Abstract
Chromosome microarray analysis (CMA) and whole exome sequencing (WES) are increasingly utilized in prenatal diagnosis of abnormal ultrasound findings, but studies on correlation between pathogenic copy number variations (pCNVs) and single-gene mutations in fetuses with nuchal translucency (NT) thickening/cystic hygroma (CH), and pregnancy outcomes, are rare. This study aimed to investigate clinical value of CMA and WES for NT thickening/CH in fetuses, explore genetic correlation between fetal NT thickening and CH, and analyze pregnancy outcomes. We retrospectively selected 215 pregnant women diagnosed with fetal NT thickening (NT > 95th)/CH who underwent invasive prenatal diagnosis at our hospital from January 2020 to June 2022. With negative chromosomal karyotype analysis (KA) and CMA results, patients voluntarily underwent WES. Patients were grouped by NT thickening/CH, and application value of KA, CMA, and WES examined. Ultrasound findings, pregnancy outcomes, and fetal growth post-birth were followed during mid/late pregnancy and post-delivery. Abnormalities in chromosomal number were detected in 28 of 215 samples, with a detection rate of 13.0%, and pCNVs were detected in 12 cases, with a detection rate of 5.6%. The most common abnormality in fetuses from both groups suggested by CMA was 22q11.21 microdeletion-microduplication syndrome. 35 patients with negative KA and CMA results underwent WES, and single gene variants were detected in 12 fetuses, with an abnormality rate of 34.3%. The incidence of adverse pregnancy outcomes was 28.2% in the NT thickening group and 82.9% in the CH group (P < 0.05). Overall, fetal NT thickening/CH was associated with genetic abnormalities, WES further improved the diagnosis of abnormal fetuses after negative KA and CMA results in both groups, and the incidence of adverse pregnancy outcomes was lower in the NT thickening group than in the CH group. The management of pregnancy outcomes could guide clinical genetic counselling.
Similar content being viewed by others
Introduction
Fetal nuchal translucency (NT) is the thickness of fluid accumulated between the skin and soft tissues of the posterior nuchal region of the foetus at 11–13 + 6 weeks of gestation and at a crown-rump length (CRL) of 45–84 mm, which can reflect the lymphatic fluid of the subcutaneous tissues of the foetus1,2. A small amount of fluid thickness can be measured in most fetuses. As the lymphatic system develops gradually around the 14th week of gestation, the lymphatic fluid in the neck returns to the heart through the internal jugular vein and the NT gradually disappears, ultimately forming the posterior nuchal folds of the skin. As an important indicator for screening fetal abnormalities in early pregnancy, NT thickening has been shown to be closely associated with chromosomal aneuploidy, such as T21 and T18, since the 1990s3,4 and is one of the first indicators used to confirm the relationship between fetal malformations and soft-index abnormalities. NT thickening may also indicate abnormalities in fetal organ development, such as congenital heart disease, respiratory, urinary, digestive, and skeletal malformations, with cardiac malformations being the most frequent3,4. A thickened NT may progress to a large cystic lymphangioma with or without fetal edema. The vast majority of fetal NT thickenings return to normal by mid-trimester.
Fetal nuchal cystic hygroma (CH) is a cystic echogenic area within the soft tissue of the skin at the back of the neck of the fetus and is mainly a lymphatic cyst5. According to the presence or absence of segregation within the cyst, it can be divided into segregated and non-segregated subtypes, with segregated subtypes accounting for more than 85% of the total number of cysts. A clinical diagnosis of fetal nuchal cystic hygroma is generally accepted as a segregated subtype6. Fetuses with CH are often associated with chromosomal abnormalities and/or structural malformations. The incidence of chromosomal abnormalities in fetuses with CH ranges from 40.0 to 63.2%, and these chromosomal abnormalities include 45,X, T18, T21 and T13 combined with cardiac malformations, skeletal malformations and so on7,8.
