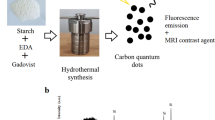
Abstract
Gd3+- free carbon dots (CDs) were synthesized by one-step solvothermal method using urea, citric acid and 3-(trifluoromethyl)aniline as precursors. Additionally, Gd3+-doped CDs were prepared by incorporating gadolinium chloride (Gd3+ ions) into the synthesis. Size selection of the purified CDs was achieved through filter membranes ranging from 3 kDa to 100 kDa. The chemical composition and optical properties of the obtained samples were characterized by Energy dispersive X-ray (EDX), Fourier-transform infrared spectroscopy (FTIR), Dynamic Light Scattering (DLS), proton relaxation time measurements, Ultraviolet–visible (UV-vis) and fluorescence spectroscopies. A comparative analysis revealed a strong size-dependent behavior in the optical properties of both Gd3+-doped and Gd3+-free CDs. Furthermore, in vitro tests confirmed the non-cytotoxicity of Gd3+-doped CDs, indicating their potential applicability in biomedicine for magnetic resonance imaging (MRI) and red fluorescence-based cell and tissue imaging.
Similar content being viewed by others
Introduction
Due to their promising physicochemical properties, such as strong photoluminescence, biocompatibility, low toxicity, environmental friendliness, and low-cost fabrication, carbon dots (CDs) have emerged as one of the most fascinating materials for imaging-guided biomedical applications1,2,3,4. The most developed bio-imaging techniques where CDs can be used as contrast agents are photoacoustic imaging (PAI)5,6, fluorescence imaging (FLI)7, and magnetic resonance imaging (MRI)8,9,10,11,12. Notably, MRI garners particular interest among these visualization techniques for its capability to produce detailed anatomical 3D representations of studied biological objects, leveraging various MRI contrast agents like heteroatom-doped CDs. For such applications, Gd3+, Fe2+, Mn2+, and Ni2+ are recognized as the predominant doping agents due to their ability to alter the physicochemical characteristics of the particles they dope and to reduce proton relaxation times. The incorporation of the metal ions into CDs induces profound alterations in their electron distribution and energy band gap, thus enabling the fine-tuning of the CDs’ optical, electrical, chemical, and magnetic properties13. One of the primary methods for achieving such adjustment involves manipulation of the CDs’ size.
Gd3+ ions are considered as optimal candidates for doping due to their distinctive paramagnetic properties14. However, it is widely recognized that free Gd3+ ions can be highly toxic (13–15 µM) because they inhibit calcium channels and cause neurologic and cardiovascular toxicity15. Minimizing the presence of free Gd3+ in Gd3+- doped CDs while preserving their superior magnetic resonance (MR) and fluorescence capabilities, would significantly enhance their applicability in bio-imaging contexts.
Numerous studies16,17,18,19,20 involving living cells and mice have been conducted to validate the low toxicity and high biocompatibility of Gd-doped CDs. These investigations revealed that Gd3+- doped CDs do not exhibit the pronounced toxicity associated with free Gd3+- ions, as the ions are complexed with the carboxyl groups within the CDs, mitigating their harmful effects. Additionally, these CDs demonstrate excellent water dispersibility and size uniformity. All these features make them excellent candidates for biomedical applications.
The quantum size effect in CDs has been the focus of numerous studies21,22,23,24. This phenomenon becomes particularly influential at the nanoscale (< 10 nm), markedly affecting the CDs’ electrical, optical, and magnetic properties. Several theoretical calculations were conducted to elucidate and verify the correlation between the size of CDs and their optical characteristics. These analyses confirmed that the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) varies with the size of the CDs: while the size of the dots increases, the gap gradually decreases. And it was deduced that the strong emission of CDs comes from the quantum-sized carbon-material structure Consequently, it was inferred that the intense luminescence exhibited by CDs is attributed to their quantum-sized carbon-based structural composition25.
For example, Kaur and Bharj26 synthesized CDs via plasma fusion and found that photoluminescence (PL) emission intensity peaks are influenced by the size of the CDs due to the quantum confinement effect. Similarly, Liu et al.23 prepared graphene quantum dots (GQD) of various sizes through a simple and eco-friendly hydrothermal method. Their findings indicated that an increase in GQD size leads to a narrower energy gap, enhancing the likelihood of photon transitions and resulting in a redshift of the PL emission peak. Li et al.27 observed distinct PL behaviors based on the size of the CDs: small CDs (1.2 nm) emit UV radiation (approximately 350 nm), medium-sized CDs (1.5–3 nm) generate visible light emission (400–700 nm) and larger CDs (3.8 nm) produce near-infrared emission (about 800 nm). They speculated that the intense emission from CDs is primarily due to the quantum-scale structure of graphite rather than the carbon-oxygen surface features. Additionally, Zhi et al.28 obtained malic acid carbon dots (MACD) fractions and showed that their PL peaks shift from blue to cyan, yellowish and red. These shifts in PL emission are attributed to the narrowing of the band gap energy as the particle size increases. Wang et al.29 synthesized amino-functionalized graphene quantum dots (AF-GQDs) with different sizes and observed that their absorption spectra exhibited a subtle blue shift with an increase in the size of the AF-GQDs. This phenomenon was linked to reduced conjugation due to a decrease in the molecular plane of the GQDs. Zhu et al.30 produced three types of GQDs with sizes of 1–4 nm, 4–8 nm, and 7–11 nm by varying the reaction temperature, and these GQDs emitted PL in the range from blue and green to yellow. To investigate the correlation between particle size and PL emission, they conducted theoretical calculations and demonstrated that the particle size is related to the HOMO-LUMO gaps; as the size increases, the gap decreases progressively. Xin et al.31 prepared four types of CDs with different sizes using a one-step electrochemical oxidation method and discovered a link between the size of the CDs and their antibacterial properties. This relationship was explained by the synergy between the bacterial absorption capacity of CDs and the reactive oxygen species stimulated by the CDs.
