
10 mmol of 3-aminopropyltriethoxysilane (APTES) was added to 50 mL of ethanol and stirred. Separately, an ethanolic solution of HQBI (10 mmol in 10 mL ethanol) was prepared. To activate the carboxylic acid groups of HQBI, EDC (10 mmol) and NHS (10 mmol) were added to the HQBI solution and stirred at room temperature for 30 min. This activated HQBI solution was then added to the APTES-functionalized SPION mixture, and the combined solution was refluxed at 80 °C for 24 h. The HQBI-APTES was isolated by the removal of the solvent under reduced pressure using a rotary evaporator. The product was washed with n-hexane and dried in a vacuum oven.
Synthesis of HQBI-SPION
The surface of the SPION was then functionalized with HQBI-APTES to introduce the ligand groups. For this, 1 g of SPION was dispersed in 50 mL of ethanol via sonication for 30 min. Subsequently, the synthesized HQBI-APTES was added to the suspension, and the mixture was refluxed at 80 °C for 24 h. The HQBI-APTES-functionalized SPION were isolated using magnetic separation, washed with ethanol to remove any unreacted APTES, and dried under vacuum at 60 °C overnight.
Synthesis of Pd@HQBI-SPION Catalyst
Palladium was immobilized on the HQBI-functionalized SPION by introducing PdCl2 and a reducing agent. 1 g of HQBI-functionalized SPION was dispersed in 50 mL of deionized water by sonication for 30 min. An aqueous solution of PdCl2 (0.1 g in 20 mL water) was added to the dispersion, and the mixture was stirred at room temperature for 2 h, immobilizing palladium on the SPION surface. The Pd@HQBI-SPION catalyst was magnetically separated, washed with deionized water and ethanol, and dried under vacuum at 60 °C overnight.
The synthesized Pd@HQBI-SPION catalyst was characterized to confirm its structural integrity, composition, and magnetic properties. Fourier Transform Infrared Spectroscopy (FTIR) was used to identify the characteristic peaks corresponding to functional groups, confirming the successful attachment of HQBI ligands. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) provided detailed images of the nanoparticles, revealing a well-dispersed morphology and uniform size distribution. Energy-Dispersive X-ray Spectroscopy (EDS) confirmed the presence of iron, oxygen, and palladium, validating the efficient immobilization process. Inductively Coupled Plasma (ICP) spectroscopy quantified the palladium loading on the SPION, which was crucial for evaluating catalytic activity. Vibrating Sample Magnetometry (VSM) measurements confirmed the superparamagnetic behaviour of the nanoparticles, essential for facile separation and recyclability of the catalyst.
General procedure for the synthesis of compound 5
1,10-Phenantroline 1 (2 mmol, 1 eq.) and benzyl bromide 2 (2 mmol, 1 eq.) were added to ethyl acetate (4 ml) and stirred under reflux for 12 h. After this time, dimedone 3 (2.2 mmol, 1.1 eq.) and Pd@HQBI-SPION (0.1 mol%) were added to the reaction mixture and stirred under reflux for 8 h. The progress and the completion of the reaction was monitored by TLC technique. After the reaction completion, the catalyst was separated using an external magnet, the mixture was quenched with water and extracted with ethyl acetate (3 × 30 mL). The combined extract was dried over Na2SO4, and solvent was removed by a rotary evaporator. The crude products were collected and recrystallized from EtOH. The products were weighted and the isolated yields of the products were calculated using the Eq. 1:
Spectral data of products
(8 S)-7-benzyl-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5a)
White solid, 94% yield, m.p. 185 –87 °C1. H NMR (500 MHz, DMSO-d6) δ 8.64 (d, J = 3.7 Hz, 1 H), 8.16 (d, J = 8.2 Hz, 1 H), 7.52 (d, J = 8.1 Hz, 1 H), 7.33 (dd, J = 8.2, 4.0 Hz, 1 H), 7.30 (d, J = 8.1 Hz, 1 H), 7.25 (d, J = 7.5 Hz, 2 H), 7.22 (t, J = 7.3 Hz, 2 H), 7.15 (t, J = 7.0 Hz, 1 H), 5.71–5.61 (m, 2 H), 5.50 (d, J = 15.4 Hz, 1 H), 4.02 (s, 1 H), 2.14 (d, J = 17.3 Hz, 1 H), 2.05 (d, J = 11.6 Hz, 2 H), 1.99 (d, J = 12.9 Hz, 1 H), 1.83 (dd, J = 14.9, 10.5 Hz, 2 H), 0.95 (s, 3 H), 0.8062 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 194.9, 167.5, 150.4, 146.4, 140.5, 139.0, 137.3, 136.3, 128.7, 128.2, 127.9, 127.3, 126.3, 120.1, 118.2, 113.2, 83.8, 54.1, 49.9, 31.5, 28.6, 26.9, 25.7. EI-MS: m/z(%) = 410 (M+, 100), 319 (93), 91 (83), 395 (62), 354 (61), 326 (23), 235 (11). Anal calc. for C27H26N2O2: C, 79.00; H, 6.38; N, 6.82. Found: C, 79.04; H, 6.33; N, 6.80.