The 2020 American College of Obstetricians and Gynecologists (ACOG) guidelines on screening for fetal chromosomal abnormalities stated that prenatal genetic testing should be routinely performed in cases of NT thickening9. Chromosome microarray analysis (CMA) is the first-line choice for prenatal diagnosis10, which can detect chromosomal abnormalities in 20-30% of fetuses with NT thickening11, but the etiology of many NT thickening cases cannot be explained by CMA, especially when combined with an underlying single-gene genetic disorder. Whole-exome sequencing (WES) is a second-generation sequencing technology that can be used for the detection of single-gene genetic disorders12 and has the advantages of a wide range of detection, high detection rates, and cost-effective testing13. It was first successfully applied in pediatrics14 and is now gradually being applied to prenatal diagnosis. Lord et al.15 and Petrovski et al.16 found that the diagnosis rate of single-gene genetic diseases in fetuses with structural abnormalities suggested by ultrasound was 10.0% and 8.5%, respectively, suggesting that WES can make up for the shortcomings of traditional prenatal diagnosis. In 2020, the American College of Medical Genetics and Genomics (ACMG) issued guidelines formally recommending that further prenatal exome testing should be performed to exclude fetal single-gene variants in cases where ultrasound reveals fetal structural abnormalities and CMA results are normal17.
Few fetal abnormalities can be detected in early pregnancy, but fetal NT thickening and CH are the two most important ultrasound abnormalities that can be detected in early pregnancy at 11–13 + 6 weeks. However, studies on the genetic correlation between these two fetal ultrasound abnormalities are rare. In 2018, Chinese scholars pointed out in a study of 216 fetuses with NT > 3 mm that the significant thickening of NT has a similar pathophysiological mechanism to the occurrence of CH18. In 2022, Sumalatha studied the outcome of pregnancies with first-trimester increased nuchal translucency and cystic hygromas19. In this study, we analyzed the KA and CMA results of all patients who underwent interventional prenatal diagnosis for NT thickening/CH and analyzed the results of further WES testing for negative CMA results, aiming to investigate the clinical value of CMA and WES for NT thickening/CH in fetuses and to explore the correlation between genetic abnormalities of fetal NT thickening and CH, in order to provide a basis for clinical genetic counselling and prenatal diagnosis decision making.
Methods
Participants
From January 2020 to June 2022, 215 pregnant women with singleton pregnancies indicating the presence of NT thickening/CH during early pregnancy (11 to 13 + 6 weeks of gestation) underwent chromosomal karyotype analysis (KA) and CMA. The genetic samples collected included 209 cases of amniotic fluid and six cases of abortion/induced labor tissue. According to the age of the pregnant woman and the gestational week of the fetus at the time of consultation, the pregnant woman and her family independently chose the appropriate sampling method after full communication and under the condition of full understanding of the relevant contents, and informed consent was signed by the woman or her relatives. This study was approved by the Ethics Committee of the affiliated Yancheng Maternity&Child Health Hospital of Yangzhou University (KY2022010).
Sample collection included the following three methods. (1) Amniocentesis sampling: after 18 weeks of gestation, scanning guided by a dedicated sonographer and transabdominal amniocentesis sampling guided by ultrasound were performed by a dedicated prenatal diagnostician. (2) Miscarriage tissue sampling: early pregnancy miscarriage tissue was selected from the chorionic villi and washed with 0.9% saline to remove the meconium tissue and avoid contamination of maternal cells as much as possible. Mid-trimester abortion tissue was obtained from the fetal skin tissue, selected samples without visible blood components, and stored at -20℃. (3) Peripheral blood sampling: peripheral venous blood (2 mL) from pregnant women and their husbands were collected and placed in EDTA anticoagulation tubes.
Study design
Fetal samples from patients with NT thickening/CH were collected and parental data were supplemented for subsequent family analysis. Ultrasound screening was performed at 16–18 weeks in the middle trimester to observe the fetus for structural malformations and to supplement the morphological data of the fetus.