Despite the significant amount of accumulated scientific data on studying the physicochemical properties of CDs, no research has been conducted specifically to clarify how the optical properties of CDs depend on the sizes of nanoparticles with and without Gd3+. In this work, we synthesized Gd3+-free and Gd3+-doped CDs by means of the solvothermal method and performed post-synthesis size selection of the CDs. We aimed to examine the size-dependent optical properties of both Gd3+−free and Gd3+-doped CDs, emphasizing the size-effect of these nanoparticles. Furthermore, we assessed the effects of these CDs on the proliferation of living cells in vitro. Notably, in vitro experiments indicated that Gd3+-doped CDs did not exhibit toxicity towards living cells, underscoring their potential for safe biomedical applications.
Results and discussion
Structure and chemical composition of CDs
Transmission Electron Microscopy (TEM) is one of the key methods for determining the structural features of fine nanoparticles, as in our case. However, it may not always be an informative tool when working with amorphous nanoparticles, which primarily consist of small molecular compounds. We attempted to capture TEM images of our particles, but due to their amorphous and small nature, we were unable to achieve good contrast. Due to poor contrast and non-representative results in the TEM images (see Fig. S1 in SM), we performed Dynamic Light Scattering (DLS) analysis instead to determine size distribution of the particles. As illustrated in Fig. 1a and b, the particle size distribution graphs indicate that the mean diameters of both types of CDs range from approximately 2 to 50 nm as a function of the used filters.
Zeta potential measurements were carried out using dynamic light scattering on a Malvern Z-sizer NANO. Figure 1c illustrates the zeta potential values of Gd3+-free and Gd3+-doped CDs, showing their pH-sensitive behavior. As one can see, the colloidal solutions of both types of CDs exhibit pH-dependent zeta potentials. A continuous decrease in zeta potential is observed as the pH increases, with Gd3+-free and Gd3+-doped CDs changing their zeta potential to the opposite sign with acidification of the medium.
At pH values below 3.8, Gd3+-free CDs exhibited positive zeta potential values can be attributed to 3-(Trifluoromethyl)aniline, an aromatic amine, used in the synthesis. This compound enriches the CDs with nitrogen, giving Gd3+-free CDs a positive zeta potential in the acid region. For Gd3+-doped CDs, the positive zeta potential was observed at pH below 7.2. The difference in the isoelectric points between the two types of CDs is likely due to the impact of the Gd ions, which carries a 3 + charge and can effectively neutralize negative surface charges, embedding itself into the Gd-CDs surface.
The chemical composition of both Gd3+-free and Gd3+-doped CDs was determined through Energy Dispersive X-ray Spectroscopy (EDX) and Fourier Transform Infrared Spectroscopy (FTIR). Figure 2a clearly illustrates that both varieties of CDs predominantly consist of carbon and oxygen atoms, with significantly lesser amounts of fluorine and, where applicable, gadolinium. Notably, the Gd3+-free CDs, as expected, exhibit no traces of gadolinium, thereby confirming their classification as gadolinium-free.
FTIR analysis was conducted to examine the molecular structure of both Gd3+-free and Gd3+-doped CDs, with the results presented in Fig. 2b. The spectra were recorded over the range of 600 to 4000 cm− 1.
The FTIR spectra revealed the presence of various functional groups that contribute to forming a protective layer on the CDs’ surface. A broad band observed between 3400 and 3000 cm− 1 corresponds to stretching vibrations ν(O‒H) of hydrogen-bonded hydroxyls. The peaks in the range of 3000–2800 cm− 1 are attributed to the stretching vibrations ν(C‒H)32. An intense band around 1600 cm− 1 likely results from bending vibrations (deformations) of free groups δ(‒NH2), water molecules or C = C bonds in benzene rings and other double bonds33,34, along with asymmetric stretching vibrations of carboxylate groups νas(‒CO2−)35. The band 1420 cm− 1 is associated with symmetric stretching vibrations of carboxylate groups νs(‒CO2−), scissoring vibrations of the CH2 fragment in vinyl groups δs(‒CH2 = CH2), and stretching vibrations ν(C = N). A band at 1070 cm–1 is attributed to ν(C‒O) stretching vibrations and may include a contribution from isopropanol36.