(8 S)-7-(2-fluorobenzyl)-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5b)
White solid, 83% yield, m.p. 197–199 °C1. H NMR (500 MHz, DMSO-d6) δ 8.55 (s, 1 H), 8.16–8.11 (m, 1 H), 7.52 (d, J = 8.0 Hz, 1 H), 7.30 (d, J = 7.9 Hz, 2 H), 7.13 (dd, J = 18.9, 8.4 Hz, 2 H), 6.94 (dd, J = 13.3, 7.1 Hz, 2 H), 6.16 (d, J = 16.4 Hz, 1 H), 5.74 (s, 1 H), 5.31 (d, J = 16.4 Hz, 1 H), 4.06 (s, 1 H), 2.07-2.00 (m, 4 H), 1.90 (d, J = 12.8 Hz, 1 H), 1.76 (d, J = 17.2 Hz, 1 H), 0.94 (s, 3 H), 0.80 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 194.9, 167.5, 159.7 (d, J = 243.8 Hz), 149.9, 146.3, 138.8, 137.0, 136.3, 128.3, 128.1, 127.3, 126.6, 123.9, 123.2, 120.1, 118.2, 113.2, 84.7, 49.9, 48.0, 40.5, 31.4, 28.7, 26.8, 25.6. EI-MS: m/z(%) = 428 (M+, 91), 344 (100), 372 (75), 319 (53), 413 (32), 109 (24), 138 (9). Anal calc. for C27H25FN2O2: C, 75.68; H, 5.88; N, 6.54. Found: C, 75.60; H, 5.91; N, 6.58.
(8 S)-7-(3-chlorobenzyl)-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5c)
White solid, 79% yield, m.p. 182–184 °C1. H NMR (500 MHz, DMSO-d6) δ 8.66–8.62 (m, 1 H), 8.19 (d, J = 8.2 Hz, 1 H), 7.52 (d, J = 8.1 Hz, 1 H), 7.36 (dd, J = 8.2, 4.0 Hz, 1 H), 7.33 (d, J = 8.1 Hz, 1 H), 7.28–7.24 (m, 1 H), 7.21 (d, J = 9.6 Hz, 3 H), 5.88 (d, J = 15.9 Hz, 1 H), 5.68 (s, 1 H), 5.36 (d, J = 15.8 Hz, 1 H), 4.05 (s, 1 H), 2.17 (d, J = 17.3 Hz, 1 H), 2.08 (s, 2 H), 2.03 (d, J = 13.2 Hz, 1 H), 1.89 (t, J = 15.0 Hz, 2 H), 0.98 (s, 3 H), 0.84 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 194.9, 167.3, 146.5, 143.5, 141.3, 138.8, 136.4, 129.9, 128.7, 128.1, 127.3, 126.7, 126.2, 125.8, 120.2, 118.4, 113.3, 84.2, 42.9, 42.2, 40.5, 31.5, 28.8, 27.5, 26.7. MS: m/z(%) = 444 (M+, 91), 446 (M + 2, 28), 319 (100), 125 (69), 138 (62), 304 (37), 235 (31). Anal calc. for C27H25ClN2O2: C, 72.88; H, 5.66; N, 6.30. Found: C, 72.78; H, 5.66; N, 6.32.