In this study, the enrolled patients were divided into two major groups according to fetal NT thickening/CH and then into two subgroups according to whether they were combined with other abnormalities suggested by ultrasound (including absence of nasal bone, pleural effusion, pericardial effusion, subcutaneous edema, and cardiac displacement).The NT thickening group was divided into group A (isolated NT thickening > 95th percentile, n = 157) and group B (NT thickening combined with other abnormalities, n = 17). The CH group was divided into Group C (isolated nuchal CH, n = 33) and Group D (nuchal CH combined with other abnormalities, n = 8). The cases were subdivided into four groups aimed at categorizing pregnancy outcomes for exploration. KA and CMA were performed in all patients, WES was performed on fetuses with normal CMA results, and pregnancy outcomes were followed.
Experimental procedure
Amniotic fluid cell culture and karyotyping were performed according to standard operating procedures20.
DNA was extracted from chorionic villi, amniotic fluid, or parental peripheral blood according to the manufacturer’s instructions. The sample volume of DNA was 250 ng, and the quality control requirements of DNA were OD260/280 values of 1.8-2.0. CMA was performed using CytoScan® 750 K Microarrays (Affymetrix, Santa Clara, CA, USA). Genomic DNA was digested, linked, amplified, purified, fragmented, labelled, hybridized, washed, and scanned, and the data were analyzed using the Chromosome Analysis Suite (ChAS 3.2) software. For the analysis and determination of CNV properties, we used publicly relevant online databases such as DECIPHER (http://www.sanger.ac.uk), OMIM (http://www.omim.org) and DGV (http://projects.tcag.ca/variation). If necessary, the discovered CNVs were further verified by RT-PCR or FISH combined with the analysis of the parents’ peripheral blood samples21,22.
Whole exome sequencing was performed on the remaining DNA samples using NGS. A HiSeq2500 genome analyzer (Illumina Inc., San Diego, CA, USA) was used. The HGMD (http://www.hgmd.cf.ac.uk/ac/index.php), gnomAD (http://gnomad.broadinstitute.org), ClinGen (https://www.clinicalgenome.org/), ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and NCBI SNP (http://www.ncbi.nlm.nih.gov/snp) were used to analyze potential pathogenic homozygous and compound heterozygous variants21,22. Sanger sequencing was used to verify that the fetuses and their parents had disease-causing or potentially disease-causing mutation sites.
Follow-up
Ultrasound scan reports and clinical information from the second trimester were reviewed, fetal systematic ultrasound and fetal ultrasound cardiogram (FUCG) results were subsequently obtained, and pregnancy outcomes, including gestational week of delivery, induction of labor, and pathological findings of the induced fetus, were recorded. Those who delivered or terminated their pregnancies in our hospital were enrolled through the e-case system, and those who did not deliver or terminate their pregnancies in our hospital were followed up by telephone until six months after delivery. All patients were followed up by telephone to assess pregnancy outcomes, and growth and development after birth, with the last follow-up occurring on 30/06/2023. All of the above information was stored in local databases (local ultrasound database, antenatal diagnostic workstation, and hospital database).
Statistical analysis
Statistical software (SPSS 26.0) was used for the data analysis. Normally distributed data, such as age and gestational age, were expressed as mean ± standard deviation, and an independent sample t-test was performed. Count data, such as rates and percentages, were compared between groups using the chi-square test or corrected chi-square test at the α = 0.05, bilateral test level, and the difference was considered to be statistically significant at P < 0.05.
Results
Study participants
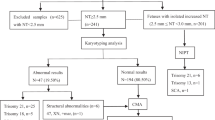
From January 2020 to June 2022, 215 pregnant women with fetal NT thickening (NT > 95th percentile)/cystic hygroma were enrolled in the study. There were 174 in the NT thickening group and 41 in the nuchal cystic hygroma group. The flow chart of prenatal diagnosis was shown in Fig. 1. The characteristics of the study population were summarized in Table 1.