Notably, a strong IR band with a maximum at 1166 cm− 1 is clearly observed, corresponding to the C-F bond37. When the fluorine atom is connected through sp3-hybridization, the C–F bond is considered covalent, with stretching vibration absorption at peaks near 1221 cm− 138 and CF3 bond at 1260 cm− 139. According to40, an asymmetric C–F stretching vibration at 1330 cm–1 appears with strong intensity.
The surface chemistry of both Gd3+-free and Gd3+-doped CDs appears very similar, suggesting that the presence of Gadolinium does not significantly affect the surface chemistry of the CDs.
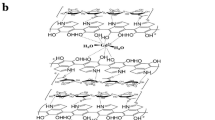
Figure 3 shows the schematic representations of the molecular structures for both fluorinated Gd3+-free and Gd3+-doped CDs. In Fig. 3a, the 3D structure of Gd3+-free CDs is depicted, where carbon atoms are illustrated in gray, oxygen atoms – in red, hydrogen atoms – in white, fluorine atoms – in turquoise, and nitrogen atoms – in blue. Figure 3b displays the 2D model of the Gd3+-doped CDs, highlighting possible places for localization for the Gd3+ ions.
Proton relaxation times measurements
Figure 4a and b present the outcomes of longitudinal (T1) and transverse (T2) proton relaxation time measurements performed on Gd3+-free and Gd3+-doped CDs. The T1 and T2 values for pure water are added for comparison. A significant distinction in relaxation times is evident between Gd3+-free and Gd3+-doped CDs. One can see that for both types of CDs with sizes below 5 kDa, the T1 and T2 values closely approximate those of pure water (4150 ± 20 ms). However, for the CDs larger than 10 kDa, the difference in both longitudinal and transverse proton relaxation times becomes markedly pronounced. In particular, the T1 and T2 values for the Gd3+- doped CDs are significantly reduced, nearly two orders of magnitude shorter than those observed for the Gd3+-free CDs, underscoring the impact of Gd3+ ion incorporation. Indeed, the sensitivity of proton relaxation times to the presence of paramagnetic ions in solutions is a well-known phenomenon41. It is noteworthy to mention the preferential incorporation of Gd3+ ions into the larger carbon dots (CDs), ranging in size from 10 to 100 kDa. This selective incorporation significantly influences the magnetic properties of the CDs, particularly determining their application in magnetic resonance imaging (MRI) as contrast agents.
Optical properties
UV-VIS absorbance
Figure 5 illustrates the absorption spectra of the Gd3+-free and Gd3+-doped CDs, with Fig. 5a and b highlighting the spectra of small (< 3 kDa) and large (10–100 kDa) Gd3+-free CDs, respectively. Conversely, Fig. 5c and d display the absorption spectra of Gd3+- doped CDs.
According to the literature data42, high-energy absorption bands exceeding 4.5 eV are attributed to π→π* transitions, specifically involving sp2 hybridized aromatic carbons, indicative of C = C aromatic bonds. The subsequent peaks observed in the range of 3.2 to 3.6 are correlated with the intrinsic absorption related to n→π* transitions in the C = O, C = N and C–F bonds present in the carbon cores. Absorption bands falling below 3 eV stem from transitions associated with surface states of the particles. It is important to note that the n→π* transitions and the broad absorption bands corresponding to surface states often exhibit overlapping features, rather than being distinctly separate. Hence, band 5 is also associated with photoinduced electronic transitions within surface chemical bonds. Regarding band 6, this feature may be ascribed to the integration of nitrogen into the sp2 carbon lattice or to surface states involving nitrogen and/or fluorine, further illustrating the complex interactions and structural nuances within the carbon dots.
The comparison reveals that the small (< 3 kDa) Gd3+-free and Gd3+-doped CDs exhibit remarkably similar absorption spectra. The spectral features, following deconvolution of the experimental data, show identical intensities and spectral positions. However, a distinct difference emerges when examining the absorption spectra of the larger CDs (10–100 kDa). The intensity of peaks, revealed through deconvolution (with Figures S2-S8 in the Supplementary Material providing detailed deconvolution processes), demonstrates a noticeable disparity. Specifically, the Gd3+- doped CDs exhibit higher absorption intensities than their Gd3+-free counterparts. In particular, the spectral band between 4.5 and 6 eV is significantly more pronounced for the Gd3+- doped CDs. Furthermore, the relative intensity of the spectral band centered around 3 eV, compared to that at 2 eV, is also elevated in the Gd3+- doped CDs, highlighting the substantial impact of gadolinium doping on the optical properties of the CDs.