(8 S)-7-(4-chlorobenzyl)-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5d)
White solid, 87% yield, m.p. 175–177 °C1. H NMR (500 MHz, DMSO-d6) δ 8.64–8.63 (m, 1 H), 8.19–8.16 (m, 1 H), 7.51 (d, J = 8.1 Hz, 1 H), 7.35 (dd, J = 8.1, 3.9 Hz, 1 H), 7.31 (d, J = 8.8 Hz, 2 H), 7.29–7.24 (m, 3 H), 5.70 (d, J = 15.6 Hz, 1 H), 5.63 (s, 1 H), 5.42 (d, J = 15.6 Hz, 1 H), 4.03 (s, 1 H), 2.1707 (d, J = 17.3 Hz, 1 H), 2.07 (s, 2 H), 2.04–1.97 (m, 1 H), 1.89–1.80 (m, 2 H), 0.9681 (s, 3 H), 0.8254 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 194.9, 167.3, 146.5, 139.7, 138.8, 137.0, 136.4, 130.9, 129.1, 128.8, 128.2, 127.9, 127.3, 120.2, 118.3, 113.3, 83.8, 53.6, 49.8, 40.5, 31.5, 28.6, 26.9, 25.7. MS: m/z(%) = 444 (M+, 75), 446 (M + 2, 23), 235 (100), 388 (88), 263 (59), 429 (42), 125 (6). Anal calc. for C27H25ClN2O2: C, 72.88; H, 5.66; N, 6.30. Found: C, 72.89; H, 5.69; N, 6.37.
(8 S)-7-(4-fluorobenzyl)-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5e)
White solid, 79% yield, m.p. 192–194 °C1. H NMR (500 MHz, DMSO-d6) δ 8.66 (d, J = 3.8 Hz, 1 H), 8.19 (d, J = 8.3 Hz, 1 H), 7.51 (d, J = 8.1 Hz, 1 H), 7.37 (dd, J = 8.2, 4.0 Hz, 1 H), 7.33 (t, J = 7.3 Hz, 3 H), 7.08 (t, J = 8.7 Hz, 2 H), 5.61 (s, 1 H), 5.56 (d, J = 15.2 Hz, 1 H), 5.49 (d, J = 15.2 Hz, 1 H), 4.02 (s, 1 H), 2.20 (d, J = 17.4 Hz, 1 H), 2.08 (s, 2 H), 2.01 (d, J = 12.9 Hz, 1 H), 1.94 (d, J = 17.3 Hz, 1 H), 1.83 (d, J = 13.1 Hz, 1 H), 0.97 (s, 3 H), 0.82 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 194.9, 167.4, 146.6, 138.9, 137.1, 136.4, 129.3, 129.2, 128.9, 128.2, 127.3, 120.2, 118.3, 114.7, 114.6, 113.3, 83.5. MS: m/z(%) = 428 (M+, 89), 319 (100), 235 (77), 344 (73), 109 (52), 413 (40), 182 (27). Anal calc. for C27H25FN2O2: C, 75.68; H, 5.88; N, 6.54. Found: C, 75.63; H, 5.89; N, 6.61.
(8 S)-7-(4-bromobenzyl)-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5f)
White solid, 90% yield, m.p. 181–183 °C1. H NMR (500 MHz, DMSO-d6) δ 8.63 (dd, J = 4.0, 1.6 Hz, 1 H), 8.17 (dd, J = 8.3, 1.6 Hz, 1 H), 7.51 (d, J = 8.1 Hz, 1 H), 7.42 (d, J = 8.3 Hz, 2 H), 7.35 (dd, J = 8.3, 4.0 Hz, 1 H), 7.31 (d, J = 8.1 Hz, 1 H), 7.19 (d, J = 8.3 Hz, 2 H), 5.72 (d, J = 15.6 Hz, 1 H), 5.63 (s, 1 H), 5.38 (d, J = 15.6 Hz, 1 H), 4.02 (s, 1 H), 2.15 (d, J = 17.3 Hz, 1 H), 2.06 (d, J = 2.9 Hz, 1 H), 2.02 (d, J = 3.7 Hz, 2 H), 1.83 (d, J = 17.2 Hz, 2 H), 0.96 (s, 3 H), 0.82 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 194.9, 167.3, 146.5, 140.2, 138.8, 137.0, 136.4, 130.8, 129.4, 128.7, 128.1, 127.3, 120.2, 119.3, 118.3, 113.3, 83.9, 53.7, 49.8, 42.1, 31.5, 28.7, 26.9, 26.0. MS: m/z(%) = 488 (M+, 87), 490 (M + 2, 84), 404 (100), 319 (77), 473 (61), 432 (54), 167 (31). Anal calc. for C27H25BrN2O2: C, 66.26; H, 5.15; N, 5.72. Found: C, 66.25; H, 5.18; N, 5.75.