KA and CMA results
Among the 215 patients, chromosomal number abnormalities were detected in 28 patients by KA, accounting for 13.0% (28/215), T21 was the most common chromosomal abnormality, accounting for 6.0% (13/215). CMA not only detected all chromosomal number abnormalities, but also 12 additional cases of pCNV, increasing the detection rate by 5.6% (12/215) ( Table 2; Fig. 1 ).
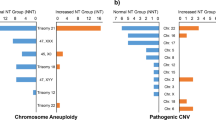
There were 23 cases of chromosomal and pCNV abnormalities in the fetal NT thickening group out of 174 cases (13.2%) and 17 cases of chromosomal and pCNV abnormalities in the fetal CH group out of 41 cases (41.5%). Six pCNVs were detected in each of the two major groups, and the detection rates increased by 3.4% (6/174) and 14.6% (6/41) by applying CMA respectively ( Table 2; Fig. 1 ). In the NT group, one case of 22q11.21 microdeletion, two cases of 22q11.21 microduplication, and two cases of 15q11.2 microdeletion were detected, and in the CH group, two cases of 22q11.21 microdeletion and two cases of 22q11.21 microduplication were detected (Table 3).
WES results
Of those with normal KA and CMA test results who continued their pregnancy, 35 underwent whole exome sequencing (WES) testing. The evaluation revealed a total of 12 fetuses (15 loci) with single gene variants, with an abnormality rate of 34.3% (12/35), which led to abnormalities in various systems. Disorders in the RAS-MAPK signalling pathway accounted for the highest proportion (50%) of patients (6/12), and involved mutations in PTPN11, RIT1, BRAF, and SOS1. Hematological disorders accounted for 25% (3/12) of the patients and involved mutations in CDAN1 and PIEZO1, cardiovascular system diseases accounted for 8.3% (1/12) of the patients and involved MYH7 mutation, one case of skeletal system disease involving EVC2 gene mutation, and one case of renal system disease involving COL4A5 gene mutation. The WES results revealed that the most common monogenic disease was Noonan syndrome, with 6 cases in both groups; haematological diseases, with 2 in the NT thickening group and 1 in the CH group; and cardiovascular diseases, with 1 in both groups ( Table 4; Fig. 1 ).
Comparison of KA/CMA/WES test results between the two groups
In the NT thickening group, there were 174 patients, KA detected 17 (9.8%) abnormalities, CMA detected a total of 23 (13.2%) abnormalities, with an additional 6 pCNVs detected in the NT group. 26 patients (26/174, 14.9%) in NT group opted for WES, and 7 abnormalities were detected (7/26, 26.9%). A total of 41 patients in the CH group and 11 (26.8%) abnormalities were detected by KA, and a total of 17 (41.5%) abnormalities were detected by CMA, which detected an additional six patients with pCNVs over KA. WES was chosen for nine (9/41, 22.0%) patients in the CH group, with five abnormal patients, accounting for 55.6% (5/9) of the sample. Statistical analysis revealed that there was a significant difference in the percentage of fetal KA-positive results and CMA-positive results between the NT/CH groups (P < 0.05, when KA was selected; P < 0.05, when CMA was selected) (Table 5; Fig. 1 ). Compared with the NT group, the CH group had a higher incidence of abnormalities.
Follow-up results
Among the 215 patients, 6 aborted/induced labor tissues were excluded, 23 of the remaining 209 patients were lost to follow-up, 186 patients were followed up successfully, and the follow-up success rate was 89.0% (186/209). 51 patients with genetic abnormalities (29 patients in the NT thickening group and 22 patients in the CH group) were terminated, except for the birth of 1 foetus with a mutation in the PTPN11 gene. Of the 163 patients with negative genetic test results, 4 had aborted tissues sent for testing, and 159 had prenatal samples sent for testing; WES was performed for 35 patients and not performed for 128 patients (Fig. 2; Table 6 ). The overall incidence of adverse pregnancy outcomes was 39.7% (69/174) and 90.2% (37/41) in the NT thickening group and CH group. It was significantly different between the two groups (X2 = 33.974, P < 0.05).