The observed spectral resemblance between the small (< 3 kDa) Gd3+-free and Gd3+-doped CDs can be attributed to their similar low-molecular chemical compositions, independent on the presence or absence of Gd3+ ions. This observation suggests that Gd3+ ions do not significantly interact with the smallest CDs. On the other hand, the noticeable spectral differences observed between the larger (10–100 kDa) Gd3+-free and Gd3+-doped CDs can be explained by the incorporation of Gd3+ ions into the latter. In particular, the enhanced UV-visible absorption intensity seen in the Gd3+-doped CDs can be attributed to their heightened polarizability. This, in turn, leads to an increased optical absorption cross-section, a result of integrating the highly charged Gd3+ ions into the larger CDs’ structure.
Fluorescence
Figure 6 display fluorescence (FL) excitation/emission (FLE) maps of Gd3+-free and Gd3+-doped CDs recorded under UV and visible light excitation, respectively. Figure 6a presents FL/FLE maps for CDs of various sizes, excited in the 250–380 nm range, while Fig. 6b displays these maps for the Gd3+-doped CDs. Across all the FL/FLE maps in Fig. 6, two distinct spectral regions are identified: one related to an excitation at 370 nm and the other at 270 nm. For smaller particles, the FL peak maximum aligns with an excitation at 370 nm, whereas for larger particles, the peak maximum corresponds to excitation at 270 nm. With increasing particle size, the absolute FL intensity excited at 370 nm (associated with smaller particles) diminishes, and the ratio of fluorescence intensities upon excitation at 270 nm to 370 nm (I270/ I370) increases. Mirroring the absorption spectra for the smaller (< 3 kDa) Gd3+-free and Gd3+-doped CDs, their FL/FLE spectral maps are virtually indistinguishable in terms of intensity and spectral characteristics. This similarity suggests that both types of CDs share an identical low-molecular-weight chemical composition, unaffected by the presence or absence of Gd3+ ions. In contrast, the FL intensities of the largest (10–100 kDa) Gd3+- doped CDs are lower compared to the Gd3+- free CDs within the same size range. Considering their higher absorption values, it can be inferred that the incorporation of Gd3+ ions enhances the likelihood of non-radiative recombination events, affecting the fluorescence properties of the CDs. FL/FLE spectral maps (at excitation range 250–380 nm) with absolute maximum values adapted for each map are given in the Supplementary (Figure S9).
Figure 6c and d show, respectively, FL/FLE maps upon excitation of Gd3+-free and Gd3+-doped CDs of various sizes in the range of 400–560 nm. Across these maps (except for Gd3+- free CDs with sizes 10–100 kDa) a prominent maximum FL intensity is observed, centered around 430 nm excitation. This FL peak, dominant in smaller particles (< 10 kDa), gradually diminishes as the size of the nanoparticles increases. This indicates that the FL/FLE spectral region, excited between 400 and 470 nm and emitting at < 600 nm, is characteristic of small, low-molecular-weight species. Conversely, the intensity of the red fluorescence in Gd3+- free CDs, excited at 540 nm, intensifies and becomes maximal for larger particles (10–100 kDa). In comparison, the intensity of similar red fluorescence in Gd3+-doped CDs is significantly reduced. This observation suggests that the incorporation of Gd3+ ions into larger CDs results in a pronounced quenching of their red fluorescence, highlighting the impact of Gd3+ ions on the fluorescence properties of the CDs, particularly in altering their emission profiles (see also Figure S10).
Cell toxicity
Figure 7 shows the evolution of cell indexes in the presence of increasing concentrations of Gd3+- free CDs (a) or Gd3+- doped CDs (b) during 24 h. The data indicate that Gd3+- free CDs do not affect cell indexes, demonstrating complete biological compatibility at the tested concentrations (as shown in Figure S11 (a) in the Supplementary Materials). However, cell indexes slightly decreased when exposed to the highest concentration of Gd3+- doped CDs (Figure S11 (b) in the Supplementary Materials), suggesting a certain degree of toxicity. The toxicity of these CDs can be qualitatively evaluated by analyzing the time dependent changes in slope values; a lower slope indicates higher cytotoxicity. Following the 24 exposures, the culture medium containing CDs was replaced with fresh medium without CDs, allowing for further assessment of any long-term effects or recovery. This had no effect on the cell indexes of cells exposed to CDs. The slopes of cell indexes for cells exposed to Gd3+- doped CDs showed an increase after the washout, indicating the absence of any lasting detrimental effects from these CDs. The impact of Gd3+- doped CDs on cell viability can be qualitatively assessed by the slope of cell index values, the lower the slope, the higher the impact. Indeed, the higher the concentration of Gd3+- doped CDs, the more significant was the decrease in the slope of the cell index. The highest concentration of Gd3+- doped CDs (10 µg/mL) resulted in a minor reduction in the slope of the cell index. At the lower concentrations (1–5 µg/mL), Gd3+- doped CDs exhibited no significant effects, highlighting the sensitivity of the cell index measurement. This slight reduction in cell index, suggesting a minimal impact potentially limited to cell adhesion, is corroborated by microscopic observations (Fig. 9), which revealed no changes in cell shape and structure, thus suggesting the absence of toxicity.