(8 S)-7-(3,4-dichlorobenzyl)-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5 g)
White solid, 77% yield, m.p. 176–178 °C1. H NMR (500 MHz, DMSO-d6) δ 8.65–8.62 (m, 1 H), 8.19 (d, J = 8.1 Hz, 1 H), 7.51 (dd, J = 8.1, 3.5 Hz, 2 H), 7.42 (s, 1 H), 7.36 (dd, J = 8.5, 4.1 Hz, 1 H), 7.33 (d, J = 8.1 Hz, 1 H), 7.22 (d, J = 7.7 Hz, 1 H), 5.83 (d, J = 15.8 Hz, 1 H), 5.68 (s, 1 H), 5.37–5.30 (m, 1 H), 4.04 (s, 1 H), 2.19 (d, J = 17.6 Hz, 1 H), 2.08 (s, 2 H), 2.03 (d, J = 13.9 Hz, 1 H), 1.93–1.85 (m, 2 H), 0.98 (s, 3 H), 0.83 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 195.8, 164.3, 146.3. 141.9, 138.0, 135.8, 133.4, 132.1, 139.8, 129.0, 127.6, 125.4, 124.4, 122.5, 121.1, 119.1, 87.0, 55.2, 54.1, 42.2, 34.5, 32.8, 26.6, 24.6. MS: m/z(%) = 478 (M+, 54), 480 (M + 2, 35), 482 (M + 4, 5), 319 (100), 158 (83), 394 (49), 463 (16). Anal calc. for C27H24Cl2N2O2: C, 67.65; H, 5.05; N, 6.67. Found: C, 67.60; H, 5.10; N, 5.79.
(8 S)-7-(3-nitrobenzyl)-11,11-dimethyl-7,8,10,11,12,14-hexahydro-13 H-8,14-methanobenzo[7,8][1,3]oxazocino[5,4-h]quinolin-13-one (5 h)
White solid, 75% yield, m.p. 168–170 °C1. H NMR (500 MHz, DMSO-d6) δ 8.54 (s, 1 H), 8.14 (dd, J = 17.9, 7.1 Hz, 3 H), 7.54–7.47 (m, 3 H), 7.34–7.28 (m, 2 H), 5.85 (d, J = 16.2 Hz, 1 H), 5.67 (s, 1 H), 5.55 (d, J = 16.4 Hz, 1 H), 4.06 (s, 1 H), 2.18 (d, J = 17.4 Hz, 1 H), 2.10 (d, J = 4.9 Hz, 1 H), 2.07–2.01 (m, 2 H), 1.91 (d, J = 15.4 Hz, 2 H), 0.97 (s, 3 H), 0.82 (s, 3 H)13. C NMR (125 MHz, DMSO-d6) δ 194.9, 167.2, 149.5, 146.5, 146.1, 138.7, 136.7, 136.4, 128.4, 128.1, 128.0, 127.4, 123.2, 120.2, 118.4, 113.5, 84.2, 54.3, 49.8, 40.6, 31.6, 28.6, 26.9, 25.6. MS: m/z(%) = 455 (M+, 63), 235 (100), 137 (74), 399 (51), 440 (50), 371 (18), 317 (7). Anal calc. for C27H25N3O4: C, 71.19; H, 5.53; N, 9.22; O, 14.05. Found: C, 71.20; H, 5.49; N, 9.21.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Sammes, P. G. & Yahioglu, G. 1, 10-Phenanthroline: a versatile ligand. Chem. Soc. Rev. 23(5), 327–334 (1994).
Summers, L. A. The phenanthrolines. In Advances in Heterocyclic Chemistry, Vol. 22, 1–69 (Elsevier, 1978).
DeMent, P. M. et al. Phenanthroline-catalyzed stereoselective formation of α-1,2-cis2-deoxy-2-fluoro glycosides. ACS Catal. 11(4), 2108–2120 (2021).