Discussion
In fetuses with NT thickening, previous studies reported that the detection rate of chromosomal abnormalities was approximately 20-30% for NT ≥ 3.0 mm, the detection rate of chromosomal abnormalities increased to 40% for NT ≥ 4.5 mm23,24, and the chromosomal abnormalities included T21, T18, T13, 45,X, triploidy, and so on. The detection rate of chromosomal abnormalities in CH fetuses ranges from 40 to 63.2%, and chromosomal abnormalities include 45,X, T18, T21, and T137,8. The results of our study suggest that the detection rate of chromosomal abnormalities in fetuses with NT thickening by karyotype was 9.8%, which was lower than the detection rate of approximately 20-30% reported in the literature. The reasons for this difference were as follows: (1) most patients in our cohort had isolated NT thickening, with a low proportion of other structural abnormalities in combination (17/174, 9.8%); (2) a greater proportion of patients were in the group with an NT thickness of 3–3.4 mm. The detection rate of chromosomal abnormalities in fetuses with CH by karyotype in our study was lower than the 43.3% reported by Corbacioglu and 63.2% reported by Schreurs7,8, mainly due to the low percentage of patients with combined structural abnormalities in our enrolled CH group. Compared with the fetuses in the NT thickening group, the incidence of combined chromosomal abnormalities was greater in fetuses with CH in early pregnancy, and there was a significant difference in the prevalence of chromosomal abnormalities between the two groups, which provides appropriate guidance for clinical genetic counselling.
At the same time, the detection rate of pCNVs in the NT thickening group was 3.4%, which was slightly higher than that reported by Egloff (2.7%)25. It could be related to the fact that the bias we enrolled (174) was much smaller than the number of cases Egloff enrolled (720). In previous studies, CMA was rarely used for the detection of CH, which was mostly mixed with NT thickening23,24. The pCNVs of the two groups partially overlapped in our studies, suggesting that NT thickening and CH have a similar genetic etiology. The 22q11.21 region contains 47 protein-coding genes, including OMIM disease-related genes such as TBX1 (602054), RTN4R (605566), and PI4KA (600286). 22q11.2 deletion syndromes mainly manifest as DiGeorge syndrome (DGS), Velovelo-cardio-facial syndrome (VCFS), and conotruncal anomaly face syndrome (CAFS)26,27. The parents of fetuses 3, 7 and 8 decided to terminate the pregnancy after receiving genetic counselling. 22q11.21 duplication syndrome has high clinical heterogeneity, and its main clinical phenotypes include mental retardation, dwarfism, dysarthria, wide eye spacing, and hypotonia. Some patients do not have obvious clinical phenotypes and have incomplete explicit characteristics (penetrance was approximately 21.9%)26,27,28. These patients usually have a family history, and the parents of the fetuses 2 and 5 decided to terminate the pregnancies after learning about the relevant information. The 15q11.2 region contains six protein-coding genes and the OMIM causative gene NIPA1 (608145), which is associated with the dominantly inherited diseases spasticity and paraplegia. According to the literature, 15q11.2 (BP1-BP2) heterozygous deletion is a neurophenotypic susceptibility locus, and carriers of 15q11.2 heterozygous deletion have major phenotypes, including developmental delays, language disorders, dyspraxia, autism, learning difficulties, and epilepsy. These phenotypes are characterized by incomplete epistasis (8-10% epistasis) and high clinical heterogeneity, and most of the 15q11.2 deletions in affected patients are inherited from their parents29. The child was followed up later by querying the system and by telephone, and follow-up data showed that his growth and development were normal at present.