Fluorescent cell labeling
In order to test the ability of Gd3+- doped CDs to serve as bimodal contrast agent, human carcinoma A549 cells were loaded with them at various concentrations (5 and 10 µg/mL). Figure 8 demonstrates the uneven distribution of fluorescence intensity on photomicrographs. The enhanced visibility within the cells is attributed to the fluorescent properties of the Gd3+- doped CDs. Notably, the most intense fluorescent spots are not located within the nucleus. While some nuclei appear slightly labeled, it’s likely that the labeling is positioned outside the nuclei, with most nuclei remaining unlabeled. It should be noted that the incorporation of Gd3+- doped CDs into cells didn’t alter the cells’ size and shape, indicating their biocompatibility. This evidence supports the potential of Gd3+- doped CDs to serve as effective fluorescent markers in cellular imaging applications, as documented in references43.
Conclusions
The Gd3+ free and Gd3+-doped CDs were successfully synthesized through a straightforward one-step solvothermal method and categorized into four size-based fractions. Our findings reveal that Gd3+ ions preferentially incorporate into larger CDs (10–100 kDa) while exhibiting minimal interaction with the smaller ones (< 3 kDa). The study indicates that the presence of Gd3+ ions in smaller CDs increases the probability of photo-induced non-radiative recombination events. Conversely, their integration into the larger CDs significantly diminishes red fluorescence, suggesting an alteration in their optical properties. In vitro experiments confirmed the global non-toxic nature of both Gd3+- free and Gd3+- doped CDs, highlighting their biocompatibility. The straightforward synthesis process, coupled with their low toxicity and the unique optical properties of the Gd3+-doped CDs, underscores their potential for biomedical applications. Specifically, their efficacy as fluorescent cell markers and low proton relaxation times offer promising opportunities for bi-modal bio-imaging (fluorescence and MRI) techniques, paving the way for advanced diagnostic and therapeutic applications.
Materials and methods
Synthesis of CDs
The synthesis of CDs was carried out according to the patented protocol44. To synthesize CDs, 3 g (15.6 mmol) of anhydrous citric acid (Sigma-Aldrich, suitable for cell culture) and 6 g (0.10 mol) of urea (Sigma-Aldrich, ACS reagent) were dissolved and made up to the 30 mL volume with DMF solvent (Sigma-Aldrich, anhydrous, 99.8%). Subsequently, 0.425 g (2.65 mmol) of 3-(trifluoromethyl)aniline (Sigma-Aldrich, ≥ 99%) was added to the solution. The resultant mixture was then equally divided into two parts. To the second portion, 242.4 mg (0.65 mmol) of gadolinium(III) chloride hexahydrate (Sigma-Aldrich, 99%) was added and dissolved with the aid of a magnetic stirrer, ensuring the formation of a clear solution. Both solutions were subsequently transferred into sealed Pyrex glass ampoules. These ampoules were then placed in a PTFE-lined stainless-steel autoclave and placed in an oven at 180 °C for 8 h. Post-synthesis, the samples were categorized as Gd3+-doped carbon dots (CDs-Gd) for the gadolinium-containing sample, and Gd3+-free carbon dots (CDs) for the sample without gadolinium.
The purification of Gd3+-free and Gd3+-doped CDs consisted of three stages: preliminary purification, dialysis and fractionation.
Preliminary purification is obligatory for coarse particles elimination. The ampoules were opened; and their contents were diluted with water to 15 mL. The colloidal solutions were then transferred to ultra-filtration spin columns (centrifugal concentrators), specifically Vivaspin® 20 (100,000 molecular weight cut-off (MWCO), Polyethersulfone (PES) membrane), and centrifuged at 3000 g for 1 h. This process resulted in a fraction with a conditional molecular weight of less than 100 kDa.
Dialysis was performed to remove low molecular components. For this purpose, dialyzing sacks made of membrane with a nominal filter rating of 3’500 MWCO (ZelluTrans ROTH® Regenerated Cellulose Tubular Membrane, flat width 19 mm, wall thickness 25 μm, volume per length 1.15 mL/cm) were utilized. This step lasted for 190 h. Liquids that permeated through the membrane pores of 100’000 MWCO were collected and transferred into dialyzing sacks 20 cm in length featuring membrane pores of 3’000 MWCO. Dialyzing sacks were filled with 15 mL of colloid solutions containing synthesized Gd3+-free and Gd3+-doped CDs and placed separately into 5000 mL vessels with deionized water. The water in these vessels was replaced three times daily over an 8-day dialysis period. Following this process, the initial fraction, labeled as “100 − 10 kDa”, was collected. The concentrations of solutions containing Gd3+-free and Gd3+-doped CDs were determined using a gravimetric technique, with values of 270 µg/mL and 130 µg/mL respectively.