Yu, F. et al. Phenanthroline-catalyzed stereoretentive glycosylations. Angew. Chem. Int. Ed. 58(21), 6957–6961 (2019).
Chen, L. et al. NH4I/1,10-phenanthroline catalyzed direct sulfenylation of N-heteroarenes with ethyl arylsulfinates. Tetrahedron 75(46), 130664 (2019).
Li, J. & Nguyen, H. M. Phenanthroline Catalysis in Stereoselective 1,2-cis glycosylations. Acc. Chem. Res. 55(24), 3738–3751 (2022).
Genovino, J., Sames, D. & Toure, B. B. Access to drug metabolites via C–H functionalization: copper-catalyzed aerobic oxidation of N,N-dimethylalkylamines in complex pharmaceuticals. Tetrahedron Lett. 56(23), 3066–3069 (2015).
Khalili, S. B. & Sardarian, A. R. KF/Al2O3: an efficient solid heterogeneous base catalyst in one-pot synthesis of benzimidazoles and bis-benzimidazoles at room temperature. Chem. Mon. 143, 841–846 (2012).
Nandakumar, M. V., Ghosh, S. & Schneider, C. Enantioselective Synthesis of a Novel Chiral 2,9-Disubstituted 1,10‐Phenanthroline and First Applications in Asymmetric Catalysis (Wiley, 2009).
Chelucci, G. &Thummel, R. P. Chiral 2, 2′-bipyridines, 1, 10-phenanthrolines, and 2,2′:6′,2″-terpyridines: syntheses and applications in asymmetric homogeneous catalysis. Chem. Rev. 2(9), 3129–3170 (2002). 102.
Breslow, R., Fairweather, R. & Keana, J. Metal-catalyzed hydration of phenanthroline nitrile. J. Am. Chem. Soc. 89(9), 2135–2138 (1967).
Taniguchi, N. Oxidative coupling of dichalcogenides with sodium sulfinates via copper-catalyzed cleavage of S–S and Se–Se bonds. J. Org. Chem. 80(3), 1764–1770 (2015).
Siva Reddy, A. & Kumara Swamy, K. Use of elemental sulfur or selenium in a novel one-pot copper-catalyzed tandem cyclization of functionalized ynamides leading to benzosultams. Org. Lett. 17(12), 2996–2999 (2015).
Bandarra, D. et al. Mo (II) complexes: a new family of cytotoxic agents? J. Inorg. Biochem. 104(11), 1171–1177 (2010).
Ikotun, O. F., Higbee, E. M., Ouellette, W. & Doyle, R. P. Pyrophosphate-bridged complexes with picomolar toxicity. J. Inorg. Biochem. 103(9), 1254–1264 (2009).
Rafique, J., FS Canto, R., Saba, S., AR Barbosa, F. & Braga, L. Recent advances in the synthesis of biologically relevant selenium-containing 5-membered heterocycles. Curr. Org. Chem. 20(2), 166–188 (2016).
Balkrishna, S. J., Bhakuni, B. S., Chopra, D. & Kumar, S. Cu-catalyzed efficient synthetic methodology for ebselen and related Se – N heterocycles. Org. Lett. 12(23), 5394–5397 (2010).
Cahard, E., Male, H. P., Tissot, M. & Gaunt, M. J. Enantioselective and regiodivergent copper-catalyzed electrophilic arylation of allylic amides with diaryliodonium salts. J. Am. Chem. Soc. 137(25), 7986–7989 (2015).
Allen, S. E., Walvoord, R. R., Padilla-Salinas, R. & Kozlowski, M. C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 113(8), 6234–6458 (2013).
Anderegg, G. & Pyridinderivate als Komplexbildner, V. I. Reaktionsenthalpie und-entropie bei der bildung der metallkomplexe von 1,10‐phenanthrolin und α, α′‐dipyridyl. Helv. Chim. Acta 46(7), 2813–2822 (1963).
Paoletti, P. Formation of metal complexes with ethylenediamine: a critical survey of equilibrium constants, enthalpy and entropy values. Pure Appl. Chem. 56(4), 491–522 (1984).
Bencini, A. & Lippolis, V. 1, 10-Phenanthroline: a versatile building block for the construction of ligands for various purposes. Coord. Chem. Rev. 254(17–18), 2096–2180 (2010).