In our study, 7 of 26 WES tests were positive in the NT group and 5 of 9 WES tests were positive in the CH group. Researchers such as Lord J and Petrovski S used WES to detect 93 and 51 fetuses with NT thickening, respectively, with diagnosis rates of 3.2% and 12%, respectively15,16. The detection rate of 26.9% in our NT group was greater than that of 3.2% in Lord’s research and 12% in Petrovski’s research. These differences may be caused by the number of patients included in our study and differences in NT thickness. WES studies on the use of isolated fetal CH are relatively rare. The major reports in the literature treated NT thickening and nuchal CH together without making a clear distinction between them15,16.
In this study, WES results showed that the most common monogenic disease was Noonan syndrome, involving the PTPN11, SOS1, BRAF and RIT1 genes, all of which could lead to abnormalities in the RAS-MAPK signalling pathway. Among them, 3 patients had PTPN11 point mutations. The PTPN11 point mutation causes Noonan syndrome type I (NS), and patients with this mutation have a special face, small body size, cardiovascular abnormalities and other conditions. It has been shown that lymphatic dysplasia in NS patients during the embryonic stage interferes with tissue migration, resulting in fetal ultrasonography revealing NT thickening and CH16. Among the 12 WES-positive fetuses in this study, 9 had new mutations, and the risk of the mutation was very low when the mother was pregnant again; therefore, prenatal diagnosis could be used to rule out related genetic diseases. One patient had an autosomal dominant inheritance from the mother, and the risk of the fetus developing the mutation was 50% when the mother became pregnant again. Two other cases were autosomal recessive, the mutation site was inherited from the parents, and the risk of the fetus developing the mutation was 25% when the mother became pregnant again. WES provides an accurate reference for clinical genetic counselling.
In addition, hematological diseases were detected in three fetuses in this study, and determining the relationship between their genotypes and ultrasound phenotypes was difficult. We referred to the Expert Consensus on Prenatal Whole Exome Sequencing, which recommends that pregnant women and their families consult a specialist hematologist, given the potential for haematological disorders to lead to serious illnesses in childhood15,30. Therefore, before the use of WES for the prenatal diagnosis of a fetus, pregnant women and their families should be fully informed about this technique. Doctors should strictly grasp the technology of the indication, which is limited to imaging (ultrasound and magnetic resonance imaging) of fetal structural abnormalities found via genetic etiology screening.
At last, pregnancy outcomes were followed up. Our results showed that the rate of adverse pregnancy was 15.3% in the NT thickening group after no abnormalities were detected by genetic testing and ultrasound. Previous studies have shown that for fetuses with NT thickening (NT > 3.5 mm), after chromosomal diseases were excluded by genetic testing and no structural malformations were found during pregnancy, the adverse pregnancy outcome rate was between 25 and 30%, and only 2.0-4.2% of fetuses were mentally retarded after birth17,31. The results of our study were slightly lower than those of the two previous studies, which could be related to the number of selected patients with different NT thicknesses. In general, the fetal prognosis was better in fetuses with NT thickening. However, the prognosis of fetuses with CH was unsatisfactory. When chromosomal abnormalities and ultrasound structural abnormalities were excluded, the adverse pregnancy outcomes were still as high as 75.0%. On the one hand, the number of fetuses in the CH group was significantly lower than that in the NT thickening group; on the other hand, these findings also suggested that the poor prognosis of the CH fetus was related not only to chromosomal disease, but also to other factors. This conclusion requires the further inclusion of more fetuses with CH in follow-up studies. When we analyzed the pregnancy outcomes of the two groups of fetuses, we found that 37.5% (6/16) of the pregnant women in the CH group who were free of fetal chromosomal diseases and fetal structural malformations still chose to induce labor, which also posed a great challenge to clinical genetic counselling.
The limitation of this study was that there were fewer cases of concomitant abnormalities in the two groups of fetuses, and fewer cases in the CH group were enrolled (19.1%), which may lead to selection bias. We will collect and include more cases in future studies. Some fetuses with negative CMA and WES results gradually develop a phenotype after birth, suggesting the urgency of the clinical application of emerging technologies, such as WGS and RNA sequencing and so on.