The fractionation procedure was implemented to segregate the synthesized particles into distinct size-based fractions, allowing for the detailed investigation of their properties within separate groups. Table 1 outlines the steps involved in the fractionation process.
Therefore, 4 fractions of Gd3+- free CDs and Gd3+- doped CDs were obtained: lower than 3 kDa, 3–5 kDa, 5–10 kDa and 10–100 kDa.
Optical characterization
After purification, UV-visible absorption and fluorescence spectra were measured. The UV-visible absorption spectra were acquired using a Shimadzu UV-2700 spectrophotometer (Shimadzu, Japan). Meanwhile, fluorescence excitation/emission maps were recorded on a Cary Eclipse Fluorescence Spectrophotometer (Agilent Technologies, USA) with a xenon flash lamp (80 Hz) as the excitation source.
Proton relaxation measurements
Proton relaxation times T1 and T2 were studied by means of Bruker 60 MHz equipment MQ60 (ND 2605, Bruker Optic GmbH, Rheinstetten, Germany). Data was analyzed using the “Bruker theminispec” application. We analyzed 300 µL of colloidal solution of the nanoparticles in the vials for all experiments.
Cell toxicity studies
Cell number measurements were conducted using a non-destructive impedance-based technique called xCELLigence (ACEA Biosciences Inc., Biotek, Colmar, France). The cells were cultured on a specialized plate with electrodes on the bottom. The system measures electrical impedance across interdigitated microelectrodes located at the bottom of the culture wells. The measurements involved applying an alternative excitation signal (with a control voltage amplitude of 20 mV) at 3 different frequencies (10, 25, and 50 kHz) through the microelectrodes in the E-plates while monitoring the voltage drop across the electrodes. The impedance data was processed over time, resulting in a cell index value. The cell index was directly related to factors such as cell number, cell surface area, and adhesion. Among these factors, cell number was found to be the primary determinant of the cell index value for a given cell line. The impedance measurement method used was non-destructive and allowed real-time continuous monitoring of cell proliferation under various conditions, including the presence of CDs and after their removal (washout), on the same cell population. To compare the effects of increasing concentrations of Gd3+- free and Gd3+- doped CDs on cell proliferation, the cell index slopes on normalized curves were measured. Each curve presented the average cell index measured in four wells. Experiments were carried out on adenocarcinomic human alveolar basal epithelial A549 cells. Cells have been seeded at 2500 /well, and allowed to grow for 48 h, until reaching a cell index value close to 1. Then culture medium has been replaced with the same composition plus increasing concentrations of NPs. Cells have been kept for 24 h then the culture medium has been replaced with fresh culture medium without NPs (washout).
Fluorescence cell labelling
Images were taken at magnification from magnification x20 using a Cytation 3 cell imaging reader (Biotek Instrument Inc., Colmar, France) on cells fixed with formalin 10% (Sigma Aldrich). Fluorescence was measured with the filter Ex586/Em 647 (Berger et al. 2015)45.
The data are presented as mean values ± SEM. Statistical analysis was performed with StatView 4.5 software (Abacus Corporation, Baltimore, MD, USA) for Windows. Data were analyzed using a one-way ANOVA followed by a Fisher’s protected least significance difference (PLSD) post hoc test. The significance level was accepted at p < 0.05.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Li, H. et al. Recent advances in carbon dots for bioimaging applications. Nanoscale Horiz.5, 218–234. https://doi.org/10.1039/C9NH00476A (2020).
Boakye-Yiadom, K. O. et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm.564, 308–317. https://doi.org/10.1016/j.ijpharm.2019.04.055 (2019).
Zhu, P. et al. Carbon Dots in Biomedicine: A Review. ACS Appl. Bio Mater.5, 2031–2045. https://doi.org/10.1021/acsabm.1c01215 (2022).
Paliienko, K. et al. Green synthesis of biocompatible Gd3+-doped ultrasmall carbon-based nanohybrids from coffee wastes. Carbon Resour. Convers.7, 100197. https://doi.org/10.1016/j.crcon.2023.09.001 (2024).
Dubyk, K. et al. Bio-distribution of carbon nanoparticles studied by photoacoustic measurements. Nanoscale Res. Lett.17, 127. https://doi.org/10.1186/s11671-022-03768-3 (2022).
Isaiev, M. et al. Application of the photoacoustic approach in the characterization of nanostructured materials. Nanomaterials12, 708. https://doi.org/10.3390/nano12040708 (2022).
Li, C. et al. Advanced fluorescence imaging technology in the near-infrared-II window for biomedical applications. J. Am. Chem. Soc.142, 14789–14804. https://doi.org/10.1021/jacs.0c07022 (2020).
Lundervold, A. S. & Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys.29, 102–127. https://doi.org/10.1016/j.zemedi.2018.11.002 (2019).