Arévalo, R. et al. Nucleophilic additions to coordinated 1,10-Phenanthroline: Intramolecular, Intermolecular, reversible, and irreversible. Chem. A Eur. J. 22(50), 17972–17975 (2016).
Accorsi, G., Listorti, A., Yoosaf, K. & Armaroli, N. 1, 10-Phenanthrolines: versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 38(6), 1690–1700 (2009).
Nikoofar, K. & Yielzoleh, F. M. A concise study on dimedone: a versatile molecule in multi-component reactions, an outlook to the green reaction media. J. Saudi Chem. Soc. 22(6), 715–741 (2018).
Teli, P., Sahiba, N., Sethiya, A., Soni, J. & Agarwal, S. Advancement in synthetic strategies of bisdimedones: two decades study. J. Heterocycl. Chem. 58(7), 1393–1407 (2021).
Esmati, M. & Zeynizadeh, B. Synthesis of GO and rGO@Fe3O4@Ni as remarkable nanocatalyst systems for solvent-free and chemoselective coupling reactions of dimedone with aromatic aldehydes. Appl. Organomet. Chem. 35(9), e6321 (2021).
Diab, H. M., Elsayed, B., Darweesh, A. F., Abdelhamid, I. A. & Elwahy, A. H. Synthesis of novel bis (sulfanediyl) bis (tetrahydropyrimido[4,5-b]quinoline-4,6-diones) linked to butenyl and butynyl spacers via thioether linkages. Polycycl. Aromat. Compd. 43(5), 4084–4102 (2023).
Pedrood, K. et al. Arylmethylene hydrazine derivatives containing 1,3-dimethylbarbituric moiety as novel urease inhibitors. Sci. Rep. 11(1), 10607 (2021).
Arora, D., Dwivedi, J., Kumar, S. & Kishore, D. Greener approach toward the generation of dimedone derivatives. Synth. Commun. 48(2), 115–134 (2018).
Heravi, M., Zadsirjan, M., Fattahi, V. & Nazari, B. Applications of dimedone in the synthesis of heterocycles: an update. Curr. Org. Chem. 20(16), 1676–1735 (2016).
van Klink, J. W., Brophy, J. J., Perry, N. B. & Weavers, R. T. β-Triketones from Myrtaceae: Isoleptospermone from Leptospermum s coparium and papuanone from Corymbia D allachiana. J. Nat. Prod. 62(3), 487–489 (1999).
Brogden, R., Heel, R., Speight, T. & Avery, G. Piroxicam: a review of its pharmacological properties and therapeutic efficacy. Drugs 22, 165–187 (1981).
Fatahi, Y., Ghaempanah, A., Maˈmani, L., Mahdavi, M. & Bahadorikhalili, S. Palladium supported aminobenzamide modified silica coated superparamagnetic iron oxide as an applicable nanocatalyst for heck cross-coupling reaction. J. Organomet. Chem. 936, 121711 (2021).
Patil, R. P., Kalantre, V. A. & Alasundkar, K. N. Recent trends of nanocatalyst for organic transformations via sustainable environmental benign route. Res. Chem. Intermed. 49(12), 5163–5203 (2023).
Li, Y. et al. Functional carbon-supported nanocatalysts for biomass conversion. Mol. Catal. 538, 113003 (2023).
Yousefnejad, F. et al. Palladium supported magnetic Fucus vesiculosus extract as a natural and novel catalyst for the synthesis of N-alkyl-2-(4-methyl-1-oxoisoquinolin-2(1H)-yl)-2-phenylacetamide derivatives. Sci. Rep. 13(1), 1272 (2023).
Bahadorikhalili, S. & Mahdavi, H. Palladium magnetic nanoparticle-polyethersulfone composite membrane as an efficient and versatile catalytic membrane reactor. Polym. Adv. Technol. 29(3), 1138–1149 (2018).
Mansoor, S. et al. Recent advancements in Se-and Te-enriched cocatalysts for boosting photocatalytic splitting of water to produce hydrogen. Res. Chem. Intermed. 49(9), 3723–3745 (2023).
Liu, Y. et al. Internal electric field enhances B refilling and carbon vacancy double modulation to promote photocatalytic hydrogen evolution. Catal. Lett. 154(3), 798–807 (2024).