In conclusion, based on negative KA and CMA tests in the two groups of fetuses, WES was performed to improve the diagnostic rate of fetuses with NT thickening/CH, and fetal pregnancy outcomes were followed up, providing clinicians with reliable clinical data, accurate genetic counselling for pregnant women and their families, and a basis for their final decision.
Data availability
The datasets supporting the major results/conclusions of this article are included within the article. Our sequencing raw data cannot be submitted to publicly available databases because the ethical approval did not permit sharing of raw sequencing data and the patients’ families did not consent to share their raw sequencing data. For further needs, please contact corresponding author.
References
Syngelaki, A. et al. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11–13 weeks’ Gestation. Ultrasound Obstet. Gynecol. 54, 468–476 (2019).
Yang, X. et al. Diagnostic yield of copy number variation sequencing in fetuses with increased nuchal translucency: A retrospective study. Arch. Gynecol. Obstet. 309, 139–144 (2024).
Bellai-Dussault, K. et al. Ultrasonographic fetal nuchal translucency measurements and cytogenetic outcomes. Jama Netw. Open. 7, e243689 (2024).
Kagan, K. O., Sonek, J. & Kozlowski, P. Antenatal Screening for chromosomal abnormalities. Arch. Gynecol. Obstet. 305, 825–835 (2022).
Vora, N. L. & Norton, M. E. Prenatal exome and genome sequencing for fetal structural abnormalities. Am. J. Obstet. Gynecol. 228, 140–149 (2023).
Malone, C. M. et al. Euploid first-trimester cystic hygroma: A more benign entity than previously thought? Fetal Diagn. Ther. 48, 667–671 (2021).
Schreurs, L. et al. First Trimester cystic Hygroma Colli: Retrospective analysis in a Tertiary Center. Eur. J. Obstet. Gynecol. Reprod. Biol. 231, 60–64 (2018).
Zhou, H. et al. Prenatal diagnosis and early childhood outcome of fetuses with extremely large nuchal translucency. Mol. Cytogenet. 16, 22 (2023).
Screening for Fetal Chromosomal Abnormalities. Acog practice bulletin summary, number 226. Obstet. Gynecol. 136, 859–867 (2020).
Levy, B. & Wapner, R. Prenatal diagnosis by chromosomal microarray analysis. Fertil. Steril. 109, 201–212 (2018).
Sinajon, P. et al. Microarray and rasopathy-disorder testing in fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 55, 383–390 (2020).
Vora, N. L. et al. Prenatal exome sequencing in anomalous fetuses: New opportunities and challenges. Genet. Med. 19, 1207–1216 (2017).
Jelin, A. C. & Vora, N. Whole exome sequencing: Applications in prenatal genetics. Obstet. Gynecol. Clin. N. Am. 45, 69–81 (2018).
Retterer, K. et al. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 18, 696–704 (2016).
Lord, J. et al. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (page): A cohort study. Lancet. 393, 747–757 (2019).
Petrovski, S. et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: A prospective cohort study. Lancet (British Edition). 393, 758–767 (2019).
Monaghan, K. G., Leach, N. T., Pekarek, D., Prasad, P. & Rose, N. C. The use of fetal exome sequencing in prenatal diagnosis: A points to consider document of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 22, 675–680 (2020).
Wang, Q. & Wang, X. Analysis for the pregnancy outcome of cystic hygroma fetuses and correlation with increased nuchal translucency in first trimester. Zhonghua Fu Chan Ke Za Zhi. 53, 665–670 (2018).
Narava, S., Balbir, S. S., Barpanda, S. & Bricker, L. Outcome of pregnancies with first-trimester increased nuchal translucency and cystic hygroma in a tertiary maternity hospital in United Arab Emirates. Int. J. Gynecol. Obstet. 159, 841–849 (2022).
Zheng, J. et al. Prenatal diagnosis of sex chromosome mosaicism with two marker chromosomes in three cell lines and a review of the literature. Mol. Med. Rep. 19, 1791–1796 (2019).