Manohar, A. et al. Synthesis and characterization of CeO2/MgFe2O4 nanocomposites for electrochemical study and their cytotoxicity in normal human dermal fibroblast (HDF) and human breast cancer (MDA-MB-231) cell lines. J. Alloys Compod. 171932 (2023). https://doi.org/10.1016/j.jallcom.2023.171932
Manohar, A. et al. Zn- doped MnFe2O4 nanoparticles for magnetic hyperthermia and their cytotoxicity study in normal and cancer cell lines. Colloids Surf. A Physicochem. Eng. Asp. 132037 (2023). https://doi.org/10.1016/j.colsurfa.2023.132037
Manohar, A. et al. Tailored Zn1-xMg0.5CuxFe2O4 nanoparticles: Optimizing magnetic hyperthermia for enhanced efficacy and investigating cytotoxicity in normal and cancer cell lines. Mater. Chem. Phys. 129050 (2024). https://doi.org/10.1016/j.matchemphys.2024.129050
Manohar, A. et al. Exploring NiFe2O4 nanoparticles: Electrochemical analysis and evaluation of cytotoxic effects on normal human dermal fibroblasts (HDF) and mouse melanoma (B16-F10) cell lines. Colloids Surf. A Physicochem. Eng. Asp. 132855 (2024). https://doi.org/10.1016/j.colsurfa.2023.132855
Li, X. et al. Metal ions-doped carbon dots: Synthesis, properties, and applications. Chem. Eng. J.430, 133101. https://doi.org/10.1016/J.CEJ.2021.133101 (2022).
Marasini, R., Thanh Nguyen, T. D., Aryal, S. Integration of gadolinium in nanostructure for contrast enhanced-magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.12. https://doi.org/10.1002/wnan.1580 (2020).
Liao, H. et al. One-pot synthesis of gadolinium(iii) doped carbon dots for fluorescence/magnetic resonance bimodal imaging. RSC Adv.5, 66575–66581. https://doi.org/10.1039/c5ra09948j (2015).
Bourlinos, A. B. et al. Gd(III)-doped carbon dots as a dual fluorescent-MRI probe. J. Mater. Chem.22, 23327–23330. https://doi.org/10.1039/c2jm35592b (2012).
Li, Y. et al. Safe and efficient magnetic resonance imaging of acute myocardial infarction with gadolinium-doped carbon dots. Nanomedicine15, 2385–2398. https://doi.org/10.2217/nnm-2020-0160 (2020).
Zeng, Y. et al. Engineered gadolinium-based nanomaterials as cancer imaging agents. Appl. Mater. Today20, 100686. https://doi.org/10.1016/j.apmt.2020.100686 (2020).
Kuznietsova, H. et al. A comparative multi-level toxicity assessment of carbon-based Gd-free dots and Gd-doped nanohybrids from coffee waste: hematology, biochemistry, histopathology and neurobiology study. Sci. Rep.131(13), 1–14. https://doi.org/10.1038/s41598-023-36496-4 (2023).
Kuznietsova, H. et al. In vitro and in vivo toxicity of carbon dots with different chemical compositions. Discov. Nano18, 1–17. https://doi.org/10.1186/s11671-023-03891-9 (2023).
He, C., Xu, P., Zhang, X. & Long, W. The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: Current state and future perspective. Carbon N Y186, 91–127. https://doi.org/10.1016/J.CARBON.2021.10.002 (2022).
Ai, L. et al. Insights into photoluminescence mechanisms of carbon dots: advances and perspectives. Sci. Bull.66, 839–856. https://doi.org/10.1016/j.scib.2020.12.015 (2021).
Liu, Z. et al. Size effect of graphene quantum dots on photoluminescence. Molecules26, 3922. https://doi.org/10.3390/molecules26133922 (2021).
Sadeghi, H. & Dorranian, D. Influence of size and morphology on the optical properties of carbon nanostructures. J. Theor. Appl. Phys.10, 7–13. https://doi.org/10.1007/s40094-015-0194-4 (2016).
Zhi, B. et al. Synthesis, applications and potential photoluminescence mechanism of spectrally tunable carbon dots. Nanoscale11, 20411–20428. https://doi.org/10.1039/C9NR05028K (2019).
Kaur, M. & Bharj, J. Influence of particle size of flame-synthesized carbon nanoparticles on band gap and photoluminescence properties. Luminescence37, 2123–2128. https://doi.org/10.1002/bio.4402 (2022).
Li, H. et al. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chemie Int. Ed.49, 4430–4434. https://doi.org/10.1002/anie.200906154 (2010).
Zhi, B. et al. Multicolor polymeric carbon dots: synthesis, separation and polyamide-supported molecular fluorescence. Chem. Sci.12, 2441–2455. https://doi.org/10.1039/d0sc05743f (2021).
Wang, S. et al. Amino-functionalized graphene quantum dots as cathode interlayer for efficient organic solar cells: Quantum dot size on interfacial modification ability and photovoltaic performance. Adv. Mater. Interfaces6, 1801480. https://doi.org/10.1002/admi.201801480 (2019).