Sadat-Ebrahimi, S. E. et al. Cu (II)-β-cyclodextrin-catalyzed synthesis of spiro [indoline-3,4′-pyrano[3,2-c]chromene]-3′-carbonitrile derivatives. Synth. Commun. 47(24), 2324–2329 (2017).
Pourjavadi, A., Safaie, N., Hosseini, S. H. & Bennett, C. Graphene oxide/poly (vinyl imidazole) nanocomposite: an effective support for preparation of highly loaded heterogeneous copper catalyst. Appl. Organomet. Chem. 29(9), 601–607 (2015).
Nasr-Esfahani, M. et al. Synthesis and characterization of Cu (II) containing nanosilica triazine dendrimer: a recyclable nanocomposite material for the synthesis of benzimidazoles, benzothiazoles, bis-benzimidazoles and bis-benzothiazoles. J. Mol. Catal. A: Chem. 379, 243–254 (2013).
Tai, V. C., Che, H. X., Kong, X. Y., Ho, K. C. & Ng, W. M. Decoding iron oxide nanoparticles from design and development to real world application in water remediation. J. Ind. Eng. Chem. (2023).
Heydari, Z., Bahadorikhalili, S., Ranjbar, P. R. & Mahdavi, M. DABCO-modified super‐paramagnetic nanoparticles as an efficient and water‐compatible catalyst for the synthesis of pyrano[3,2‐c:5,6‐c’] dichromene‐6,8‐dione derivatives under mild reaction conditions. Appl. Organomet. Chem. 32(12), e4561 (2018).
Mahdavi, V. et al. Aminoguanidine modified magnetic graphene oxide as a robust nanoadsorbent for efficient removal and extraction of chlorpyrifos residue from water. J. Environ. Chem. Eng. 9(5), 106117 (2021).
Muhammad Irfan, R. et al. Highly efficient photocatalytic syngas production from formic acid using iron-porphyrins as catalysts integrated with CdS/CNTs heterojunctions under visible light. ACS Appl. Energy Mater. 6(3), 1834–1844 (2023).
Mushtaq, A. et al. Facile synthesis of metformin loaded Mn3O4-HAp magnetic hydroxyapatite nanocomposites for T1-magnetic resonance imaging guided targeted chemo-phototherapy in vitro. Colloids Surf. A 674, 131911 (2023).
Ma’mani, L. et al. Palladium catalyst supported on N-aminoguanidine functionalized magnetic graphene oxide as a robust water-tolerant and versatile nanocatalyst. RSC Adv. 4(89), 48613–48620 (2014).
Bahadorikhalili, S., Ma’mani, L., Mahdavi, H. & Shafiee, A. Palladium catalyst supported on PEGylated imidazolium based phosphinite ionic liquid-modified magnetic silica core–shell nanoparticles: a worthy and highly water-dispersible catalyst for organic reactions in water. RSC Adv. 5(87), 71297–71305 (2015).
Alonso, F., Arroyo, A., Martín-García, I. & Moglie, Y. Cross‐Dehydrogenative Coupling of Tertiary amines and terminal alkynes catalyzed by copper nanoparticles on Zeolite. Adv. Synth. Catal. 357(16–17), 3549–3561 (2015).
Atilgan, S., Ozdemir, T. & Akkaya, E. U. Selective hg (II) sensing with improved stokes shift by coupling the internal charge transfer process to excitation energy transfer. Org. Lett. 12(21), 4792–4795 (2010).
Mahdavi, M. et al. Copper supported β-cyclodextrin grafted magnetic nanoparticles as an efficient recyclable catalyst for one-pot synthesis of 1-benzyl-1H-1,2,3-triazoldibenzodiazepinone derivatives via click reaction. RSC Adv. 6(34), 28838–28843 (2016).
Author information
Authors and Affiliations
Contributions
MHS experiment design, conceptualization, manuscript draft correction; AS methodology, manuscript draft preparation; SB methodology, manuscript draft preparation; MM conceptualization, manuscript draft correction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sayahi, M.H., Serajian, A., Bahadorikhalili, S. et al. Efficient synthesis of novel phenanthroline-dimedone derivatives using Pd@HQBI-SPION as a versatile palladium-immobilized catalyst. Sci Rep 14, 26325 (2024). https://doi.org/10.1038/s41598-024-76221-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76221-3