Xue, S. et al. Genetic examination for fetuses with increased fetal nuchal translucency by genomic technology. Cytogenet. Genome Res. 160, 57–62 (2020).
Fu, F. et al. Application of exome sequencing for prenatal diagnosis of fetal structural anomalies: Clinical experience and lessons learned from a cohort of 1618 fetuses. Genome Med. 14, 123 (2022).
Yang, D. et al. Application of chromosomal microarray analysis in 815 fetuses with increased nuchal translucency during early pregnancy. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 37, 833–838 (2020).
Ni, M. et al. Application of chromosomal microarray analysis in prenatal diagnosis of fetuses with increased nuchal translucency. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 36, 970–974 (2019).
Egloff, M. et al. Diagnostic yield of chromosomal microarray analysis in fetuses with isolated increased nuchal translucency: A French multicenter study. Ultrasound Obstet. Gynecol. 52, 715–721 (2018).
Freud, L. R. et al. Prenatal vs postnatal diagnosis of 22Q11.2 deletion syndrome: Cardiac and Noncardiac outcomes through 1 year of age. Am. J. Obstet. Gynecol. (2023).
Wang, J. et al. Preliminary study of noninvasive prenatal screening for 22Q11.2 deletion/duplication syndrome using multiplex dPCR assay. Orphanet J. Rare Dis. 18, 278 (2023).
Ehrlich, L. & Prakash, S. K. Copy-number variation in congenital heart disease. Curr. Opin. Genet. Dev. 77, 101986 (2022).
Butler, M. G. Prader-Willi Syndrome and chromosome 15Q11.2 Bp1-Bp2 region: A review. Int. J. Mol. Sci. 24, (2023).
Sparks, T. N. et al. Exome sequencing for prenatal diagnosis in nonimmune hydrops fetalis. N. Engl. J. Med. 383, 1746–1756 (2020).
Application, C. G. O. W., Lou, G., Hou, Q., Yang, K. & Guo, L. Expert consensus on the application of prenatal exome sequencing for fetal structural anomalies. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 39, 457–463 (2022).
Acknowledgements
First, we sincerely thank all the participants in this study for their cooperation and support. We thank the professionals of prenatal imaging, obstetrics, and pediatrics for contributing to the multidisciplinary consultation and clinical management of the pregnancies. We thank many nurses from our prenatal diagnostic center who contributed to the clinical follow-up. All authors read and approved the final manuscript.
Funding
This study was supported by grants from Jiangsu Maternal and Child Health Research Project (F201860), Yancheng Basic Research Programme Projects (YCBK202205, YCBK2023093 ), Yancheng Key R&D Programme for Social Development (YCBE202326, YCBE202359).
Author information
Authors and Affiliations
Contributions
Jianli Zheng analyzed and interpreted the data and wrote the manuscript. Tiantian Wang conducted the clinical data summary, analysis and patient clinical follow-up collection. Jianli Zheng, Huilin Sun, Feifei Ying and Jianbing Liu performed raw data analysis, interpretation, and Sanger sequencing. Yongjuan Guan, Fangfang Yang and Jing Wu performed the karyotype analysis. Jianbing Liu, Min Li recruited clinical information and conducted genetic counseling. Yadong Fu performed the validation tests patient clinical follow-up collection. Jianli Zheng and Jianbing Liu designed, supervised the studies and acquired funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Affiliated Yancheng Maternity&Child Health Hospital of Yangzhou University (KY2022010). Written informed consent was obtained from all participating families. This study was performed in accordance with the principles of the Helsinki Declaration.
Consent for publication
Written informed consent was obtained to publish the clinical details presented in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, J., Wang, T., Sun, H. et al. Genetic correlation between fetal nuchal translucency thickening and cystic hygroma and exploration of pregnancy outcome. Sci Rep 14, 27191 (2024). https://doi.org/10.1038/s41598-024-76628-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76628-y