Zhu, S. et al. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res.8, 355–381. https://doi.org/10.1007/S12274-014-0644-3 (2015).
Du, X. et al. Size-dependent antibacterial of carbon dots by selective absorption and differential oxidative stress of bacteria. J. Colloid Interface Sci.634, 44–53. https://doi.org/10.1016/j.jcis.2022.12.025 (2023).
Wang, X. et al. High-yield production of highly fluorinated graphene by direct heating fluorination of graphene-oxide. ACS Appl. Mater. Interfaces5, 8294–8299. https://doi.org/10.1021/am402958p (2013).
Palafox, M. et al. Scaling factors for the prediction of vibrational spectra. I. Benzene molecule. Quant. Chem.77, 661–684. https://doi.org/10.1002/(SICI)1097-461X(2000)77:3%3C661::AID-QUA7%3E3.0.CO;2-J (2000).
Larkin, P. J. Infrared and Raman Spectroscopy Principles and Spectral Interpretation 61–62 (Elsevier, 2011).
Rap, D. B., Marimuthu, A. N., Redlich, B. & Brünken, S. Stable isomeric structures of the pyridine cation (C5H5N•+) and protonated pyridine (C5H5NH+) elucidated by cold ion infrared spectroscopy. J. Mol. Spectrosc.373, 111357. https://doi.org/10.1016/j.jms.2020.111357 (2020).
Coblentz Soc C, Inc. Evaluated infrared reference spectra. in Linstrom, P. J., Mallard, W. G. (eds) NIST Chemistry Webbook, NIST Standard Reference Database Number 69 (National Institute of Standards and Technology, 2018).
Nebogatikova, N. A. et al. Fluorinated graphene dielectric films obtained from functionalized graphene suspension: preparation and properties. Phys. Chem. Chem. Phys.17, 13257–13266. https://doi.org/10.1039/C4CP04646C (2015).
Wang, X. et al. High-yield production of highly fluorinated graphene by direct heating fluorination of graphene-oxide. ACS Appl. Mater. Interfaces5, 8294–8299. https://doi.org/10.1021/am402958p (2015).
Karlick, F., Kumara, K., Datta, R., Otyepka, M. & Zboril, R. Halogeneted graphenes: Rapidly grown family of graphene deriviatives. ACS Nano2(7), 64343–64364. https://doi.org/10.1021/nn4024027 (2013).
Singh, N. P. & Yadav, R. A. Vibrational studies of trifuoromethyl benzene derivatives 1: 2-amino, 5-chloro and 2-amino. 5-bromo benzotrifuorides. Indian J. Phys.75B, 347–355 (2001).
Bloembergen, N. & Morgan, L. O. Proton relaxation times in paramagnetic solutions: Effects of electron spin relaxation. J. Chem. Phys.34, 337–346. https://doi.org/10.1142/9789812795809_0015 (1996).
Sudolská, M. et al. Nature of absorption bands in oxygen-functionalized graphitic carbon dots. J. Phys. Chem. C119, 13369–13373. https://doi.org/10.1021/acs.jpcc.5b04080 (2015).
Jiao, M. et al. Gadolinium doped red-emissive carbon dots as targeted theranostic agents for fluorescence and MR imaging guided cancer phototherapy. Chem. Eng. J.440, 135965. https://doi.org/10.1016/J.CEJ.2022.135965 (2022).
Zaderko, A. WO/2020/121119 The process for obtaining of fluoralkylated carbon quantum dots. In World Intellect. Prop. Organ (2020). https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020121119
Berger, E. et al. Pathways commonly dysregulated in mouse and human obese adipose tissue: FAT/CD36 modulates differentiation and lipogenesis. Adipocyte4, 161–180. https://doi.org/10.4161/21623945.2014.987578 (2015).
Funding
This research work was funded by EU Horizon 2020 Research and Innovation Staff Ex-change Programme (RISE) under Marie Skłodowska-Curie Action (project “UNAT” №101008159). A.N. Zaderko is grateful to French Government for financial support in frame of PAUSE program.
Author information
Authors and Affiliations
Contributions
NZ: Validation, Formal analysis, Investigation, Writing – original draft; KP: Validation, Investigation; AT: Validation, Investigation; AZ: Synthesis, Validation, Investigation, Formal analysis; AG: Validation, Investigation, Formal analysis; TB: Formal analysis, Writing – review & editing; LG: Synthesis, Investigation; RM: Formal analysis, Writing – review & editing; VS: Formal analysis, Writing – review & editing; GM: Conceptualization, Validation, Formal analysis, Supervision, Writing – original draft; VL: Conceptualization, Validation, Supervision, Funding acquisition, Project administration, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhylkybayeva, N., Paliienko, K., Topchylo, A. et al. Size dependent properties of Gd3+-free versus Gd3+-doped carbon dots for bioimaging application. Sci Rep 14, 27812 (2024). https://doi.org/10.1038/s41598-024-76500-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76500-z